Published online Apr 6, 2020. doi: 10.12998/wjcc.v8.i7.1251
Peer-review started: November 15, 2019
First decision: February 20, 2020
Revised: March 20, 2020
Accepted: March 27, 2020
Article in press: March 27, 2020
Published online: April 6, 2020
Processing time: 142 Days and 23.2 Hours
Acute myeloid leukemia (AML) harboring 11q23 translocations is classified as therapy-related AML in patients who have undergone prior treatment with cytotoxic agents. There have been only a few reports of AML that subsequently developed during imatinib mesylate (IM) treatment for gastrointestinal stromal tumors (GISTs).
A 63-year-old woman was diagnosed with a hepatic GIST recurrence in April 2012; she was administered IM 400 mg/d. In November 2015, she developed dyspnea with pancytopenia while IM treatment was continued for 42 mo. A chromosome study using a bone marrow sample showed a 46, XX karyotype with t(11;19)(q23;p13.1) in 22 of 26 analyzed metaphase cells. Fluorescence in situ hybridization using the locus-specific indicator (11q23) gene break-apart probe showed positive rearrangement in 82% of interphase cells. Reverse-transcription polymerase chain reactions subsequently confirmed the KMT2A/ELL transcript. She achieved complete response with incomplete neutrophil recovery with two decitabine treatment cycles. After the third cycle of decitabine, the disease relapsed, and she refused further treatment. She died of hemorrhagic stroke 5 mo after diagnosis. To the best of our knowledge, this is the first report of AML with KMT2A gene rearrangements in a patient with a GIST receiving IM treatment.
Physicians should consider the potential risks of developing hematologic malignancies, including therapy-related AML, in patients with GISTs receiving IM treatment.
Core tip: To the best of our knowledge, this is the first report of acute myeloid leukemia with KMT2A gene rearrangements in a patient with a gastrointestinal stromal tumor receiving imatinib mesylate (IM) treatment. Although there is no known mechanism by which IM causes acute leukemia, there have been reports supporting the speculation that IM may have a direct mutagenic effect on normal hematopoietic precursors. Physicians should consider the potential risks of developing hematologic malignancies, including therapy-related acute myeloid leukemia, in patients with gastrointestinal stromal tumors receiving IM treatment.
- Citation: Kim HJ, Baek SK, Maeng CH, Kim SY, Park TS, Han JJ. Acute myeloid leukemia with t(11;19)(q23;p13.1) in a patient with a gastrointestinal stromal tumor undergoing imatinib therapy: A case report. World J Clin Cases 2020; 8(7): 1251-1256
- URL: https://www.wjgnet.com/2307-8960/full/v8/i7/1251.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i7.1251
Gastrointestinal stromal tumors (GISTs), the most common mesenchymal tumors of the digestive tract, generally occur in the stomach (60%) and small intestine (35%)[1]. The interstitial cells of Cajal were identified as the precursor cells, and the mutational activations in receptor tyrosine kinases, c-KIT, or platelet-derived growth factor receptor-α (PDGFRA) were involved in the main pathogenesis[1,2]. This understanding of the pathogenesis led to the application of imatinib mesylate (IM), a c-KIT/PDGFRA tyrosine kinase inhibitor, for the treatment of advanced or metastatic GISTs[3].
Acute myeloid leukemia (AML) with 11q23 translocations involving the KMT2A (previously called MLL) gene has been categorized as AML with recurring genetic abnormalities according to the 2016 World Health Organization classification[4]. However, AML with 11q23 translocations should be classified as therapy-related AML (t-AML) in patients who have undergone prior treatment with cytotoxic agents, including topoisomerase inhibitors such as etoposide.
There have been only a few reports of AML that subsequently developed during IM treatment for GISTs. Herein, we describe a patient who developed AML with an 11q23 translocation while receiving IM for GISTs.
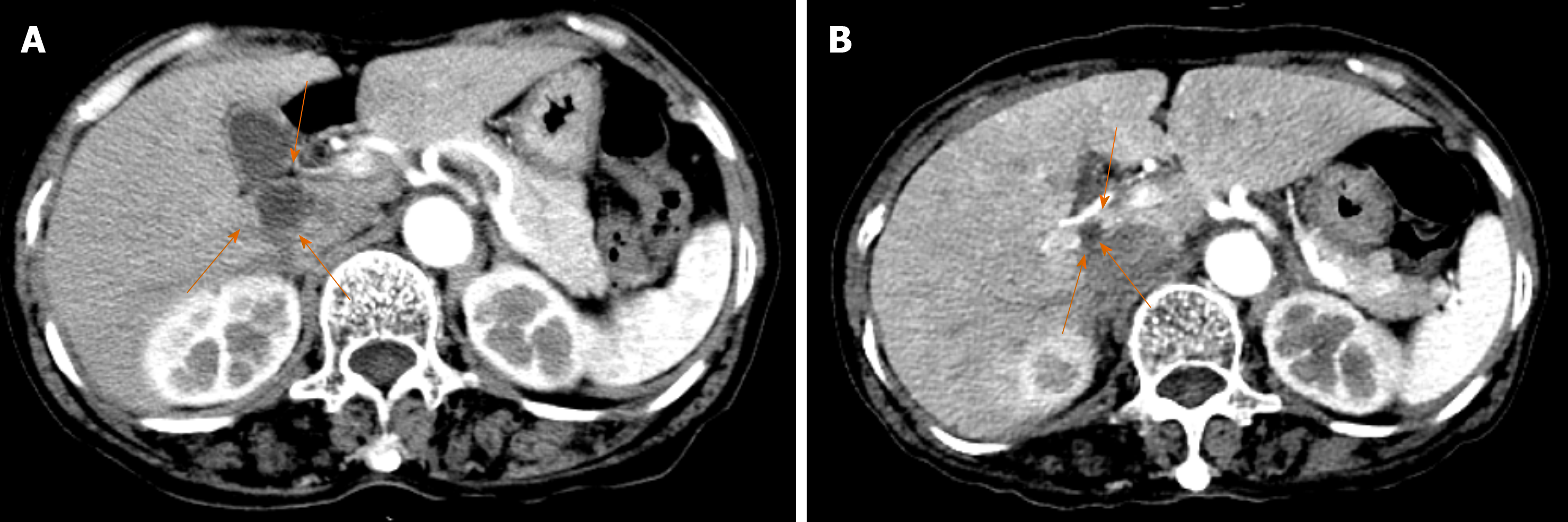
A 63-year-old woman was diagnosed with a gastric GIST in March 2007 and underwent subtotal gastrectomy. The GIST recurred in the liver in April 2012, and she was administered IM 400 mg/d (Figure 1A). She was transferred to our department in May 2012, and a partial response was observed while IM treatment was continued for 42 mo (Figure 1B). In November 2015, she developed dyspnea with pancytopenia. Her hematological data were as follows: Hemoglobin level, 4.0 g/dL; platelet count, 27 × 109/L; and white blood cell count, 1.05 × 109/L. Bone marrow examination showed hypercellularity with 70% blasts containing abundant cytoplasm, which were moderately-to-intensely basophilic, and some of them exhibited pseudopod formations and scattered fine azurophilic granules.
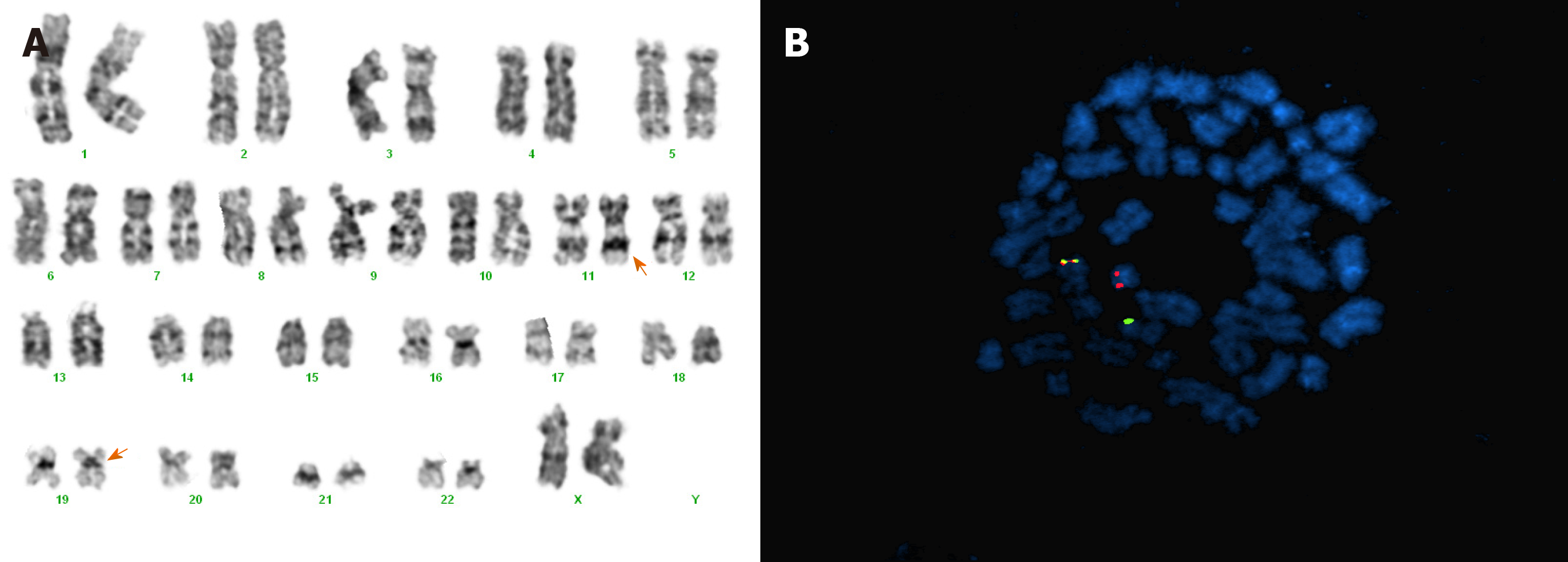
Leukemic blasts stained positively upon myeloperoxidase staining. However, the results of periodic acid–Schiff (PAS) and non-specific esterase (NSE) stains were negative. A chromosome study using a bone marrow sample showed a 46, XX karyotype with t(11;19)(q23;p13.1) in 22 out of 26 analyzed metaphase cells (Figure 2A). Fluorescence in situ hybridization using the locus-specific indicator (11q23) gene break-apart probe showed positive rearrangement in 82% of interphase cells (Figure 2B). Reverse-transcription polymerase chain reactions subsequently confirmed the KMT2A/ELL transcript.
The final diagnosis of the presented case is AML with KMT2A/ELL rearrangement.
The patient was not considered for intensive treatment because of poor performance status. Therefore, decitabine (20 mg/m2) was administered for 5 d.
She achieved a complete response with incomplete neutrophil recovery after two treatment cycles. Cytogenetic analysis showed a response with residual disease with the 46, XX, t(11;19)(q23;p13.1) karyotype remaining in 18% of metaphase cells. After the third decitabine treatment cycle, the disease relapsed with 66% blasts in the bone marrow, and cytogenic analysis showed an additional cytogenetic abnormality of del(13)(q12a14). The patient died of severe intracerebral hemorrhage 5 mo after diagnosis.
To the best of our knowledge, this is the first report of AML with KMT2A gene rearrangements in a patient with a GIST receiving IM treatment. Although there is no known mechanism by which IM causes acute leukemia, there have been reports supporting the speculation that IM may have a direct mutagenic effect on normal hematopoietic precursors.
Previously, there have been sporadic reports suggesting the association between hematologic malignancy and GISTs[5,6], and in the late 2000s, a non-random association between GISTs and myeloid leukemia was suggested in a retrospective study that enrolled 1892 patients with GISTs diagnosed between 1970 and 1996[7]. Six (0.3%) of the study patients developed AML 1.7–21 years after the diagnosis of GISTs. Although IM was not available at the time of diagnosis, some of the patients could have received IM during the course of their treatments. In another report, 12 of the 314 patients developed hematologic malignancies after the diagnosis of GISTs[8]. These studies revealed no information on KMT2A gene rearrangements.
Although the long-term experience of IM in patients with chronic myeloid leukemia (CML) was reported as only one case (0.2%) of secondary AML on an IM treatment arm after more than 10 years of follow-up[9], there have been some reports of hematologic malignancies associated with IM treatment. Despite its low prevalence (0.5%), the emergence of 11q23 translocations has been reported in patients with CML during IM treatment. In two cases, the translocation companion for 11q23 was t(11;19)[10]. Subsequent AML or MDS after treatment with second generation Bcr-Abl tyrosine kinase inhibitors including dasatinib, nilotinib, and ponatinib for CML was also reported[11-13]. Spadaro et al[14] studied the bone marrow of 49 patients with unresectable or metastatic GISTs before and during IM treatment. They found that 8 (16%) of 49 patients acquired chromosomal abnormalities, 7 of whom had trisomy 8. Three patients with trisomy 8 developed myelodysplastic syndrome (MDS), and one patient who had monosomy 7 developed MDS with excess blasts, which rapidly transformed into AML. Ganjoo et al[15] reported three cases of AML in patients with GISTs during IM treatment. Bone marrow cytogenetic analyses revealed trisomy 8 in one patient and MLL duplication in another patient. Summary of data of the patients with GISTs who subsequently developed AML is shown in Table 1. These findings suggest that IM may play a role in the acquisition of specific cytogenetic abnormalities associated with t-AML or MDS.
| No. | Age (yr) | Sex | Location of GIST | KIT expression/KIT mutation | AML type | Bone marrow cyto-genetics | Interval of GIST and leukemia | Administration of imatinib mesylate | Ref. |
| 1 | 57 | Male | Perirectal | +/ND | M4eo | 46, XY, add(6) (p25), inv (16) (p13q22), trisomy 8 | 33 | + | [12] |
| 2 | 74 | Male | ND | +/ND | NOS | ND | 38 | + | [12] |
| 3 | 70 | Female | Small bowel | +/ND | M2 | 46, XX, del(11)(9q21q23) 11q23 (MLLx2) | 27 | + | [12] |
| 4 | 63 | Female | Small bowel | +/ND | Preceded by MDS | ND | 216 | ND | [8] |
| 5 | 84 | Female | Small bowel | +/ND | NOS | ND | 21 | ND | [8] |
| 6 | 40 | Male | Small bowel | +/c.1689_1701 del | Promyelocytic leukemia | ND | 30 | ND | [8] |
| 7 | 70 | Female | Stomach | +/c.1689_1701 del | NOS | ND | 153 | ND | [8] |
| 8 | 49 | Male | Stomach | ND/ND | NOS | ND | 252 | ND | [8] |
| 9 | 58 | Female | Small bowel | +/ND | Acute myelomonocytic leukemia | ND | 20 | ND | [8] |
| 10 | 63 | Female | Stomach | +/ND | 46, XX, t(11;19)(q23;p13.1) | 96 | + | The present case |
The important issue in previous findings and the current case is whether IM has mutagenic effects on normal hematopoietic cells. Direct induction of an oncogene leading to the outgrowth of the transformed cell is one of the suggested pathogenic models for t-AML development in association with topoisomerase inhibitors[16]. Topoisomerase inhibitors stabilize double-strand breaks and delay the ligation of free DNA ends during replication. Free DNA ends can easily recombine with DNA from another chromosome, which could produce specific gene fusions such as KMT2A/MLL–MLL3. IM induces apoptosis in human CML cell lines by inhibiting topoisomerase I and II[17], supporting the idea that similar pathophysiologies may be responsible for the development of t-AML with 11q23 translocations after IM treatment in patients with GISTs. Since the pathogenesis is not yet elucidated, it is impossible to clearly demonstrate that AML occurred due to IM administration even in the present case. The risk of leukemia may be increased with IM treatment alone, but may not be increased without a combination of IM and some predisposing genetic abnormalities, such as an isocitrate dehydrogenase mutation.
In conclusion, physicians should consider the potential risks of developing hematologic malignancies, including t-AML, in patients with GISTs receiving IM treatment. Systematic collection of data on the occurrence of t-AML or MDS during long-term IM treatment in patients with GISTs should be considered along with cytogenetic analysis to better understand the mechanism of t-AML or MDS during IM treatment. Finally, further studies are warranted to identify the off-target effects of tyrosine kinase inhibitors in association with the mechanisms of therapy-related myeloid neoplasms.
Manuscript source: Unsolicited Manuscript
Specialty type: Medicine, research and experimental
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ankathil R, Diamantidis D, Morelli L S-Editor: Wang YQ L-Editor: A E-Editor: Xing YX
| 1. | Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CD, Fletcher JA. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1712] [Cited by in RCA: 1722] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 2. | Kitamura Y, Hirotab S. Kit as a human oncogenic tyrosine kinase. Cell Mol Life Sci. 2004;61:2924-2931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 149] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Heinrich MC, Owzar K, Corless CL, Hollis D, Borden EC, Fletcher CD, Ryan CW, von Mehren M, Blanke CD, Rankin C, Benjamin RS, Bramwell VH, Demetri GD, Bertagnolli MM, Fletcher JA. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008;26:5360-5367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 459] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 4. | Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5219] [Cited by in RCA: 6745] [Article Influence: 749.4] [Reference Citation Analysis (0)] |
| 5. | Herbers AH, Keuning JJ. Staging for CLL-type non-Hodgkin's lymphoma reveals a gastrointestinal stromal tumour. Neth J Med. 2005;63:74-75. [PubMed] |
| 6. | Agaimy A, Wünsch PH, Sobin LH, Lasota J, Miettinen M. Occurrence of other malignancies in patients with gastrointestinal stromal tumors. Semin Diagn Pathol. 2006;23:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Miettinen M, Kraszewska E, Sobin LH, Lasota J. A nonrandom association between gastrointestinal stromal tumors and myeloid leukemia. Cancer. 2008;112:645-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Hechtman JF, DeMatteo R, Nafa K, Chi P, Arcila ME, Dogan S, Oultache A, Chen W, Hameed M. Additional Primary Malignancies in Patients with Gastrointestinal Stromal Tumor (GIST): A Clinicopathologic Study of 260 Patients with Molecular Analysis and Review of the Literature. Ann Surg Oncol. 2015;22:2633-2639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, Baccarani M, Deininger MW, Cervantes F, Fujihara S, Ortmann CE, Menssen HD, Kantarjian H, O'Brien SG, Druker BJ; IRIS Investigators. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N Engl J Med. 2017;376:917-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 871] [Article Influence: 108.9] [Reference Citation Analysis (0)] |
| 10. | Wang W, Tang G, Cortes JE, Liu H, Ai D, Yin CC, Li S, Khoury JD, Bueso-Ramos C, Medeiros LJ, Hu S. Chromosomal rearrangement involving 11q23 locus in chronic myelogenous leukemia: a rare phenomenon frequently associated with disease progression and poor prognosis. J Hematol Oncol. 2015;8:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Larsson N, Billström R, Lilljebjörn H, Lassen C, Richter J, Ekblom M, Fioretos T. Genetic analysis of dasatinib-treated chronic myeloid leukemia rapidly developing into acute myeloid leukemia with monosomy 7 in Philadelphia-negative cells. Cancer Genet Cytogenet. 2010;199:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Krysiak K, Christopher MJ, Skidmore ZL, Demeter RT, Magrini V, Kunisaki J, O'Laughlin M, Duncavage EJ, Miller CA, Ozenberger BA, Griffith M, Wartman LD, Griffith OL. A genomic analysis of Philadelphia chromosome-negative AML arising in patients with CML. Blood Cancer J. 2016;6:e413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Issa GC, Kantarjian HM, Gonzalez GN, Borthakur G, Tang G, Wierda W, Sasaki K, Short NJ, Ravandi F, Kadia T, Patel K, Luthra R, Ferrajoli A, Garcia-Manero G, Rios MB, Dellasala S, Jabbour E, Cortes JE. Clonal chromosomal abnormalities appearing in Philadelphia chromosome-negative metaphases during CML treatment. Blood. 2017;130:2084-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Spadaro P, Ingemi M, Pitini V, Arrigo C, Soto Parra H. Myelodysplastic syndromes developing after imatinib therapy for GIST. J Clin Oncol. 2009;27:10532. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Ganjoo KN, Demetri GD, Jacobs C, Patel S. Acute myeloid leukemia in patients with gastrointestinal stromal tumors treated with Gleevec. Leuk Lymphoma. 2009;50:1882-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Heuser M. Therapy-related myeloid neoplasms: does knowing the origin help to guide treatment? Hematology Am Soc Hematol Educ Program. 2016;2016:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Baran Y, Zencir S, Cakir Z, Ozturk E, Topcu Z. Imatinib-induced apoptosis: a possible link to topoisomerase enzyme inhibition. J Clin Pharm Ther. 2011;36:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |