Published online Apr 26, 2021. doi: 10.12998/wjcc.v9.i12.2930
Peer-review started: January 10, 2021
First decision: January 24, 2021
Revised: February 3, 2021
Accepted: February 24, 2021
Article in press: February 24, 2021
Published online: April 26, 2021
Processing time: 95 Days and 11.6 Hours
Sclerosing polycystic adenosis (SPA) is a rare disease of salivary glands, similar to fibrocystic disease of the breast. It occurs over a wide age range and exhibits a slight female preference. Most SPA cases have occurred in the parotid gland. The exact nature of SPA is unclear, but its tumor nature has recently been proposed. Although SPA has a good prognosis after adequate surgery, atypical lesions might occur, ranging from mild dysplasia to carcinoma in situ in some cases. To the best of our knowledge, only five cases of SPA in the submandibular gland have been reported to date. Here, we present two new cases of SPA involving the submandibular gland.
A 50-year-old woman and a 52-year-old woman were referred to Tongji Hospital in Wuhan, China, with complaints of moderate pain, recurrent swelling, and a mass in the submandibular area. After admission, the two cases of the submandibular mass were examined physically. The boundary of the submandibular tumor was clear, and the range of motion was good. After preoperative examinations, surgery was performed on a selective basis. Postoperative histopathological examination revealed a well-defined mass with acinar structures, ducts, or cystic dilated glands of various sizes scattered in a large number of proliferative sclerosing stroma. There were flat and cuboidal cells, and eosinophils in the duct epithelium. There was also a eosinophilic substance in the lumen of dilated cysts. No atypical epithelial hyperplasia, invasive growth, or carcinoma in situ was found. Based on the above findings, the mass was diagnosed as SPA. Both patients have remained asymptomatic and no recurrence or distant metastasis had occurred by the 7-mo and 5-year follow-up, respectively.
SPA is a rare disease of the salivary gland. Even though it has a good prognosis after adequate surgery, atypical lesions may occur from mild dysplasia to carcinoma in situ. However, no recurrence, distant metastasis, or mortality has been reported for submandibular gland SPA. Clinicians and pathologists should be familiar with the characteristics of SPA in the submandibular gland to avoid misdiagnosis and overtreatment.
Core Tip: Sclerosing polycystic adenosis (SPA) is a rare disease of the salivary gland. To the best of our knowledge, only 5 cases of SPA in the submandibular gland have been reported. Here, we present two new cases of SPA involving the submandibular gland. Even though SPA has a good prognosis after adequate surgery, atypical lesions may occur from mild dysplasia to carcinoma in situ. To date, there have been no reports of recurrence, distant metastases, or mortality associated with SPA in the submandibular gland. Clinicians and pathologists should be familiar with the characteristics of this disease to avoid misdiagnosis and overtreatment.
- Citation: Wu L, Wang Y, Hu CY, Huang CM. Sclerosing polycystic adenosis of the submandibular gland: Two case reports. World J Clin Cases 2021; 9(12): 2930-2936
- URL: https://www.wjgnet.com/2307-8960/full/v9/i12/2930.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i12.2930
Sclerosing polycystic adenosis (SPA) is a rare disease of the salivary gland similar to sclerosing adenosis and fibrocystic disease of the mammary gland[1]. It was first described by Smith et al[1] in 1996 in a series of nine cases and was recognized and described with increased frequency soon after the initial description[2]. In the fourth update of the World Health Organization Classification of Head and Neck Tumors, published recently, SPA has been included in the salivary gland tumors category under the subsection of other epithelial lesions[3]. Histopathologically, it is characterized by a well-circumscribed tubulocystic proliferation of glands within an abundant sclerotic stroma, with duct morphology ranging from intercalated duct-like to apocrine, and by the presence of acini with aberrant coarse red zymogen granules[3].
Since the incidence of SPA is low, many scholars lack knowledge about SPA. In particular, many cases suggest that some areas of SPA are heteromorphic and even in situ cancer. Therefore, clinicians, especially pathologists, must be knowledgeable about the diagnosis and treatment of SPA.
To the best of our knowledge, < 80 cases of SPA have been reported in the English literature. Most SPA cases have occurred in the parotid gland, with a few cases in the submandibular gland and minor salivary glands. Here, we report two additional cases of SPA occurring within the submandibular gland.
Case 1: Complaints of a 2-year history of a mass in the left submandibular area with pain.
Case 2: Complaints of swelling in the right submandibular gland for 40 d, which with associated pain.
Case 1: A 50-year-old Chinese female patient was referred to Tongji Hospital in Wuhan, China, with complaints of a 2-year history of a mass in the left submandibular area with pain.
Case 2: A 52-year-old Chinese female patient was referred to Tongji Hospital in Wuhan, China, with complaints of swelling in the right submandibular gland for 40 d, which with associated pain.
Both patients had no remarkable medical history.
The patient was generally in good health and had no history of alcohol, tobacco, or areca nut consumption.
Case 1: There was a mass in the left submandibular area, measuring approximately 3.0 cm × 2.5 cm, with a clear boundary (i.e. well-defined borders), medium texture, acceptable mobility, and no apparent tenderness.
Case 2: The swelling of the right submandibular area measured approximately 2.0 cm × 1.5 cm, was clear in boundary, medium in texture, and mobile, with a smooth surface and mild tenderness.
Routine laboratory tests revealed no remarkable abnormality in the two patients.
Case 1: Ultrasonographic examination showed a cystic-solid mixed lesion of the left submandibular gland.
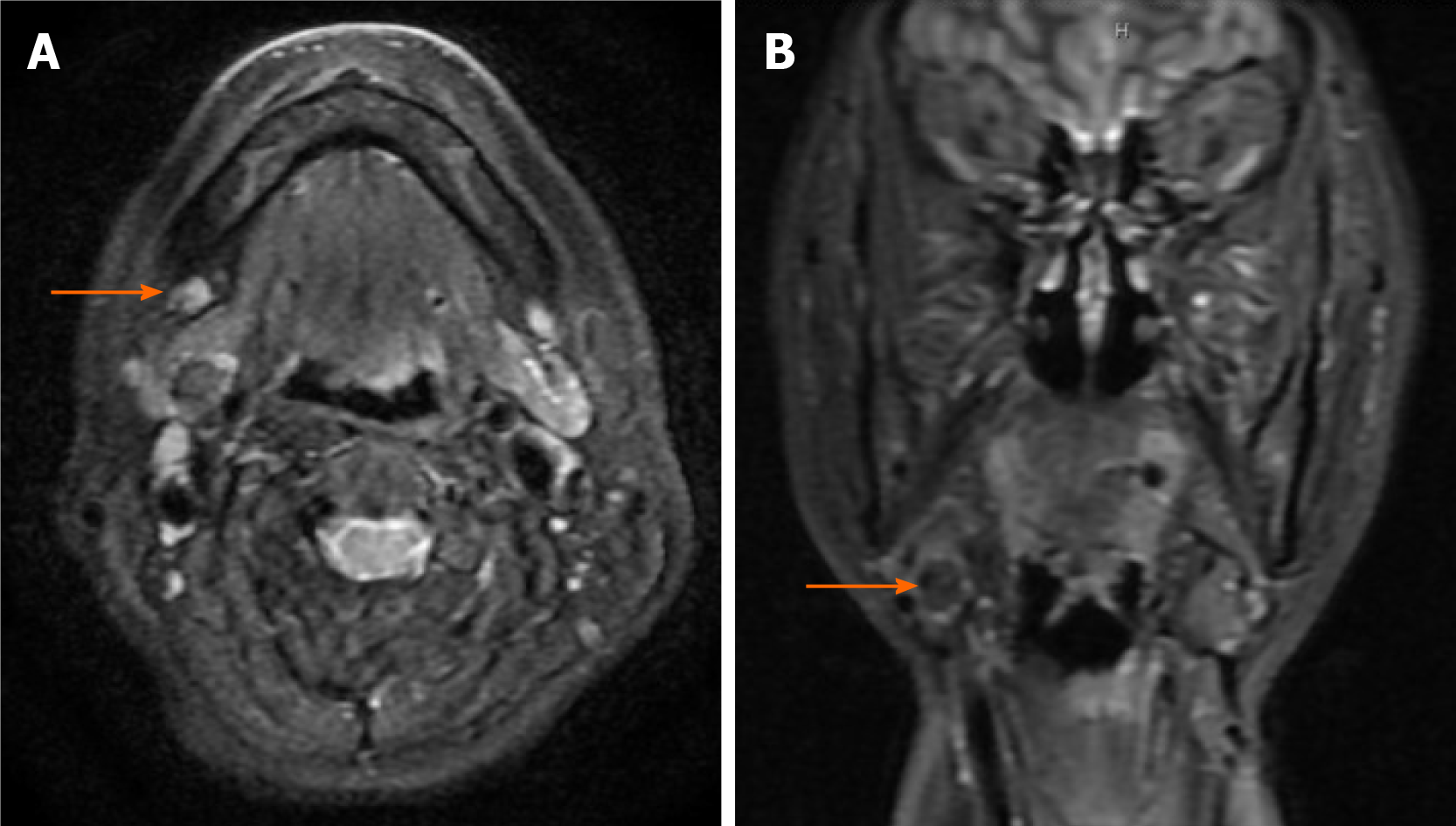
Case 2: Ultrasonographic examination revealed a mixed lesion accompanied by the partial enlargement of several lymph nodes. Moreover, magnetic resonance imaging (MRI) showed that the T2-weighted image was relatively hyperintense (Figure 1).
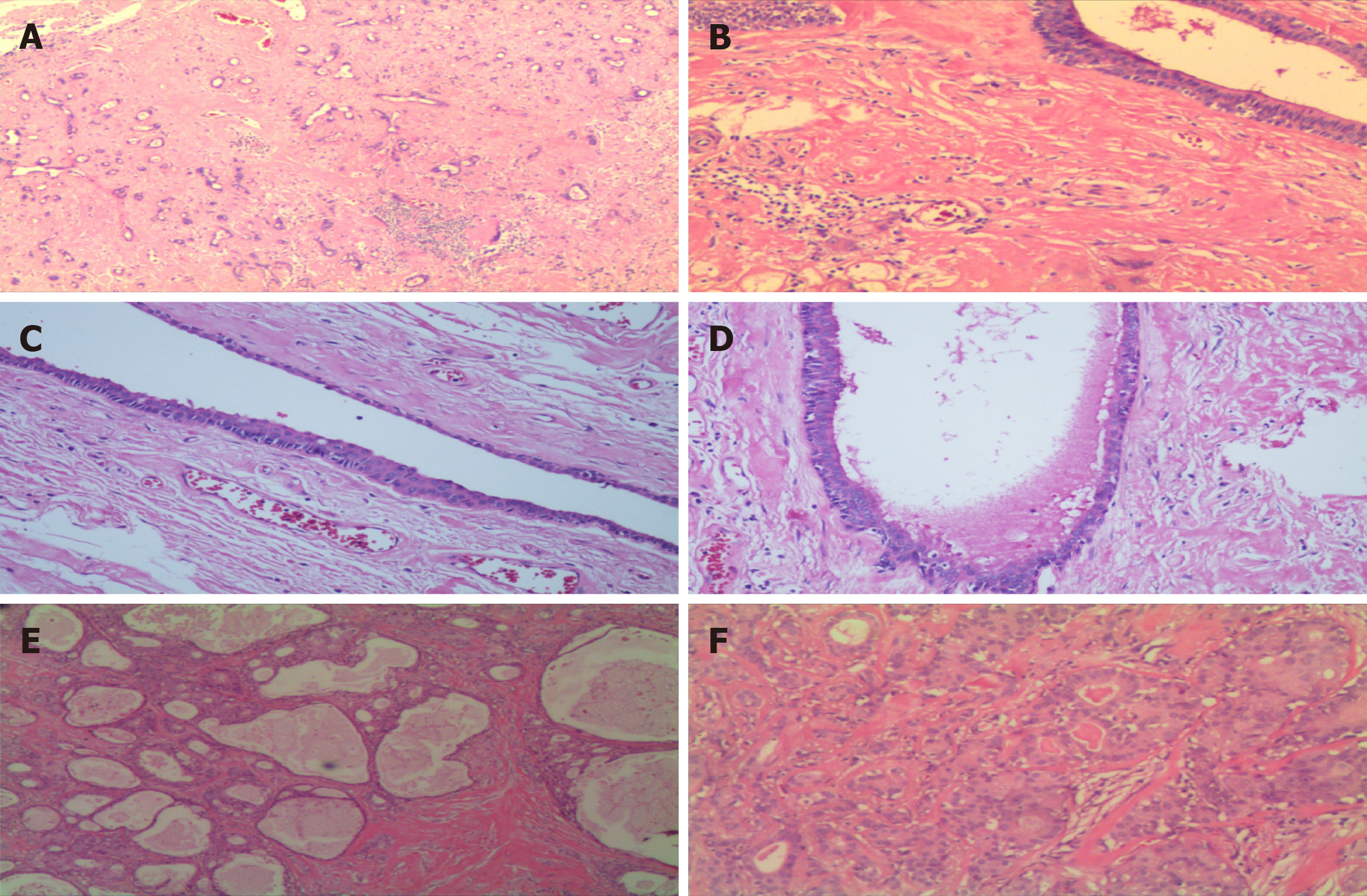
The resected specimen showed a gray-white hard nodule measuring 1.2 cm × 1.5 cm. Histopathological examination revealed a well-circumscribed mass with acinar structures, ducts, or cystic dilated glands of various sizes scattered in a massive hyperplastic sclerosing stroma (Figure 2A and B). A spectrum of flat and cuboidal epithelium, and eosinophils were seen in the duct; eosinophilic material was also present in the cysts’ lumen (Figure 2C and D). No site of atypical epithelial hyperplasia and invasive growth or in situ carcinoma was found. In addition, chronic inflammatory changes were found in the surrounding glandular tissue, with one lymph node exhibiting reactive hyperplastic changes. Based on the above findings, the mass was diagnosed as SPA.
Histopathological examination revealed a well-circumscribed nodule predominantly composed of numerous sclerotic, hypocellular, hyalinized collagen-containing ductal structures (Figure 2E). Special staining with Periodic acid-Schiff (PAS) was positive, indicating cytoplasmic PAS-diastase-resistant globules (data not shown). Immunohistochemically, the epithelial cells showed positive expression of polycystic kidney, epithelial membrane antigen, and estrogen receptor; the myoepithelial cells showed positive expression of spinal muscular atrophy, calponin, and p63, and negative expression of p53 and carcinoembryonic antigen; the proliferation cell index (Ki-67) was approximately 5% (data not shown). The above findings led to a final diagnosis of SPA.
Both patients were clinically diagnosed with a benign submandibular gland tumor, and the patients understood the situation and agreed to undergo a submandibular gland removal.
Case 1: The patient has remained asymptomatic, without recurrence or distant metastasis as of the 7-mo follow-up.
Case 2: The patient has remained asymptomatic, without recurrence or distant metastasis as of the 5-year follow-up.
SPA is a rare benign salivary gland disorder first described in 1996[1]. It has been reported in a wide age range of patients from 7 to 84 years, with a slight female predilection. The mean age of presentation is in the 4th decade, with a female-to-male ratio of 1.5-2:1[2,3]. To date, approximately 76 cases of SPA of salivary glands have been reported in the English literature, with 55 (72.4%), 5 (6.6%), and 16 (21%) involving the parotid gland, submandibular gland, and minor salivary glands of various sites in the oral cavity, respectively. Among the minor salivary glands, cases of SPA have been reported in the buccal mucosa, floor of the mouth, hard palate, retromolar area, tongue and lower lip[4-6]. Typically, SPA of the major salivary glands are slow-growing, deep-seated, round, palpable masses, occasionally with pain or a tingling sensation while SPA of the minor salivary glands is seen as asymptomatic, firm, smooth, freely movable submucosal nodules[5]. In our cases, both patients were female, about 50 years of age, and presented because of a mass in the submandibular area with associated pain. There have been seven SPA cases involving the parotid gland, and one case has been reported in the minor salivary gland in China. No case of SPA has been reported in the submandibular gland in China. To the best of our knowledge, it seems to be the first time that a Chinese SPA case arising from the submandibular gland is being reported. Seven cases occurring in the submandibular gland are summarized in Table 1[1,2,7,8]. The patients’ age range was from 28 to 69 without apparent differences in gender. Six of the seven patients presented with a mass, and the lesion’s size ranged from 1.2 to 2.4 cm in the greatest diameter.
Few studies have provided the imaging information of SPA, which is not sufficient for diagnosing SPA and can only be used as a reference for diagnosis[9]. On ultrasound, hypoechogenic microcystic areas can be found in the lesion[9]; on MRI, it has been described as a mass with small cystic areas of the relatively high intensity on T2-weighted images. Besides, fine needle aspiration biopsy (FNAB) was performed for the initial diagnosis in some cases. However, according to a recent review, FNAB could not diagnose SPA in most cases (30 of 31)[10]. In order to avoid misdiagnosis, it was recommended that aspiration should be repeated from multiple sites to obtain various cytological components of the lesion[10]. Histopathological examination is necessary to confirm the SPA diagnosis.
Histologically, SPA is typically well-circumscribed and characterized by the proliferation of microcysts, ducts, and acinar structures in a dense sclerotic stroma. The ductal cells display vacuolated, foamy, apocrine, and mucous appearances, and focal squamous metaplasia is also present[3]. The presence of acini filled with aberrant coarse red zymogen granules is also an essential characteristic of this condition[3]. Ductal epithelial dysplasia ranges from mild to severe dysplasia/carcinoma in situ, also presented in a few cases[11,12]. In our two cases, both lesions were well-circumscribed. In the first case, the acinar and ductal components were embedded in a dense sclerotic stroma (Figure 2A and B), and a spectrum of flattened, cubic, apocrine cells or eosinophils were seen in the ductal epithelium, with no dysplasia (Figure 2C). In the second case, the nodule predominantly composed of numerous sclerotic, hypocellular, hyalinized collagen-containing ductal structures (Figure 2E), and typical apocrine and secretory areas with histiocytes are also seen (Figure 2F).
The etiology and pathogenesis of SPA remain unclear. SPA was initially believed to be a reactive, non-neoplastic process similar to fibrocystic changes of the breast, and has still been categorized as a non-neoplastic epithelial lesion in the Fourth Edition of the World Health Organization Classification of Head and Neck Tumor published recently[3]. Manojlović et al[13] reported two SPA cases in two sisters in a family; therefore, they suggested a familial and possibly a genetic predisposition for this condition[13]. Swelam found co-localization of Epstein-Barr virus (EBV) and Bcl-2 in lesional cells of SPA in minor salivary glands and assumed that the viral oncogenic promotion of EBV in SPA pathogenesis occurs through increasing tumor cell survival and inhibiting apoptosis[14], while other studies have not reported the same. Moreover, a study of the X-chromosome inactivation pattern by Skálová et al[15] demonstrated the clonality in six SPA cases, suggesting that SPA cells are clonal[15]. In addition, Bishop et al[8] investigated the genetic characteristics of four SPA cases through targeted next-generation sequencing. Genetic alterations in the phosphatidylinositol 3-kinase pathway, which is recurrently mutated in a variety of tumor types, were observed in all the four cases. Among the genetic mutations, phosphatase and tensin homolog (PTEN) mutations were seen most frequently, and PTEN protein expression lost in the ductal and acinar elements was confirmed by immunohistochemistry[8]. In summary, SPA might be a neoplasm and not just a reactive process.
It is noteworthy that, as discussed by previous authors, SPA can easily be misdiagnosed as chronic sclerosing sialadenitis, pleomorphic adenoma, and mucoepidermoid carcinoma since clinicians and pathologists might be unfamiliar with this rare condition[5]. For example, among the nine cases reported by Smith et al[1], eight were initially diagnosed as pleomorphic adenomas or mucoepidermoid carcinomas[1]. The presence of remarkable lymphoplasmacytic infiltration and the absence of ductal changes of hyperplasia differentiates chronic sclerosing sialadenitis from SPA[5]. In pleomorphic adenoma, the intraductal proliferation and apocrine and sebaceous elements are lacking, differentiating it from SPA[5,16]. Mucoepidermoid carcinoma distinguished from SPA by the presence of classical cytomorphologic features, such as mucus-secreting cells and epidermoid type, and the absence of ductal changes[17].
Most SPA cases are treated with surgical excision, and recurrence has been reported in six cases of SPA in the parotid gland[9]. Although the number of recurrent cases was insufficient for statistical analysis, the results suggested that recurrence appears to be due to inadequate surgical excision and the multifocality of SPA as opposed to true recurrence. Notably, among the seven SPA cases of the submandibular gland, two cases were lost during follow-up; of the remaining five cases, none had a recurrence, and even the presence of ductal dysplasia was described in two of the five cases[1,2,7,8]. Similar to that found in SPA of the submandibular gland, no recurrence has been reported in cases of SPA in the minor salivary glands[4-6]. The prognosis of SPA is favorable, with no reports of distant metastases or mortality associated with it to date. However, four cases of malignancy have been documented in the parotid gland, including one case of invasive carcinoma arising after SPA recurred several times, necessitating surgery, reported by Canas Marques et al[12], and three cases of carcinoma in situ reported by Fulciniti et al[18], Petersson et al[11], and Mackle et al[19]. Therefore, follow-up is recommended, and a surgical excision technique should be carefully planned. To be specifical, it is recommended that SPA in the minor salivary glands should be treated with localized surgical excision with clear margins[5]. For SPA in the submandibular gland, submandibular gland excision is the treatment of choice[2]. As for primary and recurrent SPA in the parotid gland, superficial and total parotidectomy with facial nerve preservation is recommended, respectively[19].
In summary, SPA in the submandibular gland is rare, with only five cases reported previously. Here, we presented two additional cases. Based on these cases, submandibular gland excision is the treatment of choice and the prognosis is favorable, with no reports of recurrence, distant metastases or mortality to date. Therefore, clinicians and pathologists should be familiar with the characteristics of SPA in the submandibular gland to avoid misdiagnosis and overtreatment. In addition, more cases and longer follow-up are helpful to reveal the characteristics of SPA in the submandibular gland.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang L S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li JH
| 1. | Smith BC, Ellis GL, Slater LJ, Foss RD. Sclerosing polycystic adenosis of major salivary glands. A clinicopathologic analysis of nine cases. Am J Surg Pathol. 1996;20:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 76] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Gnepp DR, Wang LJ, Brandwein-Gensler M, Slootweg P, Gill M, Hille J. Sclerosing polycystic adenosis of the salivary gland: a report of 16 cases. Am J Surg Pathol. 2006;30:154-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Seethala RR, Stenman G. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Tumors of the Salivary Gland. Head Neck Pathol. 2017;11:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 277] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 4. | Noonan VL, Kalmar JR, Allen CM, Gallagher GT, Kabani S. Sclerosing polycystic adenosis of minor salivary glands: report of three cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:516-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Guduguntla P, Korlepara R, Guttikonda VR. Sclerosing Polycystic Adenosis of Hard Palate: A Rare Entity in Salivary Glands. Contemp Clin Dent. 2019;10:676-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Henrique Braz-Silva P, Motta do Canto A, Oliveira L, Martins F, Antônio Pereira da Costa A, Adolfo Costa Hanemann J. Sclerosing Polycystic Adenosis of Tongue. Bull Tokyo Dent Coll. 2018;59:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Beato Martínez A, Moreno Juara A, Candia Fernández A. [Sclerosing polycystic adenosis of the submandibular gland]. Acta Otorrinolaringol Esp. 2013;64:78-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Bishop JA, Gagan J, Baumhoer D, McLean-Holden AL, Oliai BR, Couce M, Thompson LDR. Sclerosing Polycystic "Adenosis" of Salivary Glands: A Neoplasm Characterized by PI3K Pathway Alterations More Correctly Named Sclerosing Polycystic Adenoma. Head Neck Pathol. 2020;14:630-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Eliot CA, Smith AB, Foss RD. Sclerosing polycystic adenosis. Head Neck Pathol. 2012;6:247-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Espinosa CA, Rua L, Torres HE, Fernández Del Valle Á, Fernandes RP, Devicente JC. Sclerosing Polycystic Adenosis of the Parotid Gland: A Systematic Review and Report of 2 New Cases. J Oral Maxillofac Surg. 2017;75:984-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Petersson F, Tan PH, Hwang JS. Sclerosing polycystic adenosis of the parotid gland: report of a bifocal, paucicystic variant with ductal carcinoma in situ and pronounced stromal distortion mimicking invasive carcinoma. Head Neck Pathol. 2011;5:188-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Canas Marques R, Félix A. Invasive carcinoma arising from sclerosing polycystic adenosis of the salivary gland. Virchows Arch. 2014;464:621-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Manojlović S, Virag M, Milenović A, Manojlović L, Salek Z, Skálová A. Sclerosing polycystic adenosis of parotid gland: a unique report of two cases occurring in two sisters. Pathol Res Pract. 2014;210:342-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Swelam WM. The pathogenic role of Epstein-Barr virus (EBV) in sclerosing polycystic adenosis. Pathol Res Pract. 2010;206:565-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Skálová A, Gnepp DR, Simpson RH, Lewis JE, Janssen D, Sima R, Vanecek T, Di Palma S, Michal M. Clonal nature of sclerosing polycystic adenosis of salivary glands demonstrated by using the polymorphism of the human androgen receptor (HUMARA) locus as a marker. Am J Surg Pathol. 2006;30:939-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Kawai M, Inoue T, Yonaga T, Mochizuki K, Nakazawa T, Masuyama K, Kondo T. Juvenile sclerosing polycystic adenosis cytologically mimicking Warthin tumor. Diagn Cytopathol. 2019;47:1208-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Etit D, Pilch BZ, Osgood R, Faquin WC. Fine-needle aspiration biopsy findings in sclerosing polycystic adenosis of the parotid gland. Diagn Cytopathol. 2007;35:444-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Fulciniti F, Losito NS, Ionna F, Longo F, Aversa C, Botti G, Foschini MP. Sclerosing polycystic adenosis of the parotid gland: report of one case diagnosed by fine-needle cytology with in situ malignant transformation. Diagn Cytopathol. 2010;38:368-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Mackle T, Mulligan AM, Dervan PA, O'Dwyer T. Sclerosing polycystic sialadenopathy: a rare cause of recurrent tumor of the parotid gland. Arch Otolaryngol Head Neck Surg. 2004;130:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |