Published online Apr 6, 2020. doi: 10.12998/wjcc.v8.i7.1241
Peer-review started: December 19, 2019
First decision: December 28, 2019
Revised: January 8, 2020
Accepted: March 11, 2020
Article in press: March 11, 2020
Published online: April 6, 2020
Processing time: 108 Days and 8.1 Hours
Pancreatic acinar cell carcinoma (PACC) is a rare type of malignant pancreatic cancer that represents approximately 1% of all pancreatic neoplasms. Due to its very low incidence, only a few retrospective studies are available. Although surgery is the first choice for treatment, most patients experience recurrence (mainly in the liver) and there are no clear recommendations for patients with advanced disease.
We report two patients with PACC treated with surgery who experienced tumour recurrence in the liver. Patient 1 carried a germline mutation in the APC gene. Both patients were treated with gemcitabine plus oxaliplatin and gemcitabine plus capecitabine as first- and second-line therapies, respectively. After a favourable response to chemotherapy, the patients underwent radiofrequency ablation of the remaining liver metastases. For patient 1, we documented a relapse in the liver after a disease-free period of 9 mo, and treatment with gemcitabine plus capecitabine was restarted. The patient achieved a complete response, and he remains alive without evidence of disease recurrence after six years. After radiofrequency ablation, patient 2 experienced disease-free survival for 21 mo, when peritoneal relapse was diagnosed and treated with chemotherapy. The patient achieved a stable disease state for nearly two years; nevertheless, further progressive disease was documented, and he died seven years after the first relapse.
PACC presents different biological behaviours than pancreatic adenocarcinoma. Multidisciplinary treatment involving local ablative therapies may be considered for PACC.
Core tip: Pancreatic acinar cell carcinoma (PACC) is a rare type of malignant pancreatic cancer. Surgery represents the first choice for treatment, but most patients experience relapse (mainly located in the liver), and there are no clear recommendations for the treatment of advanced disease. We report two cases of long-term PACC survivors treated with radiofrequency ablation in addition to chemotherapy for liver recurrence after surgery. This case report highlights the different biological behaviour of PACC compared to pancreatic adenocarcinoma and the importance of multidisciplinary treatment involving local ablative therapies, such as radiofrequency ablation, for recurrent PACC.
- Citation: Di Marco M, Carloni R, De Lorenzo S, Grassi E, Palloni A, Formica F, Brocchi S, Filippini DM, Golfieri R, Brandi G. Long-term survival of two patients with recurrent pancreatic acinar cell carcinoma treated with radiofrequency ablation: A case report. World J Clin Cases 2020; 8(7): 1241-1250
- URL: https://www.wjgnet.com/2307-8960/full/v8/i7/1241.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i7.1241
Pancreatic acinar cell carcinoma (PACC) is a rare type of malignant pancreatic cancer originating from acinar cells of the exocrine pancreas that represents approximately 1% of all pancreatic neoplasms[1,2]. For patients with operable disease, surgical resection is the first choice for treatment. Chemotherapy and radiotherapy have also been used for locally advanced or metastatic PACC; however, there is a lack of controlled, prospective studies due to the very low disease incidence, and there are no definitive guidelines for treating patients with advanced or recurrent disease[3]. Although PACC is associated with improved stage-specific survival compared to pancreatic ductal adenocarcinoma (PDAC), the high rate of recurrence after surgery (mainly in the liver) is the primary limitation of treatment[4-7].
Radiofrequency ablation (RFA) is a well-established treatment for hepatocellular carcinoma and represents a good alternative to surgery for colorectal liver metastases[8]. There is also a potential role and survival benefit of hepatic ablation in carefully selected patients with non-colorectal liver metastases, such as breast cancer and neuroendocrine tumours (NETs)[9,10]. However, for metastatic PDAC, the evidence is inconsistent with regard to metastasectomy or local ablative therapies, even for oligometastatic patients[11]. Moreover, only a few cases of PACC liver metastases treated with RFA have been reported in the literature. Here, we describe two PACC patients with long-term survival and recurrent PACC in the liver treated with RFA in addition to chemotherapy.
Case 1: A 43-year-old man without any symptoms who was monitored for familial adenomatous polyposis.
Case 2: A 49-year-old man presenting with a palpable abdominal mass and post-prandial abdominal pain over the previous 3 mo.
Case 1: In 1994, the patient was diagnosed with familial adenomatous polyposis (APC gene variant c.847C > T) and treated with prophylactic proctocolectomy with ileoanal pouch.
Case 2: The patient had no relevant past history.
Case 1: His brother is a carrier of the same APC gene mutation, and his father died at the age of 40 due to colorectal cancer.
Case 2: No significant personal or family history.
Case 1: Unremarkable.
Case 2: A clinical examination revealed a large, palpable abdominal mass.
Case 1: CEA, CA 19-9, and alpha-fetoprotein were negative.
Case 2: CEA, CA 19-9, neuron-specific enolase, Chromogranin A and alpha-fetoprotein were negative.
Case 1: Computed tomography (CT) revealed a hypodense mass measuring 5 cm in the head of the pancreas. F-18 fluorodeoxyglucose positron emission tomography CT (18-FDG PET-CT) showed a hypermetabolic lesion. There was no evidence of metastatic disease after a complete examination.
Case 2: CT indicated a very large, low-density tumour involving most of the pancreas with a multicystic component compressing the surrounding organs, without evidence of metastatic disease. No pathologic deposits were found by somatostatin-analogue scintigraphy.
Pathological examination of surgical specimens revealed PACC with positive retroperitoneal surgical margin (R1), vascular and perineural invasion and regional lymph node negativity.
Histologic examination of surgical specimens revealed PACC with a neuroendocrine component < 25% with negative regional lymph nodes.
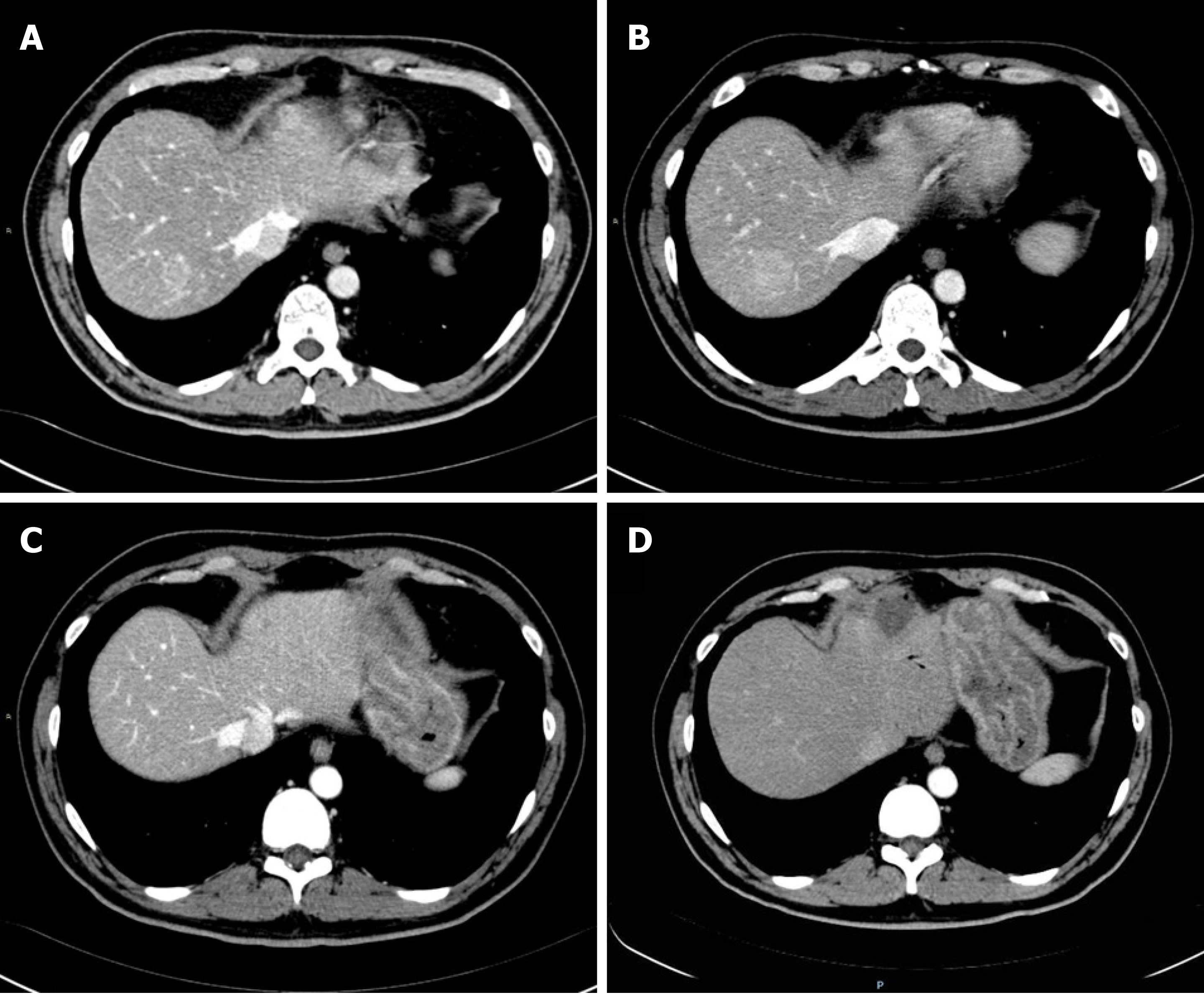
Considering the lack of evidence of distant metastases based on CT, the patient underwent a pylorus-preserving pancreaticoduodenectomy in October 2007, and the histopathological examination revealed PACC in the head of the pancreas measuring 5 cm in diameter, a positive retroperitoneal surgical margin (R1), vascular and perineural invasion and regional lymph node negativity (pT3N0Mx, AJCC 8th edition). Due to his slow recovery after surgery, which was characterized by a bilio-enteric fistula, no adjuvant chemotherapy or radiotherapy was administered, and the patient started a follow-up programme. He remained disease-free until August 2009, when CT and 18-FDG-PET-CT showed two hepatic lesions in segments II and VII. The lesion in segment II was biopsied and was demonstrated to be PACC metastasis (Figure 1A). Considering the lack of evidence in the literature, a decision was made to use a first-line treatment that was currently administered to PDAC patients. He was treated with gemcitabine and oxaliplatin (gemcitabine 1000 mg/m2 plus oxaliplatin 100 mg/m2 in a 14-d cycle). However, the first radiologic assessment after six courses of therapy demonstrated an increase in the diameter of the previously reported metastases (Figure 1B) and the appearance of new small lesions. In January 2010, the chemotherapy regimen was switched to gemcitabine plus capecitabine (gemcitabine 1000 mg/m2 on days 1 and 8 and capecitabine 1300 mg/m2 for 14 d). Except for neutropoenia (G3) and anaemia (G2), the treatment was well tolerated and was administered for a total of 22 cycles until April 2012; the patient achieved a partial response, and only the lesion localized to segment II remained detectable by CT (Figure 1C). This lesion was treated in June 2012 with RFA without any complications (Figure 1D). The patient was then treated with another four cycles of gemcitabine plus capecitabine until November 2012, when he started a follow-up programme. In March 2013, both CT and 18-FDG PET-CT showed a hepatic lesion highly suspicious of relapse, and the patient restarted treatment with gemcitabine plus capecitabine, achieving a complete response according to 18-FDG PET-CT and CT after three cycles. Considering the absence of any sign of disease and the long-term treatment, chemotherapy was stopped. Since June 2013, there have been no signs of tumour relapse.
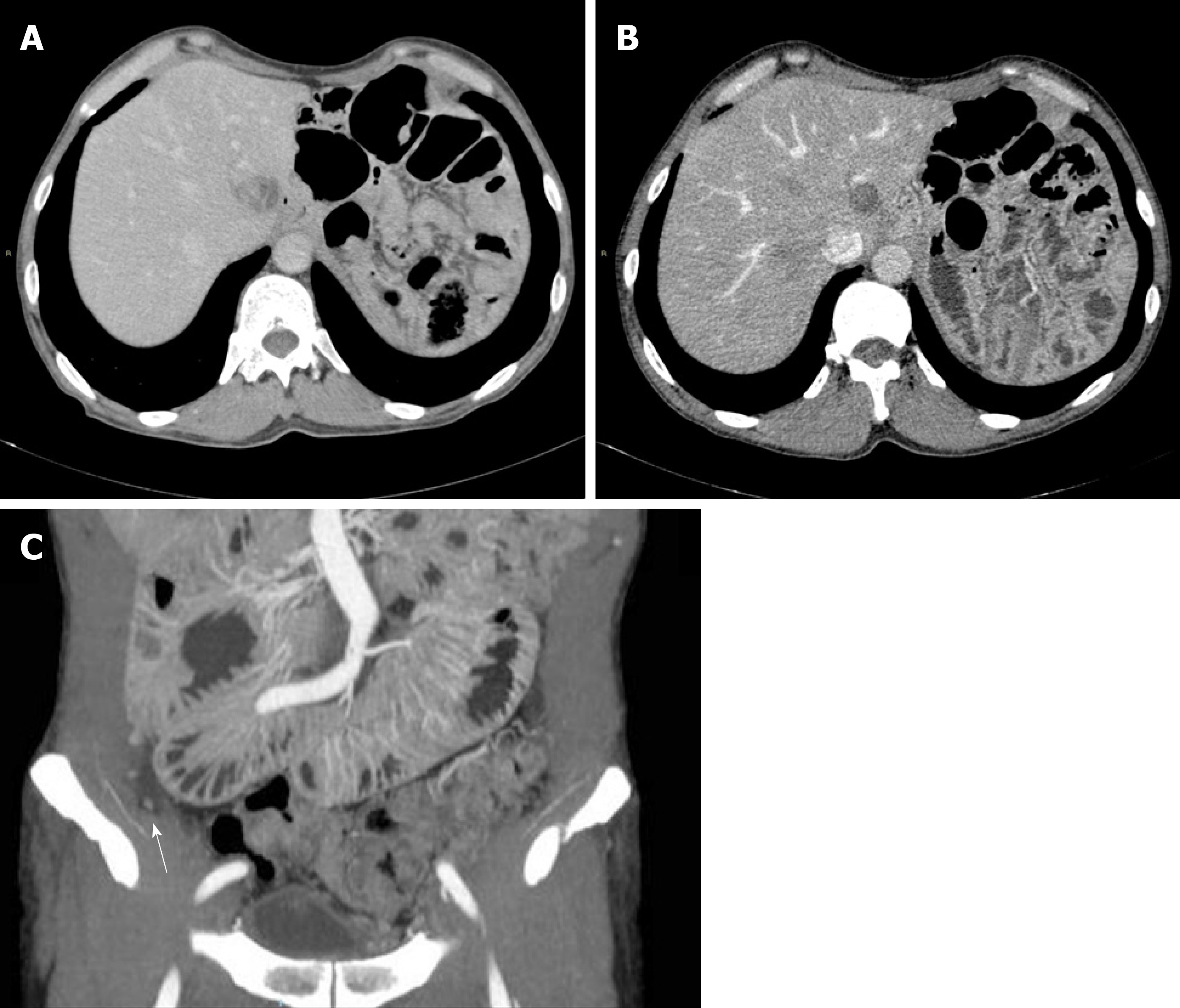
Considering the relevant symptoms caused by the mass and the lack of evidence of distant metastases by CT, in January 2009, the patient was treated with total pancreatectomy, total gastrectomy, splenectomy, extended right hemicolectomy and left adrenalectomy. Pathology revealed a PACC with a neuroendocrine component < 25% in the pancreas, measuring 20 cm in diameter with negative regional lymph nodes (pT3N0Mx, AJCC 8th edition). A postoperative CT showed two hepatic metastases at segments I and V (Figure 2A). He was started on treatment with gemcitabine plus oxaliplatin and achieved stable disease for 27 wk until October 2009. Due to peripheral neuropathy (oxaliplatin-related neurotoxicity), the patient was treated with gemcitabine plus capecitabine for eight cycles and achieved a partial response. Considering the long-term disease control, RFA was performed for the remaining hepatic metastasis at segment I in September 2010, and another eight cycles of gemcitabine plus capecitabine were administered until July 2011 (Figure 2B). The patient remained disease-free until June 2012, when both CT and 18-FDG-PET-CT indicated peritoneal relapse (Figure 2C). Treatment with gemcitabine plus capecitabine was restarted, but after four cycles, progressive disease in the peritoneum was documented. In November 2012, therapy was switched to capecitabine plus irinotecan (irinotecan 200 mg/m2 on day 1 and capecitabine 2000 mg/m2 on days 1 to 15 every 21 d), leading to good disease control for nearly two years. Twenty cycles were administered, with a considerable reduction in 18F-FDG uptake (a standardized uptake value of 2.3 vs 4.8) and stable disease based on CT. In November 2014, CT showed progression in the peritoneum; we performed a biopsy of a peritoneal nodule, and pathology confirmed the initial diagnosis of PACC. Considering both the lack of evidence in the literature in favour of other treatments and the long time since the last administration of gemcitabine plus capecitabine, this treatment was reintroduced. However, this systemic treatment caused severe myelotoxicity after four cycles, and the patient was thus administered only metronomic capecitabine until July 2015, when chemotherapy was stopped due to persistent anaemia and thrombocytopaenia. CT was performed in December 2015 and documented further progressive disease in the peritoneum and new liver metastases. The patient died in March 2016, seven years after the first relapse.
Since June 2013, there have been no signs of tumour relapse.
CT was performed in December 2015 and documented further progressive disease in the peritoneum and new liver metastases. The patient died in March 2016, seven years after the first relapse.
PACC is a rare tumour accounting for approximately 1% of all exocrine pancreatic neoplasms and mostly occurs in late adulthood, with a peak incidence in the sixth decade of life and a male to female ratio of 3.6:1[3]. PACC often has a large size at detection, with a diameter > 10 cm; nevertheless, most patients have no specific symptoms, such as weight loss (52%), abdominal pain (32%), nausea and vomiting (20%), melena (12%), weakness, anorexia or diarrhoea (8%)[5]. There are some remarkable differences in the driver mutations and patterns of genetic alterations in PACC compared to PDAC. The patient described in case 1 was affected by familial adenomatous polyposis, presenting the APC gene variant c.847C > T. Alterations in APC are frequently involved in the pathogenesis of PACC; indeed, alterations in APC, mainly represented by loss (48%) and methylation (56%), are observed in approximately one-half of PACC patients; in contrast, mutations, such as in our patient, are less frequently observed (7%)[12]. For this reason, it is not surprising that people with familial adenomatous polyposis appear to be particularly predisposed to developing PACC[13], although there are only a few cases reported in the literature of patients with familial adenomatous polyposis affected by PACC[14]. Studies have reported lower rates of EGFR and KRAS mutations in PACC than in PDAC, though more than 70% of PACCs display a reduction in or loss of DCC expression based on immunohistochemistry. This appears to be an early genetic change that is different from what occurs in PDAC[15]. Furthermore, the differences in genetic alterations between PACC and PDAC are reflected in the different prognoses. For example, in a retrospective study involving 865 patients with PACC, the five-year stage-specific survival was significantly better for resected PACC than PDAC (stage I: 52.4% vs 28.4%; II: 40.2% vs 9.8%; III: 22.8% vs 6.8%; and IV: 17.2% vs 2.8%)[4]. Nevertheless, approximately half of these patients have metastatic disease at diagnosis; the liver is the most common site of metastasis, and a high rate of disease recurrence after surgery has been documented by several studies and ranges from 50% (median follow-up 27.1 mo) to 100%, with recurrence mainly located in the liver[5,6,16].
With this high rate of disease recurrence and a relatively less aggressive biological behaviour than PDAC, some authors have suggested that aggressive multimodal treatments such as multiple lines of chemotherapy combined with loco-regional techniques or reiterative surgery should be considered for patients with advanced or recurrent disease, as overall survival might improve[16,17]. Hartwig et al[18] compared the long-term survival of six patients with limited metastatic disease (3 patients with synchronous hepatic metastases, 1 patient with synchronous omental metastases and 2 patients with metachronous liver metastases) who underwent both primary and metastatic lesion resection with that of nonmetastatic patients, and there were no differences between the two groups. Other promising survival outcomes have been reported in some case series and case reports for patients who underwent an aggressive surgical approach[19-22].
RFA is based on protein denaturation with thermal coagulation caused by electrodes that are directly inserted into the centre of the tumour. It is a well-established treatment for hepatocellular carcinoma and represents a good alternative to surgery for colorectal and NET liver metastases[8,10]; however, there are only a few reports about RFA for the treatment of metastatic PACC (Table 1). As an example, Armstrong et al[23] reported a survival of over five years for metastatic PACC treated with multiple RFAs, cryotherapy, stereotactic radiosurgery and several lines of chemotherapy based on genomic profiling and cell line development. In addition, a case series of Butturini et al[16] described a patient with recurrent PACC in the liver 28 mo after surgery; the patient was not suitable for surgical resection and was treated with chemotherapy (gemcitabine, oxaliplatin and capecitabine), RFA and chemoembolization, achieving an overall survival of 45 mo. Cananzi et al[24] described an 11-year survival outcome of a patient with PACC who had liver metastasis and was treated with reiterative surgery, RFA and multiple lines of chemotherapy. Additionally, a patient who was treated with radiotherapy for primary tumour and RFA for hepatic metastases after responding well to gemcitabine plus oxaliplatin was reported by Béchade et al[25]. However, the follow-up was too brief to draw conclusions.
| Report | Metastatic at diagnosis | First-line therapy | Relapse | Second-line therapy | Overall survival |
| Béchade et al[25], 2016 | Two liver metastases | GEMOX 6 cycles + radiation therapy for the primary lesion + radiofrequency treatment for the two liver metastases | - | - | Disease-free at 6 mo after radiotherapy and RFA |
| Cananzi et al[24], 2013 | Multiple liver metastases | 1 Docetaxel, irinotecan and cetuximab 2 February 2002: Distal pancreatosplenectomy with right hepatectomy; adjuvant chemotherapy with docetaxel, irinotecan and cetuximab | Liver 5 mo | 1 July 2002: Gemcitabine, oxaliplatin and cetuximab and then continued only cetuximab (toxicity) 2 May 2006: Resection of the liver and peritoneal metastases, extensive lymphadenectomy, followed by oxaliplatin (hypersensitivity reaction) and later carboplatin, gemcitabine, cetuximab and bevacizumab 3 October 2007 and January 2008: 2 RFAs (17 and 20 mo after the second surgery) for hepatic metastases, followed by carboplatin (later stopped), gemcitabine, cetuximab and bevacizumab (later stopped); PD at 35 mo after the second surgery 4 April 2009: Repeat para-aortic lymphadenectomy, intraoperative hepatic RFA, followed by gemcitabine, capecitabine, and cetuximab 5 April 2010: Right/left partial adrenalectomy, partial peritonectomy, extensive lymphadenectomy, followed by liposomal doxorubicin 6 August 2010: Nab-paclitaxel with panitumumab 7 March 2012: Resection of a retroperitoneal mass and completion of left adrenalectomy 8 June 2012: Left frontal brain metastasis resection 9 August 2012: Nab-paclitaxel and panitumumab | Over 11 years after diagnosis and over 10 years after the first surgery |
| Armstrong et al[23], 2011 | One liver metastasis | October 2003: Distal pancreatectomy and splenectomy + RFA on liver metastasis. Adjuvant therapy: 3 cycles of gemcitabine and cisplatin followed by radiation to the pancreatic bed concomitant with five weekly cycles of cisplatin | Liver 6 mo | 1 April 2004: Hepatic RFA + 5 cycles of weekly paclitaxel with thalidomide; continued only paclitaxel (toxicity) 2 February 2005: Capecitabine 3 August 2005: Imatinib 4 October 2005: Etoposide 5 December 2005: Liposomal doxorubicin 6 February 2007: Hepatic intra-arterial brachytherapy with SIR-spheres 7 January 2008: RFA for 1 liver lesion retreated with cryotherapy 8 May 2008: stereotactic radiosurgery of a pericardial lymph node 9 July 2008: sorafenib and temozolomide 10 November 2008: external beam radiation of a progressive liver lesion 11 January 2009: nab-paclitaxel and bevacizumab, stopped for toxicity | DOD at 68 mo |
| Butturini et al[16], 2011 | No | Whipple (T3N0MX stage II R0) | Multiple liver metastases 28 mo | 1 Gemcitabine, capecitabine and oxaliplatin 2 RFA + chemoembolization | DOD at 45 mo |
In contrast to previously reported cases, our patients were treated with only chemotherapy and RFA, without other local ablative therapies or reiterative surgery. In addition, our patients presented resectable disease at diagnosis, whereas most of the previously reported patients had metastasis at diagnosis. For our patients, the decision to apply RFA was made based on the oligometastatic nature of the disease and the good response to chemotherapy. For cases 1 and 2, one liver metastasis was treated with RFA, resulting in a disease-free survival of 9 and 21 mo, respectively. No relevant complications or recurrence of the treated metastases occurred. In both patients, chemotherapy was administered after RFA. We believe that this case study may help to obtain a better understanding of the real impact of local ablative therapies such as RFA for this rare disease. Nevertheless, a limitation of this report is the small number of cases, which was due to the rarity of PACCs. For this reason, it is impossible to offer strong recommendations.
Due to the very low incidence of this disease, there are no prospective studies focusing on chemotherapy for PACC, and data are available from only case series and case reports; making it is unclear what is the most appropriate first-line chemotherapy regimen. Nevertheless, combination fluoropyrimidine-based chemotherapies appear to be the best choice, as they are associated with higher rates of disease control than gemcitabine-based chemotherapies, as shown in a systematic review by Glazer et al[26]. Additionally, platinum-based chemotherapy shows promising results in terms of overall survival. In a retrospective study by Brunetti et al[27], which included 23 PACC patients treated with chemotherapy, 10 of 12 patients who had an overall survival equal to or longer than the median reported overall survival were treated with platinum-based chemotherapy. Furthermore, according to Yoo et al[28], oxaliplatin-containing regimens may have improved activity against PACC than gemcitabine. The activity of platinum salts may be explained by the fact that approximately half of PACC patients (45%) have inactivating genomic alterations in DNA repair genes and that BRCA2 mutations are detected in 20% of PACC patients[29]. No data support the activity of gemcitabine alone, and promising results were not obtained with the combination of gemcitabine plus nab-paclitaxel[20,27]. Our findings are in line with previous studies that showed the efficacy of fluoropyrimidine-based chemotherapy. Both patients obtained a partial response to gemcitabine plus capecitabine, and in case 1, we also observed a complete response to the treatment of the second relapse. In addition, patient 2 was treated with capecitabine plus irinotecan as a third-line therapy and achieved stable disease for nearly two years.
We report two cases of long-term PACC survivors who were effectively treated with RFA in addition to fluoropyrimidine-based chemotherapy. Our cases highlight the different biological behaviour of PACC and PDAC and the importance of multidisciplinary treatment involving local ablative therapies for metastatic PACC. As previously demonstrated in the treatment of liver metastases of other types of cancers, we suggest that RFA should also be considered for PACC patients and that it may improve the prognosis of oligometastatic PACCs, which are chemo-responsive.
Manuscript source: Unsolicited Manuscript
Specialty type: Medicine, research and experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yu PF, Rawat K, Aktekin A S-Editor: Wang YQ L-Editor: A E-Editor: Liu MY
| 1. | Mulkeen AL, Yoo PS, Cha C. Less common neoplasms of the pancreas. World J Gastroenterol. 2006;12:3180-3185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Wisnoski NC, Townsend CM, Nealon WH, Freeman JL, Riall TS. 672 patients with acinar cell carcinoma of the pancreas: a population-based comparison to pancreatic adenocarcinoma. Surgery. 2008;144:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 3. | Chaudhary P. Acinar Cell Carcinoma of the Pancreas: A Literature Review and Update. Indian J Surg. 2015;77:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Schmidt CM, Matos JM, Bentrem DJ, Talamonti MS, Lillemoe KD, Bilimoria KY. Acinar cell carcinoma of the pancreas in the United States: prognostic factors and comparison to ductal adenocarcinoma. J Gastrointest Surg. 2008;12:2078-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Holen KD, Klimstra DS, Hummer A, Gonen M, Conlon K, Brennan M, Saltz LB. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol. 2002;20:4673-4678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 200] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Seo S, Yoo C, Kim KP, Ryoo BY, Chang HM, Hong SM, Lee JH, Song KB, Hwang DW, Kim KH, Hwang S, Kim SC. Clinical outcomes of patients with resectable pancreatic acinar cell carcinoma. J Dig Dis. 2017;18:480-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Matos JM, Schmidt CM, Turrini O, Agaram NP, Niedergethmann M, Saeger HD, Merchant N, Johnson CS, Lillemoe KD, Grützmann R. Pancreatic acinar cell carcinoma: a multi-institutional study. J Gastrointest Surg. 2009;13:1495-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Tsitskari M, Filippiadis D, Kostantos C, Palialexis K, Zavridis P, Kelekis N, Brountzos E. The role of interventional oncology in the treatment of colorectal cancer liver metastases. Ann Gastroenterol. 2019;32:147-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 9. | Wong J, Cooper A. Local Ablation for Solid Tumor Liver Metastases: Techniques and Treatment Efficacy. Cancer Control. 2016;23:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Eriksson J, Stålberg P, Nilsson A, Krause J, Lundberg C, Skogseid B, Granberg D, Eriksson B, Akerström G, Hellman P. Surgery and radiofrequency ablation for treatment of liver metastases from midgut and foregut carcinoids and endocrine pancreatic tumors. World J Surg. 2008;32:930-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Ghidini M, Petrillo A, Salati M, Khakoo S, Varricchio A, Tomasello G, Grossi F, Petrelli F. Surgery or Locoregional Approaches for Hepatic Oligometastatic Pancreatic Cancer: Myth, Hope, or Reality? Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Furlan D, Sahnane N, Bernasconi B, Frattini M, Tibiletti MG, Molinari F, Marando A, Zhang L, Vanoli A, Casnedi S, Adsay V, Notohara K, Albarello L, Asioli S, Sessa F, Capella C, La Rosa S. APC alterations are frequently involved in the pathogenesis of acinar cell carcinoma of the pancreas, mainly through gene loss and promoter hypermethylation. Virchows Arch. 2014;464:553-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Pittman ME, Brosens LA, Wood LD. Genetic Syndromes with Pancreatic Manifestations. Surg Pathol Clin. 2016;9:705-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Moussata D, Senouci L, Berger F, Scoazec JY, Pinson S, Walter T, Lombard-Bohas C, Saurin JC. Familial adenomatous polyposis and pancreatic cancer. Pancreas. 2015;44:512-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Bergmann F, Aulmann S, Sipos B, Kloor M, von Heydebreck A, Schweipert J, Harjung A, Mayer P, Hartwig W, Moldenhauer G, Capper D, Dyckhoff G, Freier K, Herpel E, Schleider A, Schirmacher P, Mechtersheimer G, Klöppel G, Bläker H. Acinar cell carcinomas of the pancreas: a molecular analysis in a series of 57 cases. Virchows Arch. 2014;465:661-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Butturini G, Pisano M, Scarpa A, D'Onofrio M, Auriemma A, Bassi C. Aggressive approach to acinar cell carcinoma of the pancreas: a single-institution experience and a literature review. Langenbecks Arch Surg. 2011;396:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Ohara Y, Oda T, Enomoto T, Hisakura K, Akashi Y, Ogawa K, Owada Y, Domoto Y, Miyazaki Y, Shimomura O, Kurata M, Ohkohchi N. Surgical resection of hepatic and rectal metastases of pancreatic acinar cell carcinoma (PACC): a case report. World J Surg Oncol. 2018;16:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Hartwig W, Denneberg M, Bergmann F, Hackert T, Hinz U, Strobel O, Büchler MW, Werner J. Acinar cell carcinoma of the pancreas: is resection justified even in limited metastatic disease? Am J Surg. 2011;202:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Jauch SF, Morris VK, Jensen CT, Kaseb AO. Multimodal approach and long-term survival in a patient with recurrent metastatic acinar cell carcinoma of the pancreas: A case report. Pancreatology. 2016;16:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Kruger S, Haas M, Burger PJ, Ormanns S, Modest DP, Westphalen CB, Kleespies A, Angele MK, Hartwig W, Bruns CJ, Kirchner T, Werner J, Heinemann V, Boeck S. Acinar cell carcinoma of the pancreas: a rare disease with different diagnostic and therapeutic implications than ductal adenocarcinoma. J Cancer Res Clin Oncol. 2016;142:2585-2591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Sumiyoshi T, Shima Y, Okabayashi T, Kozuki A, Iwata J, Saisaka Y, Tokumaru T, Nakamura T, Morita S. Long-term survival following pancreatectomy and s-1 chemotherapy for pancreatic acinar cell carcinoma with peritoneal dissemination: a case report and literature review. Medicine (Baltimore). 2015;94:e378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Nagata S, Tomoeda M, Kubo C, Yoshizawa H, Yuki M, Kitamura M, Takenaka A, Uehara H, Katayama K, Nakanishi K, Takahashi H, Ohigashi H, Ishikawa O, Tomita Y. Intraductal polypoid growth variant of pancreatic acinar cell carcinoma metastasizing to the intrahepatic bile duct 6 years after surgery: a case report and literature review. Pancreatology. 2012;12:23-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Armstrong MD, Von Hoff D, Barber B, Marlow LA, von Roemeling C, Cooper SJ, Travis P, Campbell E, Paz-Fumagalli R, Copland JA, Colon-Otero G. An effective personalized approach to a rare tumor: prolonged survival in metastatic pancreatic acinar cell carcinoma based on genetic analysis and cell line development. J Cancer. 2011;2:142-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Cananzi FC, Jayanth A, Lorenzi B, Belgaumkar A, Mochlinski K, Sharma A, Mudan S, Cunningham D. "Chronic" metastatic pancreatic acinar cell carcinoma. Pancreatology. 2013;13:549-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Béchade D, Desjardin M, Salmon E, Désolneux G, Bécouarn Y, Evrard S, Fonck M. Pancreatic Acinar Cell Carcinoma. Case Rep Gastroenterol. 2016;10:174-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Glazer ES, Neill KG, Frakes JM, Coppola D, Hodul PJ, Hoffe SE, Pimiento JM, Springett GM, Malafa MP. Systematic Review and Case Series Report of Acinar Cell Carcinoma of the Pancreas. Cancer Control. 2016;23:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Brunetti O, Aprile G, Marchetti P, Vasile E, Casadei Gardini A, Scartozzi M, Barni S, Delfanti S, De Vita F, Di Costanzo F, Milella M, Cella CA, Berardi R, Cataldo I, Scarpa A, Basile D, Mazzuca F, Graziano G, Argentiero A, Santini D, Reni M, Cascinu S, Silvestris N. Systemic Chemotherapy for Advanced Rare Pancreatic Histotype Tumors: A Retrospective Multicenter Analysis. Pancreas. 2018;47:759-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 28. | Yoo C, Kim BJ, Kim KP, Lee JL, Kim TW, Ryoo BY, Chang HM. Efficacy of Chemotherapy in Patients with Unresectable or Metastatic Pancreatic Acinar Cell Carcinoma: Potentially Improved Efficacy with Oxaliplatin-Containing Regimen. Cancer Res Treat. 2017;49:759-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Chmielecki J, Hutchinson KE, Frampton GM, Chalmers ZR, Johnson A, Shi C, Elvin J, Ali SM, Ross JS, Basturk O, Balasubramanian S, Lipson D, Yelensky R, Pao W, Miller VA, Klimstra DS, Stephens PJ. Comprehensive genomic profiling of pancreatic acinar cell carcinomas identifies recurrent RAF fusions and frequent inactivation of DNA repair genes. Cancer Discov. 2014;4:1398-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |