Published online Apr 26, 2021. doi: 10.12998/wjcc.v9.i12.2838
Peer-review started: November 23, 2020
First decision: January 7, 2021
Revised: January 16, 2021
Accepted: February 22, 2021
Article in press: February 22, 2021
Published online: April 26, 2021
Processing time: 141 Days and 2 Hours
Rhabdomyolysis is a serious complication of heat stroke. Unlike that in acute kidney injury, the risk of muscle bleeding in rhabdomyolysis is often ignored and can substantially increase via the widespread use of anticoagulants, leading to the formation of intramuscular hematoma.
During the summer, a middle-aged man and an elderly man were diagnosed with heat stroke, rhabdomyolysis, and acute renal impairment. Low-dose enoxaparin sodium was initiated for prophylaxis of deep vein thrombosis after the disease was stabilized with continuous renal replacement therapy. After that, the patients' hemoglobin decreased progressively, and no obvious intracranial, thoracic, digestive, or skin bleeding tendency was found. However, one of the patients had hip muscle pain, and computed tomography and color ultrasound confirmed that the patients separately had lumbar back and hip intermuscular hematoma. After discontinuation of anticoagulant drugs and monitoring of the steady increase in hemoglobin, the intermuscular hematomas were gradually absorbed. Following the use of prophylactic anticoagulation therapy, the patients' hemoglobin showed a progressive downward trend. Hematoma formation in the lumbosacral and buttock muscles was confirmed after excluding bleeding in typical regions (such as the digestive tract, thoracic cavity, and abdominal cavity). Anticoagulant drugs were discontinued immediately, and nutritional support was increased. Subsequently, the hemoglobin levels gradually increased, and the hematoma volumes gradually decreased.
Patients with rhabdomyolysis have a risk of muscle bleeding, and inappropriate use of anticoagulants may lead to an increased risk or even to the formation of an intermuscular hematoma. When continuous blood loss is found in the body, the possibility of bleeding in the muscles and more typical sites should be considered.
Core Tip: We report two patients with rhabdomyolysis caused by heat stroke who developed an intermuscular hematoma during prophylactic anticoagulation therapy. We used heparinized continuous renal replacement therapy, and the patients’ conditions gradually stabilized after a few days. However, in the use of anticoagulants to prevent deep vein thrombosis, the two patients had a progressive decline in hemoglobin. After comprehensive examination, common blood loss was excluded, such as gastrointestinal bleeding, retroperitoneal hematoma, and pleural and abdominal hematoma, and the diagnosis of intramuscular hematoma of the lumbosacral region and buttock was finally confirmed.
- Citation: Yuan SY, Xie KF, Yang J. Intramuscular hematoma in rhabdomyolysis patients treated with low-molecular-weight heparin: Report of two cases. World J Clin Cases 2021; 9(12): 2838-2844
- URL: https://www.wjgnet.com/2307-8960/full/v9/i12/2838.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i12.2838
Rhabdomyolysis, a serious complication of heat stroke, is correlated with mitochondrial abnormalities, glucose and lipid metabolism, and inflammatory myopathy. Usually, it manifests as muscle soreness, stiffness, muscle weakness, brown urine, soy sauce urine, etc. Muscle swelling, compartment syndrome, and even acute kidney injury may occur in the later stage[1]. When rhabdomyolysis is encountered in clinical practice, our focus should be on clearing the accumulation of metabolites caused by the decomposition of myocytes after acute necrosis and on protecting organ function as soon as possible. However, the risk of muscle bleeding caused by rhabdomyolysis is often ignored. The risk of deep vein thrombosis in critically ill patients is of increasing concern, as it is associated with increased use of anticoagulants[2]. In rhabdomyolysis patients, the improper use of anticoagulants may lead to a significant increase in the risk of muscle bleeding, leading to the formation of intramuscular hematoma. At present, there are no reports of intermuscular hematoma caused by rhabdomyolysis due to heat stroke. We report two patients with rhabdomyolysis caused by heat stroke who presented with an intermuscular hematoma under anticoagulant therapy.
Case 1: A 49-year-old man visited our emergency room (ER) complaining of syncope and persistent high fever.
Case 2: A 66-year-old man visited the ER complaining of fever, fatigue, and syncope.
Case 1: The patient fainted while working in the field and was taken to the ER by his family. The monitored vital signs showed a temperature of 40 °C, and his body temperature did not decline, even after conventional antipyretic drugs and physical methods were used.
Case 2: Four days prior, the patient had symptoms of influenza, which led to fever and fatigue. While taking a bath, he fainted in the bathroom behind closed doors. After waking up, he could not climb out of the tub on his own. The next day, he was found by his family and was brought to the ER. This period lasted approximately 27 h.
Case 1: Prior to this, the patient had no underlying disease.
Case 2: Four years previous, the patient had been diagnosed with hypertension and received 5 mg amlodipine tablets once a day. His blood pressure was consistently monitored and controlled.
There were no special circumstances in the patients' personal and family history.
Case 1: In the ER, the monitored vital signs showed a temperature of 40 °C, heart rate of 93 bpm, respiratory rate of 32 breaths per minute, blood pressure of 98/61 mmHg, and oxygen saturation in room air of 98%. Physical examination revealed that all the skin of the body was red with scattered red spots and a Glasgow Coma scale of 3/15, without any other pathological signs.
Case 2: The patient’s vital signs were stable. On physical examination, it was found that the skin was bruised at compression sites such as the chest and knee, and wet rales were observed on auscultation at the bottom of both lungs.
Case 1: Blood analysis revealed mild leukocytosis (13.65 × 109/L) with predominant neutrophils (80.8%) with a platelet count of 96 × 109/L and normal hematocrit. Prothrombin and partial thromboplastin times were normal, and D-dimers were slightly increased at 1511.00 µg/L. Serum C-reactive protein was increased at 9.37 mg/L (normal range < 12 mg/L). Blood biochemistry revealed that peripheral blood glucose was 8.7 mmol/L, creatinine was 192 µmol/L, uric acid was 905 µmol/L, alanine aminotransferase was 67 IU/L, aspartate aminotransferase was 123 IU/L, creatine kinase (CK) was 5460 IU/L, CK myocardial isoenzyme was 162.2 IU/L, lactate dehydrogenase was 450 IU/L, and myoglobin was 56800 ng/L. Electrocardiogram revealed sinus tachycardia. Arterial blood was normal.
Case 2: Blood analysis revealed mild leukocytosis (16.12 × 109/L) with predominant neutrophils (90.1%) and normal hematocrit and platelet count. Prothrombin and partial thromboplastin times were normal, and D-dimers were slightly increased at 1535.00 µg/L. Serum C-reactive protein was increased at 247 mg/L, and procalcitonin was ≥ 100 ng/mL. Blood biochemistry revealed that peripheral blood glucose was 6.0 mmol/L, creatinine was 487.3 µmol/L, uric acid was 1446 µmol/L, alanine aminotransferase was 428.7 IU/L, aspartate aminotransferase was 2590.6 IU/L, CK was 179785 IU/L, lactate dehydrogenase was 4274.7 IU/L, and CK myocardial isoenzyme was 3925 IU/L. Electrocardiogram findings and arterial blood were normal.
Case 1: In the ER, computed tomography (CT) of the head and lung showed no obvious abnormality.
Case 2: In the ER, CT of the head showed no obvious abnormality, but CT of the lung showed diffuse exudative lesions in both lower lobes and the medial segment of the right middle lobe.
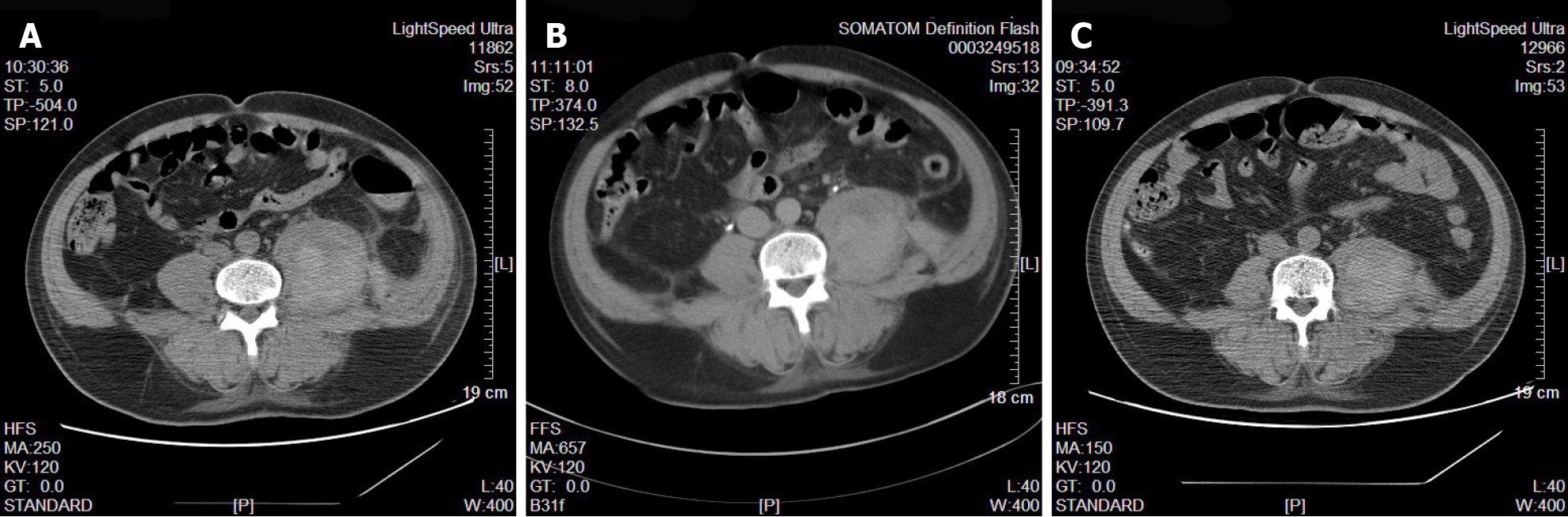
Case 1: In the intensive care unit (ICU), after several days of ICU treatment, the patient's vital signs were basically stable, and myocardial enzymatic indexes were significantly improved compared with those before treatment. To further effectively prevent deep vein thrombosis (DVT), low-molecular-weight heparin was used. However, subsequent monitoring of routine blood tests revealed a slow and progressive decline in hemoglobin levels. At this time, the patient did not complain of body pain and discomfort. We excluded digestive system diseases leading to blood loss through stomach content and fecal occult blood examinations, and no obvious pleural or peritoneal effusion was found by bedside ultrasound examination. In addition, the coagulation function was generally normal. We continuously monitored blood analysis and further refined CT examinations of the head, lungs, and whole abdomen. The CT results showed that the left lumbosacral muscles were locally swollen and less homogeneous in density, suggesting that the lumbosacral muscle hemorrhage had formed an intermuscular hematoma (Figure 1A).
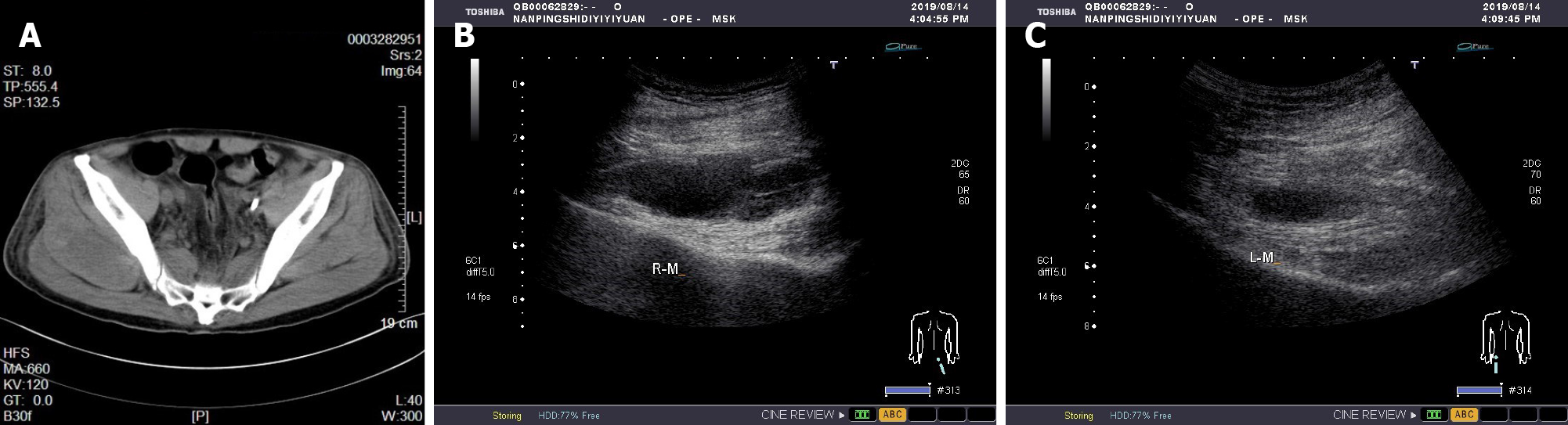
Case 2: Similar to case 1, we started prophylactic anticoagulant therapy when the patient's condition became stable after treatment in the ICU. A similar progressive decrease in hemoglobin occurred, and the patient complained of obvious buttock muscle soreness. We immediately improved CT and bedside color Doppler examination of the buttocks. CT revealed a new uneven density in the soft tissues of both hips (Figure 2A). Bedside color Doppler ultrasound indicated the formation of bilateral gluteal intermuscular hematoma.
The final diagnoses were heat stroke, rhabdomyolysis, acute kidney disease injury, and lumbosacral intermuscular hematoma.
The final diagnoses were heat stroke, rhabdomyolysis, acute kidney disease injury, pulmonary infection, hypertension, and buttock intermuscular hematoma.
Both patients were diagnosed with rhabdomyolysis and acute kidney disease injury. We immediately performed continuous renal replacement therapy (CRRT) to remove accumulated metabolites, manage volume, etc. Heparinized anticoagulation was used, and the activated partial thromboplastin time was monitored to adjust the heparin dose. After admission to the ICU, we continued to monitor vital signs, provide organ function and nutritional support, prevent DVT, and perform other measures. In addition, we dynamically monitored the indicators of blood inflammation, liver and kidney function, electrolytes, and coagulation function and adjusted the drug regimen in a timely manner. Case 1's core body temperature remained high on admission to the ICU, and we added a variety of physical cooling methods to rapidly reduce and maintain the core body temperature to a normal range. At the time of admission to the ICU, case 2 showed clear evidence of pulmonary infection, and his procalcitonin level was significantly elevated, so he was given additional broad-spectrum antibiotic therapy. The procalcitonin levels were continuously monitored to guide antibiotic therapy. After treatment, the condition of the two patients gradually stabilized, and CRRT was stopped on the 3rd and 8th d for cases 1 and 2, respectively. To continue prophylactic anticoagulant therapy, enoxaparin sodium (0.3 mL) was added once a day for case 1 and subcutaneously every other day for case 2. However, subsequent monitoring of routine blood tests revealed a slow and progressive decline in hemoglobin levels. Initially, we thought that the cause of the hemoglobin reduction might have been related to inadequate nutrient intake and high body fat consumption caused by infection. At this time, we excluded digestive system diseases leading to blood loss through stomach content and fecal occult blood examinations, and no obvious pleural or peritoneal effusion was found by bedside ultrasound examination. No obvious damage was found in the dynamic monitoring of coagulation function. We offered an enhanced enteral nutrition regimen, but continued monitoring of routine blood tests still indicated a persistent decrease in hemoglobin. To this end, we needed to consider additional possibilities. We then performed CT scans of the head, chest, and whole abdomen. The cause of the persistent blood loss was finally found to be the formation of a lumbosacral intermuscular hematoma and buttock intermuscular hematoma. When the cause of hemorrhage was clear, we immediately stopped using anticoagulant drugs and infused suspended red blood cells appropriately to correct the anemia. CT and bedside color Doppler ultrasonography were used to check the absorption of the intramuscular hematoma, and we continued to monitor routine blood tests until the condition was stable. Follow-up monitoring of the patients routinely found that the hemoglobin steadily increased, and CT and color Doppler ultrasound suggested a gradual decrease in the volume of the intramuscular hematoma (Figure 1B and C and Figure 2B and C).
The two patients were transferred to the general ward after hemoglobin stabilization. A few weeks later, color Doppler ultrasonography showed that the lumbosacral and gluteal intermuscular hematoma was basically absorbed.
Heat stroke is a life-threatening clinical syndrome characterized by an increase in core temperature of > 40 °C and abnormalities in the central nervous system, such as altered mental status, convulsions, or coma, and is associated with multiple organ damage due to an imbalance in body heat production and dissipation caused by exposure to a thermal environment and/or intense exercise[1]. According to the different causes and susceptible populations, heat stroke can be divided into classical heat stroke and exertional heat stroke. Due to the involvement of thermal injury factors, rhabdomyolysis caused by heat stroke is significantly different from general exercise rhabdomyolysis. The increase in CK within 24 h of onset of the former is often not prominent and then gradually increases, often peaking 5-7 d after onset, but its peak value is higher than that of exercise rhabdomyolysis, reaching up to 400000 U/L. Myoglobin is often > 1000 ng/mL, and the peak can reach 70000-80000 ng/mL[3]. According to the classification of heat stroke, cases 1 and 2 were diagnosed with exertional heat stroke and classical heat stroke, respectively. CK and myoglobin in case 1 peaked on the second day of admission, with the highest values reaching 61400 IU/L and 77847 ng/mL, respectively. Case 2 showed peak CK and myoglobin values of 264600 IU/L and 326376 ng/mL, respectively, on the day of admission. We used continuous renal replacement therapy in time after the patient entered the ICU; otherwise, these values would have been higher.
Venous thromboembolism, including DVT and pulmonary embolism, is a disease with high morbidity and mortality[4]. In recent years, increasing attention has been given to the prevention of VTE, which means that the use of anticoagulants has become increasingly frequent, especially for critically ill patients in the ICU. However, we know that anticoagulants and antiplatelet drugs lead to a certain risk of bleeding. Common bleeding-related risks include thrombocytopenia[5], active gastrointestinal bleeding[6], retroperitoneal hematoma[7], etc., but muscle bleeding is easily ignored because it is relatively rare. A retrospective study by Hiraga et al[8] showed that the incidence of muscle hematoma was 0.4% among 694 patients with ischemic stroke who received antithrombotic therapy. The initial symptoms of hematoma included pain (n = 3) and syncope (n = 1), and the patients were not correctly diagnosed at the onset of hematoma formation. Artzner et al[9] conducted a two-center retrospective study to analyze the incidence of spontaneous iliopsoas muscle hematoma among 40 ICU inpatients, of whom 50% received dialysis and 95% received prophylactic or therapeutic doses of heparin. Watanabe et al[10] reported a patient treated with warfarin for atrial fibrillation complicated with bilateral lumboiliac hematoma, rhabdomyolysis, and acute kidney injury, which was attributed to improper use of warfarin. The two cases reported here were both heat stroke patients who had rhabdomyolysis and acute kidney injury and were treated with heparin-based CRRT. After CRRT treatment was completed and no coagulation dysfunction was found, an appropriate dose of enoxaparin sodium prophylactic anticoagulant therapy was used. Subsequently, the levels of hemoglobin continued to decrease, and no obvious bleeding sites were found. For one patient, the formation of a left psoas major intermuscular hematoma was observed when examining the bleeding sites. The other patient complained of right gluteal pain, which was confirmed by CT and color Doppler ultrasound. Immediately after the discovery of the intramuscular hematoma, the patient was discontinued from enoxaparin sodium anticoagulation therapy and transfused to correct the anemia. Subsequently, his hemoglobin level stabilized, and the extent of the intramuscular hematoma decreased.
Given the diagnosis of and treatment experience with case 1, we used prophylactic anticoagulation with subcutaneous enoxaparin with a further dose reduction of 0.3 mL every other day. However, muscle bleeding remained at the noninjected site. Given the results with the above two cases, we have reason to believe that rhabdomyolysis patients have a risk of muscle bleeding and that improper use of anticoagulants may increase this risk or even lead to the formation of intramuscular hematomas. In addition, when continuous blood loss is found in the body, the possibility of bleeding in the muscles and more typical sites should be considered.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng Q S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Liu SY, Song JC, Mao HD, Zhao JB, Song Q; Expert Group of Heat Stroke Prevention and Treatment of the People’s Liberation Army; and People’s Liberation Army Professional Committee of Critical Care Medicine. Expert consensus on the diagnosis and treatment of heat stroke in China. Mil Med Res. 2020;7:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 2. | Reynolds PM, Van Matre ET, Wright GC, McQueen RB, Burnham EL, Ho PJM, Moss M, Vandivier RW, Kiser TH; Colorado Pulmonary Outcomes Research Group (CPOR). Evaluation of Prophylactic Heparin Dosage Strategies and Risk Factors for Venous Thromboembolism in the Critically Ill Patient. Pharmacotherapy. 2019;39:232-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | People’s Liberation Army Professional Committee of Critical Care Medicine. Expert consensus on standardized diagnosis and treatment for heat stroke. Mil Med Res. 2016;3:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 4. | Tran HA, Gibbs H, Merriman E, Curnow JL, Young L, Bennett A, Tan CW, Chunilal SD, Ward CM, Baker R, Nandurkar H. New guidelines from the Thrombosis and Haemostasis Society of Australia and New Zealand for the diagnosis and management of venous thromboembolism. Med J Aust. 2019;210:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 5. | Greinacher A. CLINICAL PRACTICE. Heparin-Induced Thrombocytopenia. N Engl J Med. 2015;373:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 408] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 6. | Li L, Geraghty OC, Mehta Z, Rothwell PM; Oxford Vascular Study. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet. 2017;390:490-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 280] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 7. | Sbrana F, Pasanisi EM. A massive retroperitoneal hematoma during low-molecular-weight-heparin therapy. Intern Emerg Med. 2016;11:153-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Hiraga A, Nakagawa Y, Kamitsukasa I, Suzuki T, Kuwabara S. Muscle haematoma due to antithrombotic treatment for ischaemic stroke. J Clin Neurosci. 2015;22:1160-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Artzner T, Clere-Jehl R, Schenck M, Greget M, Merdji H, De Marini P, Tuzin N, Helms J, Meziani F. Spontaneous ilio-psoas hematomas complicating intensive care unit hospitalizations. PLoS One. 2019;14:e0211680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Watanabe Y, Koutoku H, Nagata H, Oda Y, Kikuchi H, Kojima M. Rhabdomyolysis with Acute Kidney Injury Caused by Bilateral Iliopsoas Hematoma in a Patient with Atrial Fibrillation. Intern Med. 2019;58:2887-2890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |