Published online May 16, 2021. doi: 10.12998/wjcc.v9.i14.3394
Peer-review started: December 30, 2020
First decision: February 12, 2021
Revised: February 21, 2021
Accepted: March 3, 2021
Article in press: March 3, 2021
Published online: May 16, 2021
Processing time: 119 Days and 18.6 Hours
Tenosynovial giant cell tumors (TGCTs) are a frequent benign proliferative disease originating from the synovial membrane. However, TGCTs rarely occur in the spine. The purpose of this paper is to report a case of TGCT occurring in the cervical spine. Although the disease is rare, it is essential to consider the possibility of TGCT in axial skeletal lesions. Awareness of spinal TGCTs is important because their characteristics are similar to common spinal tumor lesions.
A 49-year-old man with a 2-year history of neck pain and weakness in both lower extremities was referred to our ward. Imaging revealed a mass extending from the left epidural space to the C4-5 paravertebral muscles with uneven enhancement. The tumor originated in the synovium of the C4-5 lesser joint and eroded mainly the C4-5 vertebral arch and spine. Puncture biopsy was suggestive of a giant cell-rich lesion. The patient had pulmonary tuberculosis, and we first administered anti-tuberculosis treatment. After the preoperative requirements of the anti-tuberculosis treatment were met, we used a posterior cervical approach to completely remove the mass after fixation with eight pedicle screws. The mass was identified as a TGCT by postoperative immunohistochemical analysis. Recurrence was not detected after 1 year of follow-up.
Spinal TGCTs are often misdiagnosed. The radiological changes are not specific. The ideal treatment comprises complete excision with proper internal fixation, which can significantly reduce postoperative recurrence.
Core Tip: This paper reviews a rare case of a tenosynovial giant cell tumor (TGCT) growing in the spine, eroding the C4-5 vertebral arches and the spinous processes, the radiological features of which mimic those of other neoplastic lesions. The definitive diagnosis of TGCT is made by immunohistochemistry. The ideal treatment comprised complete resection of the mass and appropriate internal fixation. By reviewing the diagnostic and therapeutic history and analyzing the clinical and radiological manifestations, a better understanding of the characteristics of TGCTs of the spine can be achieved, helping to improve their diagnosis and treatment.
- Citation: Zhu JH, Li M, Liang Y, Wu JH. Tenosynovial giant cell tumor involving the cervical spine: A case report . World J Clin Cases 2021; 9(14): 3394-3402
- URL: https://www.wjgnet.com/2307-8960/full/v9/i14/3394.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i14.3394
Tenosynovial giant cell tumors (TGCTs) are fibrous histiocytic tumors originating from the tendon sheath, synovium, or sac. The pathological features are mononuclear synovial cell proliferation, osteoclast-like multinucleated giant cells, and hemosiderin macrophages, and TGCTs rarely cause bone destruction[1]. In most cases, TGCTs involve the small joints of the fingers and hands (approximately 90%), and they can also occur in the elbows and knees (approximately 10%). In rare cases, TGCTs appear on the synovial membrane of the spinal accessory joints[2].
Here, we report the case of a patient with paralysis of both lower limbs caused by compression of the spinal cord due to bone destruction caused by a TGCT behind C4-5, which we surgically treated.
By reviewing the diagnostic and therapeutic history of this case and analyzing the clinical and radiological manifestations, a better understanding of the characteristics of TGCTs of the spine can be achieved, helping to improve their diagnosis and treatment. Although TGCTs are rare in the spine, they are similar to common spinal tumors in many features and TGCTs should be considered in the differential diagnosis when we diagnose axial skeletal lesions.
A 49-year-old man with a 2-year history of neck pain and weakness in both lower extremities was referred to our ward.
The patient was treated conservatively at the outpatient clinic of Xiangya Hospital, Central South University, over the previous four months. After 4 mo of strict conservative treatment, including nutritional support, pain relief, and herbal medicine interventions, the patient's symptoms were not relieved, and the weakness in both lower limbs progressed even more.
The patient had a history of pulmonary tuberculosis in both upper lungs for 4 years and was not on regular anti-tuberculosis medication.
The patient had no specific personal or family history.
The patient had decreased muscle strength (grade 3) in both lower extremities, significant sensory loss in the left thumb and index finger, active bilateral tendon reflexes, no Hoffmann's sign, and no significant abnormalities in the remaining extremities on physical examination.
The tuberculosis infection T-cell spotting test was positive, but sputum smears on three consecutive days were negative for acid-fast bacilli. The erythrocyte sedi
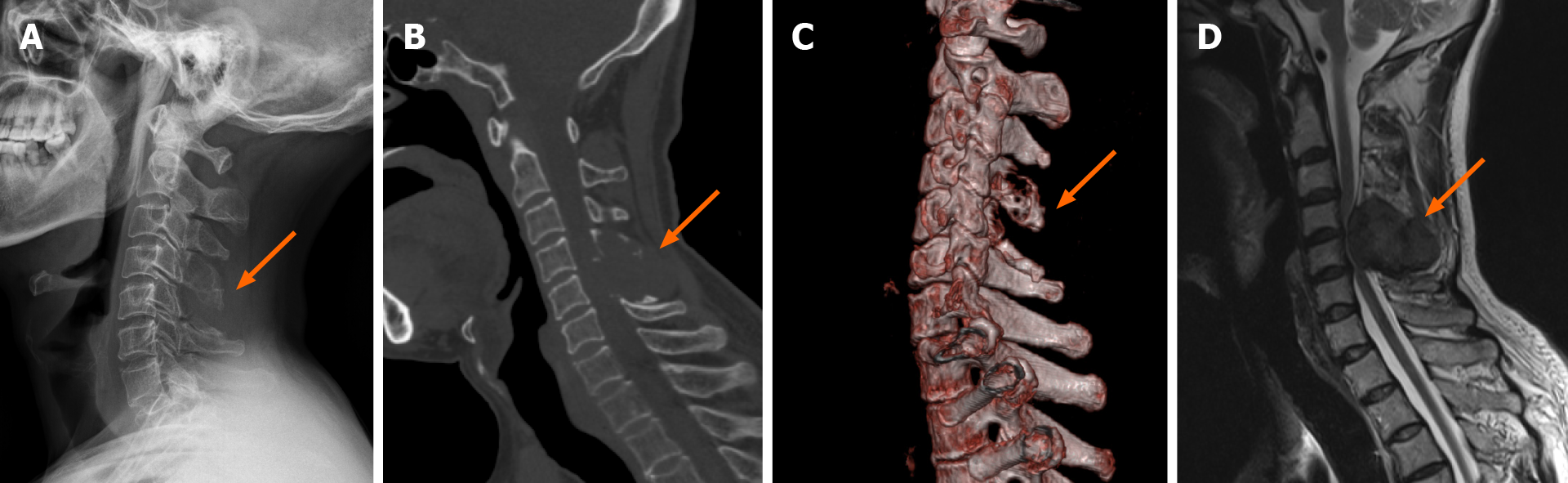
Plain X-ray radiography of the cervical spine showed destructive lesions in the appendage area of the C4-5 vertebrae (Figure 1A). A computed tomography (CT) scan of the cervical spine showed bone destruction and a soft tissue mass in the appendage area of the C4-5 vertebrae (Figure 1B). Spiral CT three-dimensional reconstruction showed the outline of bone destruction in the appendage area of C4-5 (Figure 1C). The nature of the mass was to be determined, considering the possibility of a benign bone tumor. A contrast-enhanced magnetic resonance imaging (MRI) scan revealed a neoplastic lesion extending from the left epidural space to the paravertebral muscles at C4-5. The lesion was isointense on T1-weighted imaging (T1WI) and heterogeneously hypointense on T2-weighted imaging (T2WI) and showed heterogeneous enhan
The patient was diagnosed with a TGCT.
After the preoperative requirements of the antituberculosis treatment were met, we were ready to perform surgery. After anesthesia was successfully induced, cranial ring arch traction was established. The operation was performed via the posterior approach of the cervical spine. The posterior structure of C2-C7 was exposed by incision and dissection to the paravertebral muscles on both sides. Bilateral pedicle screws were implanted at C2 and C7, and a pair of lateral mass screws were implanted bilaterally at C3, on the left side at C4, on the right side at C5, and bilaterally at C6. After that, titanium rods were connected to the screws. After fixation, the corresponding lamina was removed, and the egg-sized mass was visible, with an obvious boundary at the lower edge of C3, at C4/5, and at the upper edge of the C6 spinous process (Figure 2A). The cervical spinal canal as well as the nerve roots was decompressed until the spinal cord was relaxed and free of pressure; spinal cord pulsations were observed to return, and exploration of the spinal canal showed patency (Figure 2B). The surgical field was then soaked in distilled water, rinsed with saline, and thoroughly hemostatic. Next, an autologous cancellous bone graft was placed, and a silicone drain was left in place. Finally, the wound was sutured layer by layer and covered with a sterile dressing, the circumference of the neck was protected, the cranial ring arch was removed, and the patient was sent to the postanesthesia care unit and returned to the ward. The entire procedure took 4.5 h.
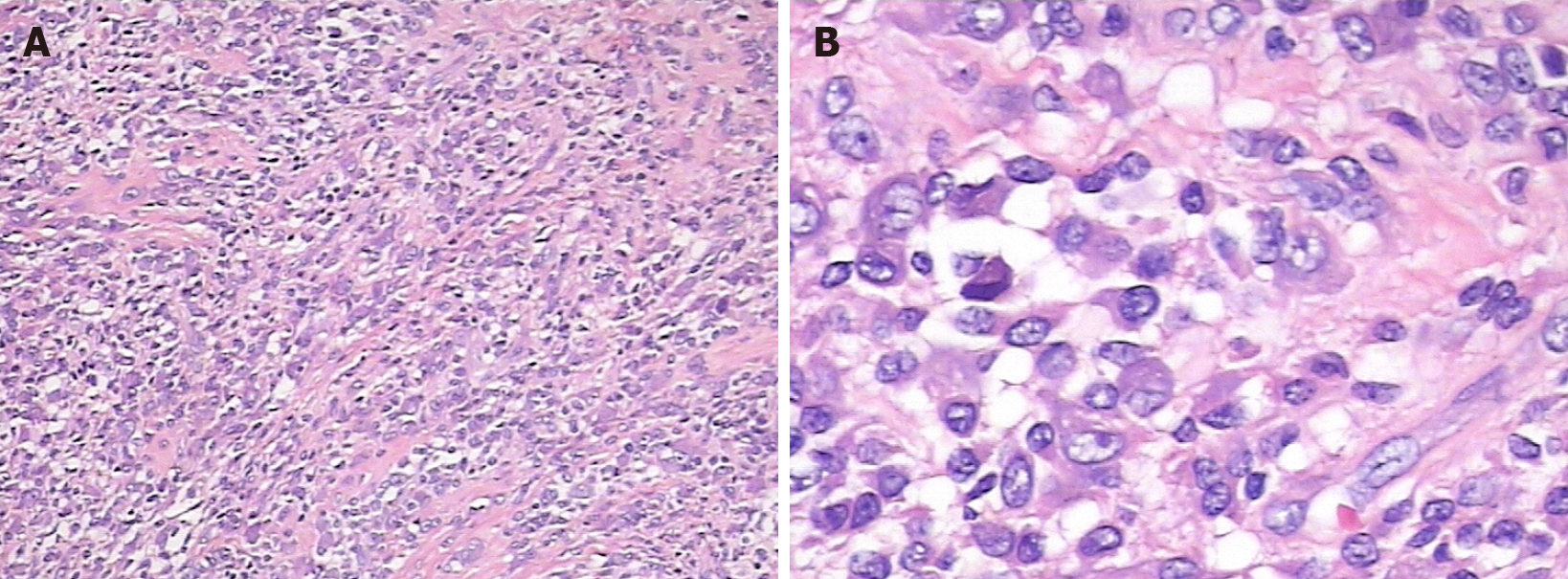
The tumor and adjacent normal tissue were completely removed and sent for cryosection and routine pathological examinations (Figure 2C). Lymphatic, plasma-like, and multinucleated cellular infiltrates were seen in the spine at the C4-5 level, and routine pathology and immunohistochemistry were needed to exclude hematopoietic tumors (Langerhans’ histiocytic hyperplasia, etc.), as shown by the cryosection examination results. Tumor tissues were taken for routine pathology combined with immunohistochemistry, with the following results: CD138 (-), CD68 (+), H3.3G34W (-), EMA (-), SATB2 (-), vimentin (+), S-100 (+), CD1a (-), Langerin (-), CD163 (-), and Ki67 (+, 10%), suggesting tendon sheath giant cells (Figure 3). Comprehensive consideration of the histopathological and immunohistochemical findings indicated that the tumor was a TGCT.
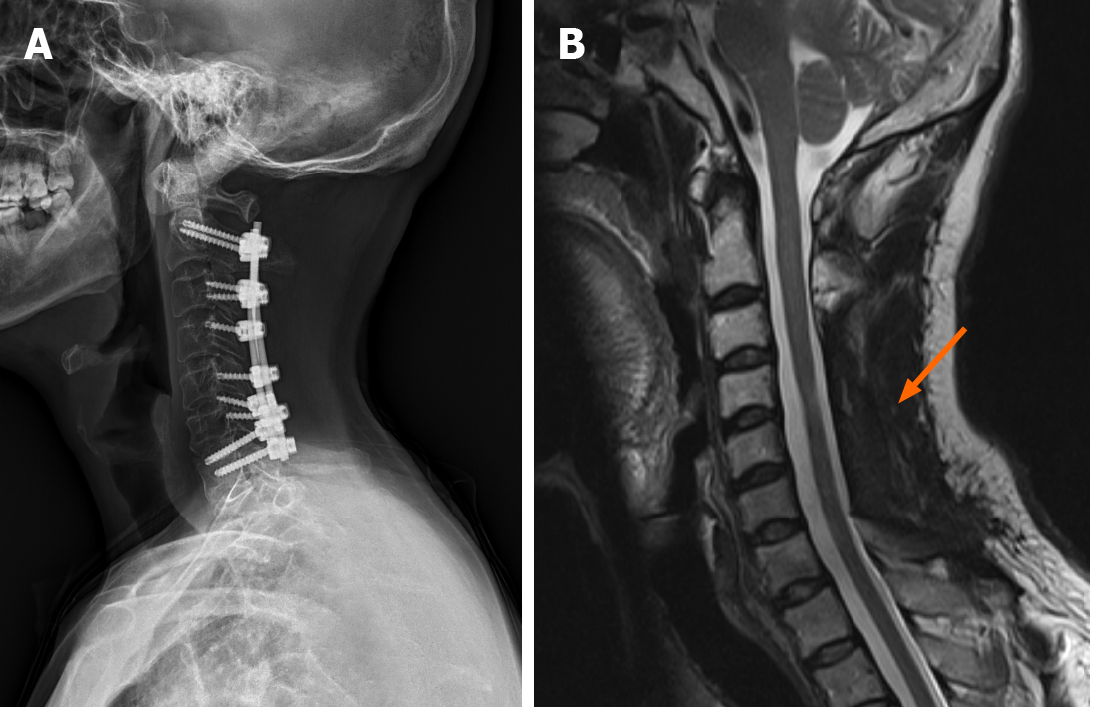
X-ray radiography performed 4 d after the surgery showed that the screws and titanium rods were in a good position, with no obvious loosening. The neck pain disappeared, the muscle strength of both lower extremities returned to grade 4, and limb sensation returned to normal after 2 wk of postoperative rehabilitation. There were no signs of internal fixation loosening on X-ray examination and no signs of local recurrence on MRI at the follow-up 1 year postoperatively (Figure 4).
TGCTs generally occur in the fingers, as well as in the ankles, wrists, and small joints of the lower limbs, but rarely in the spine[3]. Kleinman et al[3] first reported the case of a 65-year-old woman with a TGCT located in the cervical spine in 1980[3]. According to Wang et al[4], the cervical spine is the most common site of spinal TGCTs, followed by the lumbar spine and thoracic spine[4]. TGCTs of the spine usually originate from the synovium of the facet joints, grow diffusely outside the joints, and invade nearby vertebrae[5].
The most common origin of TGCTs has been reported to be the synovial membranes of the facet joints and bursa, depending on the location and growth characteristics, which can be classified as localized or diffused[6]. The diffuse type of TGCT affects the synovial membranes of large joints, such as the knees, hips, ankles, and elbows, while the localized type usually involves the tendons of the hands and feet[6]. TGCTs are also divided into intraarticular and extraarticular according to the site of growth. Intraarticular, diffuse TGCTs are also known as hyperpigmented villous nodular synovitis (PVNS); extraarticular tumors are slowly growing lesions with an excellent prognosis and can usually be removed completely. The etiology of PVNS is still controversial. Some scholars believe that it is an abnormality in lipid metabolism secondary to inflammatory trauma, others insist that it is a response to chronic trauma and recurrent bleeding, and others insist that it is a tumor[7-9]. The different types of TGCTs share the same morphological features on microscopy, mainly consisting of large synovial-like monocytes, small mononuclear histiocytes, and osteoclast-like giant cells.
Although there is no conclusive evidence regarding the cell of origin of TGCTs, most authors agree that TGCTs originate from fibroblasts and histiocytes of the synovium[10]. West et al[11] determined that the colony-stimulating factor 1 (CSF1) gene encoding the CSF1 receptor ligand is translocated in only 2% to 16% of tumor cells, suggesting that only a minority of TGCT cells are tumor cells[11]. By reviewing 81 cases of TGCT, Rao et al[12] demonstrated that the process of diffuse TGCT (also known as PVNS) formation is neoplastic[12]. TGCTs have been classified as fibrous histiocytic tumors in the World Health Organization (2012) classification of soft tissue and bone tumors.
TGCTs of the cervical spine originate in the synovium of the trabecular joint and grow out of the joint, but in one case, it was reported that the tumor bulged into the trabecular joint[6]. In our case, the tumor most likely originated in the cervical facet joints in the corresponding segments and grew slowly extra-articularly, eroding the spinous processes and vertebral plates, with clear borders of the lesion observed intraoperatively. Typically, giant cell tumors of the tendon sheaths present as slow-growing, extraarticular masses with clear borders that cause little discomfort[13]. If the tumor does not compress the spinal cord, the patient may be asymptomatic, but if it compresses the spinal cord and nerve roots, it may manifest as a series of neurological symptoms, so the size and location of the tumor determine whether the tumor can be detected at an early stage[14]. In our case, the patient initially developed a series of symptoms related to compression of the spinal cord and the C6 nerve root, which led to the discovery of a giant cell tumor of the cervical spine.
The classical appearance of tenosynovial cell tumors on X-rays is only a soft tissue mass, but sometimes they can also show calcification and, in rare cases, a periosteal reaction, which is not classical and makes the differential diagnosis difficult[15]. CT images of TGCTs show low soft tissue density; lesions may occasionally show high density with iron-containing heme, and images of eroded bone may be seen inci
In our reported case, CT revealed bone destruction and a soft tissue mass of an undetermined nature in the C4-5 adnexal region, and a benign bone tumor was considered likely. MRI indicated bone destruction and soft tissue formation in the C4-5 adnexal region, and a benign bone tumor with aggressive manifestations was considered likely (most likely an osteoblastoma). SPECT demonstrated slightly increased bone metabolism in the high cervical and 9th thoracic vertebrae, with a high likelihood of the lesion being benign. None of the radiographs could confirm the diagnosis of a TGCT, and we considered the differential diagnosis to include a giant cell tumor of bone, nerve sheath tumor, neurofibroma, tendinous fibroma, synovial sarcoma, and osteoblastoma, so we chose to perform a biopsy to help confirm the diagnosis. The pathological results of preoperative CT-guided biopsy showed only a small amount of giant cell-rich tissue, and the final mass dissected intraoperatively was sent for routine pathological testing and further immunohistochemistry to confirm the diagnosis of a TGCT. This suggests that single-modality imaging and preoperative and intraoperative pathological examinations may not always confirm the diagnosis and that further immunohistochemical analysis will ultimately lead to accurate conclusions and provide better guidance for further postoperative treatment.
Currently, the primary treatment for TGCTs of the spine is surgery, and internal fixation is not necessary due to the absence of bone destruction and intervertebral instability. In our case, there was significant bone destruction, and to remove the tumor completely, a portion of the posterior cervical column had to be removed, so we performed internal fixation after tumor removal. The primary consideration for surgical intervention is its propensity for local recurrence, which is closely related to the extent of surgical resection. Furlong et al[17] showed that in the follow-up of patients with TGCTs of the spine, no recurrence was found from 4 mo to 9 years after major or extensive tumor resection in five of the patients, while four of them experienced recurrence after incomplete resection for various reasons and thus also underwent secondary resection[17]. Furlong et al[17] also concluded that the diffuse growth pattern, the number of osteoclast-like giant cells, and the degree of epidural involvement were all associated with local recurrence, whereas the size and location of the tumor at the time of resection, patient sex, collagen content, and presence or absence of trabecular joint involvement were not associated with patient prog
There is no clear data in the literature to support whether radiation therapy after primary tumor resection helps prevent postoperative recurrence. The current consensus is that radiotherapy is given only to patients who, for various reasons, are unable to have their lesions removed[17]. TGCTs express high levels of CSF1R, which suggests that some chemotherapy regimens may also be effective for treatment. 1p11-13 is the most common region of structural rearrangement in TGCTs, and the most common chromosomal translocation is t(1:2)(p13:q37), where genetic fusions of CSF1 to COL6A3 were identified in molecular pathology studies[11,18]. CSF1, also known as macrophage colony-stimulating factor, is an important inflammatory mediator of inflammatory arthritis, and CSF1 expression is upregulated in both chronic inflam
We report a rare case of TGCT originating from the synovium of the C4-5 facet joint. The radiological changes were not specific and puncture biopsy was only informative; immunohistochemistry was needed for final confirmation of the diagnosis. The ideal treatment comprised complete resection of the mass and appropriate internal fixation, with complete resection or incomplete resection determining the likelihood of postoperative revision. Adjuvant radiotherapy and chemotherapy are currently used in cases of unresectable, residual, or recurrent disease. A review of this case will provide a better understanding of the characteristics of TGCTs of the spine and improve their diagnosis and treatment.
We would like to thank our patient for generously allowing us to share his case and Dr. Zhou JY for providing the pathological images.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sabouri AS S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 671] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 2. | Bui-Mansfield LT, Youngberg RA, Coughlin W, Chooljian D. MRI of giant cell tumor of the tendon sheath in the cervical spine. J Comput Assist Tomogr. 1996;20:113-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Kleinman GM, Dagi TF, Poletti CE. Villonodular synovitis in the spinal canal: case report. J Neurosurg. 1980;52:846-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Wang K, Zhu B, Yang S, Liu Z, Yu M, Liu X. Primary diffuse-type tenosynovial giant cell tumor of the spine: a report of 3 cases and systemic review of the literature. Turk Neurosurg. 2014;24:804-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Furuhata R, Iwanami A, Tsuji O, Nagoshi N, Suzuki S, Okada E, Fujita N, Yagi M, Matsumoto M, Nakamura M, Watanabe K. Tenosynovial giant cell tumor of the cervical spine: a case report. Spinal Cord Ser Cases. 2019;5:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Yamada S, Oshima K, Hamada K, Sotobori T, Joyama S, Hashimoto N, Outani H, Tanaka Y, Nakanishi K, Araki N. Giant cell tumor of the tendon sheath arising from a membrane surrounding the posterior arch of C1: a case report. Spine J. 2016;16:e353-e357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Gezen F, Akay KM, Aksu AY, Bedük A, Seber N. Spinal pigmented villonodular synovitis: a case report. Spine (Phila Pa 1976). 1996;21:642-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Granowitz SP, D'Antonio J, Mankin HL. The pathogenesis and long-term end results of pigmented villonodular synovitis. Clin Orthop Relat Res. 1976;335-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Weidner N, Challa VR, Bonsib SM, Davis CH Jr, Carrol TJ Jr. Giant cell tumors of synovium (Pigmented villonodular synovitis) involving the vertebral column. Cancer. 1986;57:2030-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | O'Connell JX, Fanburg JC, Rosenberg AE. Giant cell tumor of tendon sheath and pigmented villonodular synovitis: immunophenotype suggests a synovial cell origin. Hum Pathol. 1995;26:771-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | West RB, Rubin BP, Miller MA, Subramanian S, Kaygusuz G, Montgomery K, Zhu S, Marinelli RJ, De Luca A, Downs-Kelly E, Goldblum JR, Corless CL, Brown PO, Gilks CB, Nielsen TO, Huntsman D, van de Rijn M. A landscape effect in tenosynovial giant-cell tumor from activation of CSF1 expression by a translocation in a minority of tumor cells. Proc Natl Acad Sci USA. 2006;103:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 385] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 12. | Rao AS, Vigorita VJ. Pigmented villonodular synovitis (giant-cell tumor of the tendon sheath and synovial membrane). A review of eighty-one cases. J Bone Joint Surg Am. 1984;66:76-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 253] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Ushijima M, Hashimoto H, Tsuneyoshi M, Enjoji M. Giant cell tumor of the tendon sheath (nodular tenosynovitis). A study of 207 cases to compare the large joint group with the common digit group. Cancer. 1986;57:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Motamedi K, Murphey MD, Fetsch JF, Furlong MA, Vinh TN, Laskin WB, Sweet DE. Villonodular synovitis (PVNS) of the spine. Skeletal Radiol. 2005;34:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Karasick D, Karasick S. Giant cell tumor of tendon sheath: spectrum of radiologic findings. Skeletal Radiol. 1992;21:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Parmar HA, Sitoh YY, Tan KK, Teo J, Ibet S M, Hui F. MR imaging features of pigmented villonodular synovitis of the cervical spine. AJNR Am J Neuroradiol. 2004;25:146-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 17. | Furlong MA, Motamedi K, Laskin WB, Vinh TN, Murphey M, Sweet DE, Fetsch JF. Synovial-type giant cell tumors of the vertebral column: a clinicopathologic study of 15 cases, with a review of the literature and discussion of the differential diagnosis. Hum Pathol. 2003;34:670-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Nilsson M, Höglund M, Panagopoulos I, Sciot R, Dal Cin P, Debiec-Rychter M, Mertens F, Mandahl N. Molecular cytogenetic mapping of recurrent chromosomal breakpoints in tenosynovial giant cell tumors. Virchows Arch. 2002;441:475-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Douglass TG, Driggers L, Zhang JG, Hoa N, Delgado C, Williams CC, Dan Q, Sanchez R, Jeffes EW, Wepsic HT, Myers MP, Koths K, Jadus MR. Macrophage colony stimulating factor: not just for macrophages anymore! Int Immunopharmacol. 2008;8:1354-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Cupp JS, Miller MA, Montgomery KD, Nielsen TO, O'Connell JX, Huntsman D, van de Rijn M, Gilks CB, West RB. Translocation and expression of CSF1 in pigmented villonodular synovitis, tenosynovial giant cell tumor, rheumatoid arthritis and other reactive synovitides. Am J Surg Pathol. 2007;31:970-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Fiocco U, Sfriso P, Lunardi F, Pagnin E, Oliviero F, Scagliori E, Cozzi L, Vezzù M, Molena B, Scanu A, Panziera C, Nardacchione R, Rubaltelli L, Dayer JM, Calabrese F, Punzi L. Molecular pathways involved in synovial cell inflammation and tumoral proliferation in diffuse pigmented villonodular synovitis. Autoimmun Rev. 2010;9:780-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Blay JY, El Sayadi H, Thiesse P, Garret J, Ray-Coquard I. Complete response to imatinib in relapsing pigmented villonodular synovitis/tenosynovial giant cell tumor (PVNS/TGCT). Ann Oncol. 2008;19:821-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Ravi V, Wang WL, Lewis VO. Treatment of tenosynovial giant cell tumor and pigmented villonodular synovitis. Curr Opin Oncol. 2011;23:361-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Cassier PA, Gelderblom H, Stacchiotti S, Thomas D, Maki RG, Kroep JR, van der Graaf WT, Italiano A, Seddon B, Dômont J, Bompas E, Wagner AJ, Blay JY. Efficacy of imatinib mesylate for the treatment of locally advanced and/or metastatic tenosynovial giant cell tumor/pigmented villonodular synovitis. Cancer. 2012;118:1649-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 25. | Brownlow N, Russell AE, Saravanapavan H, Wiesmann M, Murray JM, Manley PW, Dibb NJ. Comparison of nilotinib and imatinib inhibition of FMS receptor signaling, macrophage production and osteoclastogenesis. Leukemia. 2008;22:649-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Cassier PA, Italiano A, Gomez-Roca CA, Le Tourneau C, Toulmonde M, Cannarile MA, Ries C, Brillouet A, Müller C, Jegg AM, Bröske AM, Dembowski M, Bray-French K, Freilinger C, Meneses-Lorente G, Baehner M, Harding R, Ratnayake J, Abiraj K, Gass N, Noh K, Christen RD, Ukarma L, Bompas E, Delord JP, Blay JY, Rüttinger D. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. Lancet Oncol. 2015;16:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 289] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 27. | Tap WD, Wainberg ZA, Anthony SP, Ibrahim PN, Zhang C, Healey JH, Chmielowski B, Staddon AP, Cohn AL, Shapiro GI, Keedy VL, Singh AS, Puzanov I, Kwak EL, Wagner AJ, Von Hoff DD, Weiss GJ, Ramanathan RK, Zhang J, Habets G, Zhang Y, Burton EA, Visor G, Sanftner L, Severson P, Nguyen H, Kim MJ, Marimuthu A, Tsang G, Shellooe R, Gee C, West BL, Hirth P, Nolop K, van de Rijn M, Hsu HH, Peterfy C, Lin PS, Tong-Starksen S, Bollag G. Structure-Guided Blockade of CSF1R Kinase in Tenosynovial Giant-Cell Tumor. N Engl J Med. 2015;373:428-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 426] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 28. | Brahmi M, Vinceneux A, Cassier PA. Current Systemic Treatment Options for Tenosynovial Giant Cell Tumor/Pigmented Villonodular Synovitis: Targeting the CSF1/CSF1R Axis. Curr Treat Options Oncol. 2016;17:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 29. | Lamb YN. Pexidartinib: First Approval. Drugs. 2019;79:1805-1812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |