Published online Mar 26, 2020. doi: 10.12998/wjcc.v8.i6.1042
Peer-review started: December 10, 2019
First decision: December 30, 2019
Revised: January 7, 2020
Accepted: March 5, 2020
Article in press: March 5, 2020
Published online: March 26, 2020
Processing time: 106 Days and 18.6 Hours
Acute-on-chronic liver failure (ACLF), which includes hepatic and multiple extra-hepatic organ failure, is a severe emergency condition that has high mortality. ACLF can rapidly progress and requires an urgent assessment of condition and referral for liver transplantation. Bacterial infections (BIs) trigger ACLF and play pivotal roles in the deterioration of clinical course.
To investigate the clinical characteristics and 28-d outcomes of first BIs either at admission or during hospitalization in patients with hepatitis B virus (HBV)-ACLF as defined by the Chinese Group on the Study of Severe Hepatitis B (COSSH).
A total of 159 patients with HBV-ACLF and 40 patients with acute decompensation of HBV-related chronic liver disease combined with first BIs were selected for a retrospective analysis between October 2014 and March 2016. The characteristics of BIs, the 28-d transplant-free survival rates, and the independent predictors of the 28-d outcomes were evaluated.
A total of 194 episodes of BIs occurred in 159 patients with HBV-ACLF. Among the episodes, 13.4% were community-acquired, 46.4% were healthcare-associated, and 40.2% belonged to nosocomial BIs. Pneumonia (40.7%), spontaneous bacterial peritonitis (SBP) (34.5%), and bloodstream infection (BSI) (13.4%) were the most prevalent. As the ACLF grade increased, the incidence of SBP showed a downward trend (P = 0.021). Sixty-one strains of bacteria, including 83.6% Gram-negative bacteria and 29.5% multidrug-resistant organisms, were cultivated from 50 patients with ACLF. Escherichia coli (44.3%) and Klebsiella pneumoniae (23.0%) were the most common bacteria. As the ACLF grade increased, the 28-d transplant-free survival rates showed a downward trend (ACLF-1, 55.7%; ACLF-2, 29.3%; ACLF-3, 5.4%; P < 0.001). The independent predictors of the 28-d outcomes of patients with HBV-ACLF were COSSH-ACLF score (hazard ratio [HR] = 1.371), acute kidney injury (HR = 2.187), BSI (HR = 2.339), prothrombin activity (HR = 0.967), and invasive catheterization (HR = 2.173).
For patients with HBV-ACLF combined with first BIs, pneumonia is the most common form, and the incidence of SBP decreases with increasing ACLF grade. COSSH-ACLF score, acute kidney injury, BSI, prothrombin activity, and invasive catheterization are the independent predictors of 28-d outcomes.
Core tip: Bacterial infections (BIs) play pivotal roles in the deterioration of clinical course in patients with acute-on-chronic liver failure (ACLF). This study aimed to investigate the clinical characteristics of first BIs in patients with hepatitis B virus-ACLF, as defined by the Chinese Group on the Study of Severe Hepatitis B. Healthcare-associated and nosocomial BIs were the most common types. Pneumonia was the most common form of BI. Gram-negative bacteria accounted for 83.6%, and multidrug-resistant organisms accounted for 29.5% of bacteria detected. The 28-d transplant-free survival rates of the patients were very low. Chinese Group on the Study of Severe Hepatitis B-ACLF score, acute kidney injury, bloodstream infection, prothrombin activity, and invasive catheterization were the independent predictors of 28-d outcomes.
- Citation: Li C, Su HB, Liu XY, Hu JH. Clinical characteristics and 28-d outcomes of bacterial infections in patients with hepatitis B virus-related acute-on-chronic liver failure. World J Clin Cases 2020; 8(6): 1042-1055
- URL: https://www.wjgnet.com/2307-8960/full/v8/i6/1042.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i6.1042
Acute-on-chronic liver failure (ACLF) is a common syndrome that occurs in combination with organ failure and has high mortality rate[1]. Although the definitions of ACLF differ between Eastern and Western countries, in the simplest terms, ACLF is an acute deterioration in patients with chronic liver disease (CLD) (usually cirrhosis) and has high short-term mortality[2-4]. Alcoholic liver disease is the major etiology in patients with ACLF from Europe and North America, but hepatitis B virus (HBV) is the major etiology in Asia-Pacific and Africa. Based on the European Association for the Study of the Liver definition of ACLF, the Chinese Group on the Study of Severe Hepatitis B (COSSH) has recently developed new criteria for HBV-ACLF and established a relevant prognostic score based on the characteristics of Chinese patients with HBV-CLD. In patients with chronic hepatitis B, total bilirubin (TBIL) ≥ 12 mg/dL and international normalized ratio (INR) ≥ 1.5 are included in the new criteria for HBV-ACLF. The COSSH-ACLF criteria bridge a gap in the European Association for the Study of the Liver-ACLF definition for HBV-ACLF diagnosis. The new criteria increased the number of patients diagnosed with HBV-related ACLF by 20%, thereby increasing their opportunity to receive intensive management[5].
Bacterial infections (BIs) play pivotal roles in the development and progression of ACLF[6,7]. BIs mainly trigger the occurrence of ACLF in patients with cirrhosis. About 32.6% of patients with ACLF are triggered by BIs in the Chronic Liver Failure Consortium ACLF in Cirrhosis study[8]. The 30-d survival rates of patients with BIs triggered ACLF (71.6%) were distinct from those with non-BIs triggered ACLF (33.8%); moreover, BIs triggered ACLF was independently associated with 30- and 90-d mortality[6].
The study of COSSH show that patients with HBV-CLD combined with BIs are prone to ACLF[5]. However, the clinical characteristics, survival rates, and prognostic effects of BIs in the COSSH definition for patients with HBV-ACLF are unclear. Patients with COSSH-HBV-ACLF combined with first BIs were selected for this retrospective study. This study aimed to evaluate the clinical characteristics of BIs, infection sites, etiology, and 28-d outcomes of patients. To avoid some confounding factors, we did not include patients in the intensive care unit (ICU).
Patients with HBV-ACLF and acute decompensation (AD) of HBV-related CLD combined with first BIs who were hospitalized at the Fifth Medical Center of Chinese PLA General Hospital of China from October 2014 to March 2016 were selected from the electronic database for retrospective analysis. Patients with diseases resulting in bilirubin elevation, such as hemolytic, congenital non-hemolytic, and obstructive jaundice, malignant tumor, and extrahepatic diseases that seriously influence life were excluded. Patients with hospital length of stay < 3 d or incomplete laboratory results, patients in ICU, and pregnant women were also excluded from the electronic database. All patients with HBV-ACLF received antiviral therapy with nucleoside analogues after admission. Those patients were assessed for nutritional status by nutritionist after hospitalization. The target of energy intake for those patients was 30-35 kcal/kg·d.
The inclusion criteria were as follows: Presence of CLD, AD manifestation, and first BIs either at admission or during hospitalization. The exclusion criteria were as follows: Viral infections other than HBV and hepatic lesions because of other factors, such as alcoholic hepatitis, autoimmune liver diseases, drug-induced liver injury, Wilson’s Disease, hemochromatosis, and schistosomiasis.
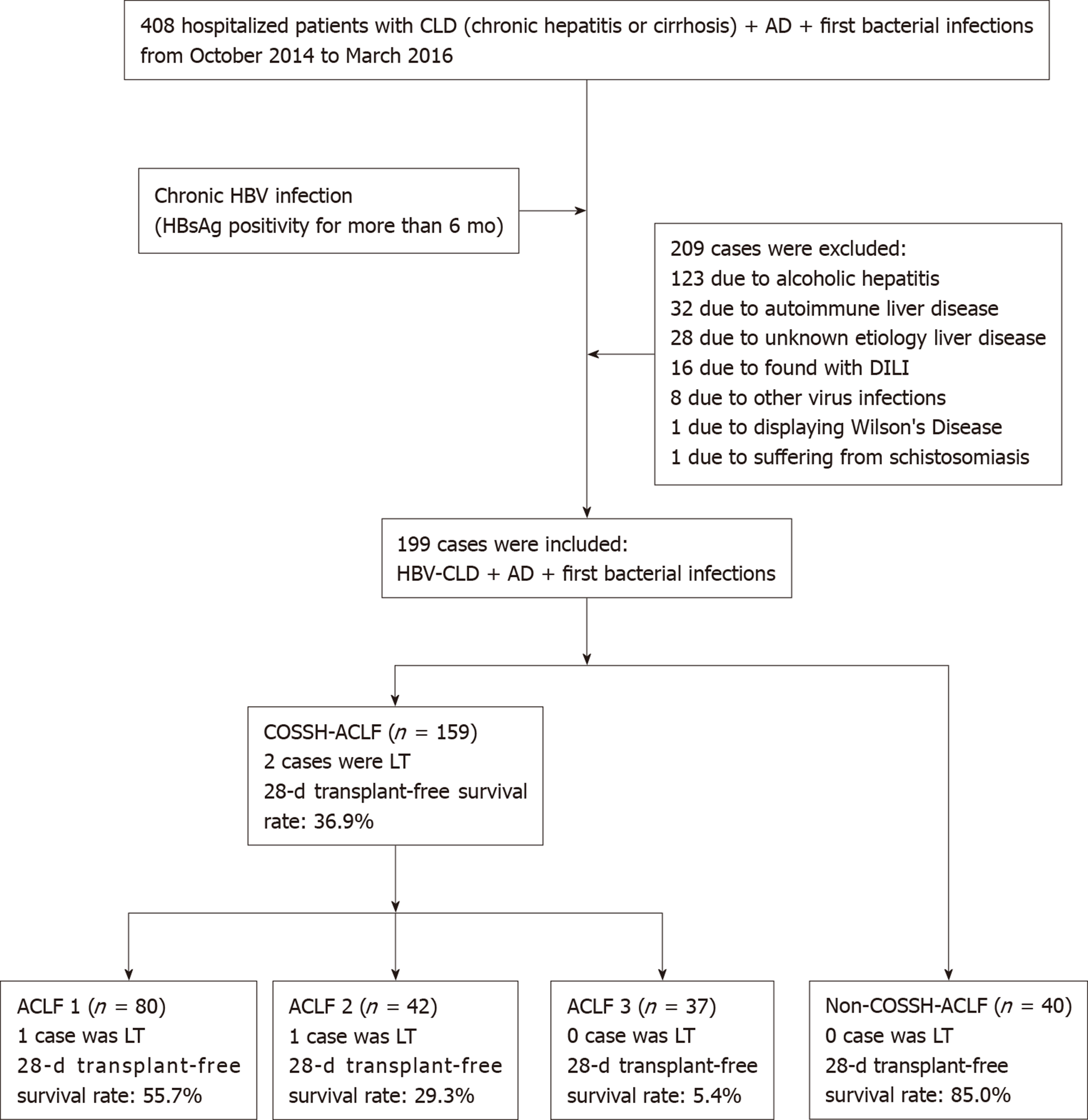
A total of 408 patients satisfied the inclusion criteria, and 209 patients were excluded based on the exclusion criteria. Finally, 199 patients were selected. According to the definition of COSSH-HBV-ACLF, 159 patients were diagnosed with HBV-ACLF and 40 patients were diagnosed with AD-HBV-CLD. Eighty cases were ACLF grade 1 (ACLF-1), 42 cases were ACLF grade 2 (ACLF-2), and 37 cases were ACLF grade 3 (ACLF-3). The patients were followed 28 d after their first BIs. We considered death as the primary endpoint. Information on prognosis was verified through medical records and telephone contact. Two patients (one case with ACLF-1 and one case with ACLF-2) with HBV-ACLF received orthotopic liver transplantation during the follow-up period (Figure 1).
Based on the COSSH-HBV-ACLF criteria, patients with HBV-CLD were divided into an HBV-ACLF (abbreviated as ACLF) group and an AD of HBV-CLD (abbreviated as AD) group. According to the degree and number of organ failures, ACLF was divided into ACLF-1, ACLF-2, and ACLF-3. The severity of ACLF was assessed using COSSH-ACLF scores. COSSH-ACLF scores were calculated using the formula 0.741 × INR + 0.523 × HBV-SOFA + 0.026 × age + 0.003 × TBIL. HBV-SOFA was assessed based on the severity of kidney injury, hepatic encephalopathy (HE), circulation, and respiratory function by COSSH-ACLF criteria[5].
Complications of ACLF were diagnosed based on the following criteria. HE was defined based on the West Haven criteria[9]. Acute variceal bleed was diagnosed based on the Baveno V endoscopic criteria[10]. Acute kidney injury (AKI) was defined using the criteria of revised consensus recommendations of the International Club of Ascites[11].
First BIs were referred to as the first BIs that occurred either at admission or during hospitalization in patients. BIs were diagnosed based on the following criteria. Spontaneous bacterial peritonitis (SBP) was diagnosed based on polymorphonuclear cell count in ascitic fluid ≥ 250/mm3 with/without a positive fluid culture[12]. Pneumonia was diagnosed based on the presence of radiological evidence of consolidation with at least two of the following criteria: Fever higher than 38 °C or hypothermia less than 35 °C; dyspnea; cough; purulent sputum; pleuritic chest pain; and white blood cells > 1000/mm3 or < 4000/mm3[13]. Bloodstream infection (BSI) was diagnosed based on the growth of a non-common skin contaminant from ≥ 1 blood culture (BC) and of a common skin contaminant from ≥ 2 BCs drawn on separate sites with signs of infection[14]. Spontaneous bacterial empyema (SBE) was diagnosed based on polymorphonuclear cell count in pleural fluid ≥ 250/mm3[15]. Urinary tract infection (UTI) was diagnosed based on urine white blood cells count > 10/high-power field with a positive urine Gram stain or urine culture or uncountable leucocytes per field if negative cultures[16]. Cellulitis was diagnosed based on the clinical signs of infection of the skin associated with discomfort, erythema, swelling, and warmth of the affected area[17]. Unproven BIs were diagnosed based on the presence of fever and leucocytosis that requires antibiotic therapy without any identifiable source[15]. In the present study, we defined spontaneous bacterial empyema, UTI, cellulitis, and unproven BIs as other BIs.
Systemic inflammation response syndrome (SIRS) was assessed according to the recommendations of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference[18]. The definitions of community-acquired (CA), healthcare-associated (HCA), and nosocomial infections were based on the time of acquisition[19,20]. Multidrug-resistant organisms (MDROs) was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories[21].
Categorical variables are expressed as number (%), and continuous variables are described as the mean ± SD or median (inter-quartile range, Q1–Q3). Continuous variables were compared by Student’s t test. Mann–Whitney U test was used to compare the parameters under nonnormal distribution. Categorical data were compared by χ2 or Fisher’s exact test. Trend test of categorical data was carried out using linear-by-linear association approach. Cumulative survival probability curves were calculated by Kaplan–Meier method and compared by log-rank test. Univariate COX proportional hazard regression analysis was first used to screen candidate factors. Candidate variables (P < 0.05) were used as input into a multivariate COX proportional hazard regression analysis following a forward stepwise approach (P-in: 0.05 and P-out: 0.01). All statistical analyses were conducted using SPSS 19.0 software (IBM, Armonk, NY). For all analyses, P < 0.05 was considered statistically significant.
This study involved 199 patients, including 159 patients with HBV-ACLF and 40 patients with AD-HBV-CLD, with an average age of 48.0 ± 10.9 years. About 85.4% of the patients were male, and 86.4% had cirrhosis. At the time of BIs, 96.5% had ascites, 47.7% had AKI, 53.3% had HE, 19.6% had acute variceal bleeding, 45.2% had SIRS, and 19.1% had invasive catheterization. Moreover, 18.1% of the patients developed multiple-site BIs.
TBIL, direct bilirubin, alanine aminotransferase, aspartate amino transferase, creatinine, INR, AKI ratio, HE ratio, and COSSH-ACLF scores in the ACLF group were higher than those in the AD group (P < 0.05) (Table 1). The prothrombin activity (PTA) in the ACLF group was lower than that in the AD group (P < 0.05) (Table 1). No differences in the other clinical indicators were observed between the two groups (P > 0.05) (Table 1).
| Characteristic | Total (n = 199) | AD (n = 40) | ACLF (n = 159) | χ2/t/Z value | P value |
| Age (yr) | 48.0 ± 10.9 | 49.0 ± 12.1 | 47.7 ± 10.5 | 0.667 | 0.506 |
| Male (%) | 170 (85.4) | 34 (85.0) | 136 (85.5) | 0.007 | 0.932 |
| Cirrhosis (%) | 172 (86.4) | 35 (87.5) | 137 (86.2) | 0.049 | 0.825 |
| SIRS (%) | 90 (45.2) | 15 (37.5) | 75 (47.2) | 1.206 | 0.272 |
| Temperature (°C) | 37.1 (36.6, 38.0) | 37.4 (36.6, 38.2) | 37.1 (36.6, 38.0) | -0.500 | 0.617 |
| MAP (mmHg) | 87.8 ± 14.2 | 86.6 ± 14.4 | 88.1 ± 14.2 | -0.580 | 0.562 |
| Invasive catheterization (%) | 38 (19.1) | 5 (12.5) | 33 (20.8) | 1.410 | 0.235 |
| Multiple sites of BIs (%) | 36 (18.1) | 6 (15.0) | 30 (18.9) | 0.323 | 0.570 |
| HBV DNA (log10 IU/ml) | 4.1 (2.5, 6.0) | 3.4 (1.2, 5.7) | 4.4 (2.6, 6.2) | -1.259 | 0.208 |
| WBC (×109/L) | 8.7 (6.0, 13.6) | 8.0 (4.7, 11.3) | 8.9 (6.1, 14.0) | -1.477 | 0.140 |
| NEUT (×109/L) | 6.7 (4.1, 11.1) | 5.9 (3.6, 8.6) | 6.9 (4.2, 11.3) | -1.261 | 0.207 |
| HGB (g/L) | 107.8 ± 24.9 | 108.2 ± 25.0 | 107.7 ± 24.9 | 0.129 | 0.897 |
| PLT (×109/L) | 63.0 (42.0, 91.0) | 60.0 (47.0, 90.3) | 63.0 (41.0, 96.0) | -0.092 | 0.927 |
| ALB (g/L) | 27.0 (24.0, 31.0) | 26.5 (23.0, 31.5) | 28.0 (25.0, 31.0) | -1.088 | 0.277 |
| TBIL (μmol/L) | 330.6 ± 158.4 | 226.5 ± 142.9 | 356.7 ± 151.6 | -4.909 | < 0.001 |
| DBIL (μmol/L) | 227.8 ± 109.4 | 159.0 ± 100.6 | 245.2 ± 104.9 | -4.684 | < 0.001 |
| ALT (IU/L) | 81.0 (38.0, 183.0) | 48.0 (29.3, 129.5) | 87.0 (42.0, 246.0) | -2.774 | 0.006 |
| AST (IU/L) | 119.0 (71.0, 221.0) | 73.5 (60.0, 175.3) | 126.0 (82.0, 234.0) | -2.549 | 0.011 |
| ALP (IU/L) | 141.0 (106.0, 188.0) | 133.0 (101.0, 181.5) | 143.0 (111.0, 192.0) | -1.184 | 0.236 |
| GGT (IU/L) | 50.0 (33.0, 77.0) | 46.0 (29.3, 70.8) | 54.0 (34.0, 78.0) | -1.193 | 0.233 |
| Cr (μmol/L) | 101.0 (80.0, 148.0) | 83.0 (72.0, 96.0) | 112.0 (84.0, 162.0) | -4.480 | < 0.001 |
| Na (mmol/L) | 133.0 (130.0, 136.0) | 134.0 (130.3, 136.0) | 133.0 (130.0, 136.0) | -0.916 | 0.360 |
| INR | 2.2 (1.7, 2.8) | 1.6 (1.4, 2.0) | 2.3 (1.9, 3.1) | -6.429 | < 0.001 |
| PTA (%) | 30.0 ± 13.1 | 42.2 ± 12.6 | 26.9 ± 11.4 | 7.455 | < 0.001 |
| CRP (mg/L) | 18.3 (11.1, 40.4) | 25.1 (13.0, 43.0) | 17.5 (10.4, 40.1) | -1.112 | 0.266 |
| PCT (ng/mL) | 1.2 (0.6, 2.3) | 1.1 (0.4, 2.1) | 1.2 (0.7, 2.4) | -1.182 | 0.237 |
| Ascites (%) | 192 (96.5) | 37 (92.5) | 155 (97.5) | 1.101 | 0.126 |
| AKI (%) | 95 (47.7) | 9 (22.5) | 86 (54.1) | 12.782 | < 0.001 |
| HE (%) | 106 (53.3) | 6 (15.0) | 100 (62.9) | 29.449 | < 0.001 |
| AVB (%) | 39 (19.6) | 5 (12.5) | 34 (21.4) | 1.601 | 0.206 |
| COSSH-ACLF scores | 6.5 (5.9, 7.5) | 5.4 (5.2, 5.9) | 6.8 (6.1, 7.9) | -7.851 | < 0.001 |
A total of 194 episodes of BIs occurred in 159 patients with HBV-ACLF, of which 13.4% belonged to CA BIs, 46.4% belonged to HCA BIs, and 40.2% belonged to nosocomial BIs. Forty-seven episodes of BIs occurred in 40 patients with AD of HBV-CLD, of which 19.1% belonged to CA BIs, 57.4% belonged to HCA Bis, and 23.4% belonged to nosocomial BIs. The percentage of nosocomial BIs in the ACLF group was higher than that in the AD group (P = 0.032) (Table 2). Pneumonia, SBP, and BSI were the most common forms of BIs in the ACLF and AD groups. Pneumonia was the most common form in the ACLF group, and SBP was the most common form in the AD group (Table 2).
| Characteristic | Total (n = 241) | AD (n = 47) | ACLF (n = 194) | χ2 value | P value |
| Source of acquisition | |||||
| CA BIs (%) | 35 (14.5) | 9 (19.1) | 26 (13.4) | 1.007 | 0.316 |
| HCA BIs (%) | 117 (48.5) | 27 (57.4) | 90 (46.4) | 1.851 | 0.174 |
| Nosocomial BIs (%) | 89 (36.9) | 11 (23.4) | 78 (40.2) | 4.586 | 0.032 |
| Site of BIs | |||||
| Pneumonia (%) | 93 (38.6) | 14 (29.8) | 79 (40.7) | 1.909 | 0.167 |
| SBP (%) | 87 (36.1) | 20 (42.6) | 67 (34.5) | 1.054 | 0.305 |
| BSI (%) | 32 (13.3) | 6 (12.8) | 26 (13.4) | 0.013 | 0.908 |
| Other BIs (%) | 29 (12.0) | 7 (14.9) | 22 (11.3) | 0.451 | 0.502 |
| SBE (%) | 11 (4.6) | 3 (6.4) | 8 (4.1) | 0.076 | 0.782 |
| UTI (%) | 7 (2.9) | 3 (6.4) | 4 (2.1) | 1.207 | 0.272 |
| Cellulitis (%) | 2 (0.8) | 0 (0.0) | 2 (1.0) | Fisher | 1.000 |
| Unproven BIs (%) | 9 (3.7) | 1 (2.1) | 8 (4.1) | 0.048 | 0.827 |
SBP was the most common form of BI in the AD and ACLF-1 groups. Pneumonia was the most common form of BIs in the ACLF-2 and ACLF-3 groups (Table 3). The trend test displayed that as the ACLF grade increased, the incidence of SBP showed a downward trend (trend value = 5.291, P = 0.021). No significant changes were observed in pneumonia (trend value = 2.357, P = 0.125), BSI (trend value = 0.605, P = 0.437), or other BIs (trend value = 0.083, P = 0.774).
| Site of BIs | AD (n = 47) | ACLF-1 (n = 92) | ACLF-2 (n = 53) | ACLF-3 (n = 49) | χ2 value | P value |
| SBP (%) | 20 (42.6) | 39 (42.4) | 16 (30.2) | 12 (24.5) | 6.093 | 0.107 |
| Pneumonia (%) | 14 (29.8) | 36 (39.1) | 20 (37.7) | 23 (46.9) | 3.006 | 0.391 |
| BSI (%) | 6 (12.8) | 9 (9.8) | 10 (18.9) | 7 (14.3) | 2.468 | 0.481 |
| Other BIs (%) | 7 (14.9) | 8 (8.7) | 7 (13.2) | 7 (14.3) | 1.635 | 0.651 |
Fourteen strains of bacteria were cultivated from 13 cases in the AD group, in which 28.6% belonged to CA, 21.4% belonged to HCA, and 50.0% belonged to nosocomial BIs. Twelve strains of bacteria (85.7%) were Gram negative, including eight strains of Escherichia coli (E. coli), three strains of Klebsiella pneumoniae (K. pneumoniae), and one strain of Enterobacter aerogenes. Two strains of Gram-positive bacteria were detected, including one strain of Staphylococcus aureus (S. aureus) and one strain of Staphylococcus epidermidis (S. epidermidis). Sixty-one strains of bacteria were cultivated from 50 cases in the ACLF group, in which 16.4% belonged to CA, 44.3% belonged to HCA, and 39.3% belonged to nosocomial BIs. Fifty-one strains of bacteria (83.6%) were Gram negative, including 27 strains of E. coli, 14 strains of K. pneumoniae, four strains of Pseudomonas aeruginosa, two strains of Acinetobacter baumannii, one strain of Enterobacter cloacae, one strain of Aeromonas hydrophila, one strain of Aeromonas guinea, and one strain of Stenotrophomonas maltophilia. Ten strains of Gram-positive bacteria were observed, including five strains of Enterococcus faecium, two strains of S. epidermidis, two strains of S. aureus, and one strain of Streptococcus oralis. E. coli and K. pneumoniae were the most common bacteria in the AD and ACLF groups. No significant difference in the proportion of Gram-negative or -positive bacteria was found between the two groups (P > 0.05). In the ACLF group, the most common cultivated bacteria for SBP, BSI, and other BIs were E. coli, but K. pneumoniae was the most common cultivated bacterium for pneumonia (Table 4).
| Bacterial isolate | Total Bis (n = 61) | Pneumonia (n = 12) | SBP (n = 19) | BSI (n = 26) | Other Bis (n = 4) |
| Source of acquisition | |||||
| CA BIs (/total, %) | 10 (16.4) | 0 (0) | 4 (21.1) | 6 (23.1) | 0 (0) |
| HCA BIs (/total, %) | 27 (44.3) | 5 (41.7) | 8 (42.1) | 11 (42.3) | 3 (75.0) |
| Nosocomial BIs (/total, %) | 24 (39.3) | 7 (58.3) | 7 (36.8) | 9 (34.6) | 1 (25.0) |
| Gram-negative bacteria (/total, %) | 51 (83.6) | 12 (100.0) | 16 (84.2) | 20 (76.9) | 3 (75.0) |
| Escherichia coli (/total, %) | 27 (44.3) | 1 (8.3) | 14 (73.7) | 10 (38.5) | 2 (50.0) |
| Klebsiella pneumoniae (/total, %) | 14 (23.0) | 5 (41.7) | 2 (10.5) | 6 (23.1) | 1 (25.0) |
| Pseudomonas aeruginosa (/total, %) | 4 (6.6) | 4 (33.3) | 0 (0) | 0 (0) | 0 (0) |
| Acinetobacter baumannii (/total, %) | 2 (3.3) | 0 (0) | 0 (0) | 2 (7.7) | 0 (0) |
| Other (/total, %) | 4 (6.6) | 2 (16.7) | 0 (0) | 2 (7.7) | 0 (0) |
| Gram-positive bacteria (/total, %) | 10 (16.4) | 0 (0) | 3 (15.8) | 6 (23.1) | 1 (25.0) |
| Enterococcus faecium (/total, %) | 5 (8.2) | 0 (0) | 2 (10.5) | 3 (11.5) | 0 (0) |
| Staphylococcus aureus (/total, %) | 2 (3.3) | 0 (0) | 0 (0) | 2 (7.7) | 0 (0) |
| Staphylococcus epidermidis (/total, %) | 2 (3.3) | 0 (0) | 0 (0) | 1 (3.8) | 1 (25.0) |
| Other (%) (/total, %) | 1 (1.6) | 0 (0) | 1 (5.3) | 0 (0) | 0 (0) |
| MDROs (/total, %) | 18 (29.5) | 3 (25.0) | 5 (26.3) | 8 (30.8) | 2 (50.0) |
| Multidrug-resistant Gram-negative organisms (/Gram-negative bacteria, %) | 10 (19.6) | 3 (25.0) | 3 (18.8) | 3 (15.0) | 1 (33.3) |
| Multidrug-resistant Gram-positive organisms (/Gram-positive bacteria, %) | 8 (80.0) | 0 (0) | 2 (66.7) | 5 (83.3) | 1 (100.0) |
In the AD group, five strains of bacteria (35.7%) were MDROs, including four strains of E. coli and one strain of S. aureus. In the ACLF group, 18 strains of bacteria (29.5%) were MDROs, including ten strains of Gram-negative bacteria (four strains of E. coli, two strains of K. pneumoniae, two strains of Acinetobacter baumannii, one strain of Enterobacter cloacae, and one strain of Pseudomonas aeruginosa) and eight strains of Gram-positive bacteria (five strains of Enterococcus faecium, two strains of S. aureus, and one strain of S. epidermidis). In the ACLF group, the MDRO rate of cultivated Gram-positive bacteria (80.0%) was higher than that of cultivated Gram-negative bacteria (19.6%) (χ2 = 11.900, P = 0.001), and the proportion of multidrug-resistant Gram-positive organisms in BSI (83.3%) was higher than that of multidrug-resistant Gram-negative organisms (15.0%) (χ2 = 7.164, P = 0.007) (Table 4).
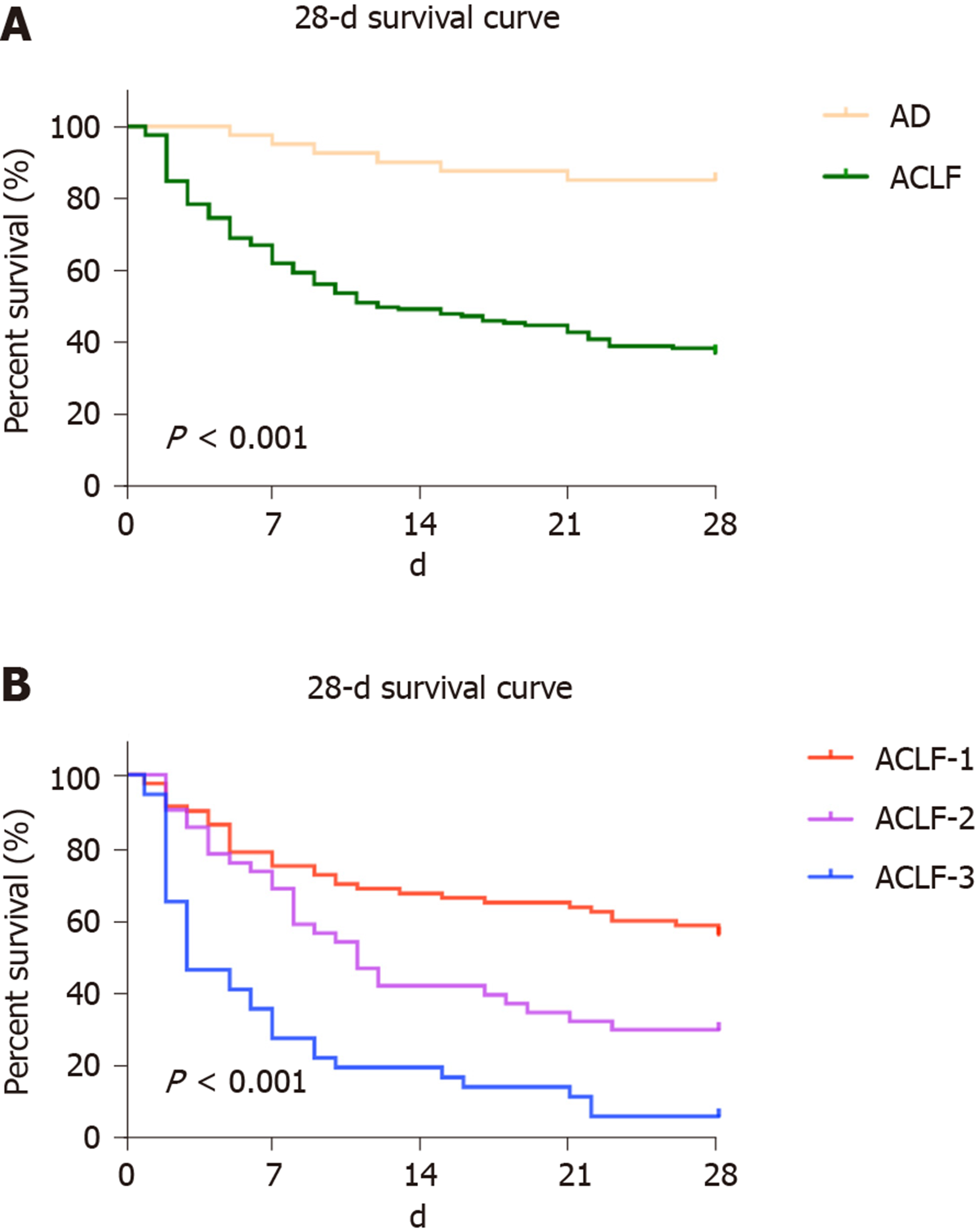
Using the Kaplan–Meier method, the 28-d transplant-free survival rate in the ACLF group (36.9%) was significantly lower than that in the AD group (85.0%) (χ2 = 25.488, P < 0.001) (Figure 2A). Generally, differences were observed in the 28-d transplant-free survival rates in the ACLF-1, ACLF-2, and ACLF-3 groups (χ2 = 47.422, P < 0.001) (Figure 2B). The 28-d transplant-free survival rate (55.7%) of ACLF-1 was higher than those of ACLF-2 (29.3%) (χ2 = 7.824, P = 0.005) and ACLF-3 (5.4%) (χ2 = 44.330, P < 0.001). The 28-d transplant-free survival rate of ACLF-2 was higher than that of ACLF-3 (χ2 = 13.398, P < 0.001) (Figure 2B). As the ACLF grade increased, the 28-d transplant-free survival rates decreased (trend value = 28.557, P < 0.001).
According to the 28-d outcomes, patients with HBV-ACLF were divided into the survivor and non-survivor groups, and two patients who underwent orthotopic liver transplantation were excluded. Univariate Cox regression was conducted on the baseline indices of the cohort. Categorical variables were differentiated and assigned with values based on their sign, and continuous variables were assigned with values according to actual numerical values. Age, SIRS, invasive catheterization, grade of ACLF, WBC, neutrophil count, alanine aminotransferase, aspartate amino transferase, creatinine, INR, PTA, procalcitonin, AKI, HE, pneumonia, SBP, BSI, multiple sites of BIs, and COSSH-ACLF scores were screened out as meaningful variables and used as inputs for multivariate Cox regression (P < 0.05) (Table 5). Multivariate Cox regression showed that COSSH-ACLF scores (hazard ratio [HR] = 1.371), AKI (HR = 2.187), BSI (HR = 2.339), PTA (HR = 0.967), and invasive catheterization (HR = 2.173) were the independent predictors of 28-d outcomes of the patients (P < 0.05) (Table 5).
| Variable | Survivors (n = 58) | Nonsurvivors (n = 99) | Univariate Cox regression | Multivariate Cox regression | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |||
| Age (yr) | 45.5 ± 9.7 | 49.3 ± 10.7 | 1.023 (1.005-1.042) | 0.013 | ||
| Male (%) | 53 (91.4) | 82 (82.8) | 0.693 (0.410-1.170) | 0.170 | ||
| Cirrhosis (%) | 50 (86.2) | 85 (85.9) | 0.956 (0.543-1.684) | 0.877 | ||
| SIRS (%) | 17 (29.3) | 58 (58.6) | 2.214 (1.480-3.310) | < 0.001 | ||
| Temperature (°C) | 37.1 (36.6, 38.0) | 37.1 (36.6, 38.0) | 1.055 (0.861-1.293) | 0.604 | ||
| MAP (mmHg) | 86.9 ± 11.4 | 88.9 ± 15.5 | 1.003 (0.987-1.019) | 0.696 | ||
| Invasive catheterization (%) | 6 (10.3) | 26 (26.3) | 2.026 (1.290-3.181) | 0.002 | 2.173 (1.320-3.579) | 0.002 |
| Grade of ACLF | ||||||
| ACLF-1 (%) | 44 (75.9) | 35 (35.4) | Reference | < 0.001 | ||
| ACLF-2 (%) | 12 (20.7) | 29 (29.3) | 1.977 (1.205-3.243) | 0.007 | ||
| ACLF-3 (%) | 2 (3.4) | 35 (35.4) | 4.648 (2.862-7.548) | < 0.001 | ||
| HBV DNA (log10 IU/ml) | 3.6 (2.3, 5.5) | 4.7 (2.8, 6.6) | 1.083 (0.997-1.177) | 0.060 | ||
| WBC (×109/L) | 7.5 (5.6, 11.3) | 10.4 (6.9, 15.0) | 1.054 (1.022-1.088) | 0.001 | ||
| NEUT (×109/L) | 5.4 (3.4, 8.6) | 8.5 (5.1, 12.7) | 1.058 (1.023-1.094) | 0.001 | ||
| HGB (g/L) | 105.0 ± 21.6 | 109.3 ± 26.3 | 1.003 (0.994-1.011) | 0.516 | ||
| PLT (×109/L) | 64.0 (46.8, 88.5) | 63.0 (40.0, 98.0) | 1.000 (0.995-1.005) | 0.984 | ||
| ALB (g/L) | 28.0 (25.0, 31.0) | 28.0 (23.0, 31.0) | 0.984 (0.945-1.024) | 0.435 | ||
| TBIL (μmol/L) | 353.6 ± 130.9 | 356.4 ± 163.7 | 1.000 (0.999-1.001) | 0.836 | ||
| DBIL (μmol/L) | 257.4 ± 88.3 | 236.8 ± 113.7 | 0.998 (0.996-1.000) | 0.091 | ||
| ALT (IU/L) | 76.5 (35.3, 127.8) | 103.0 (49.0, 352.0) | 1.001 (1.000-1.001) | 0.003 | ||
| AST (IU/L) | 118.0 (71.0, 165.0) | 136.0 (85.0, 367.0) | 1.001 (1.000-1.001) | < 0.001 | ||
| ALP (IU/L) | 158.5 (125.8, 211.3) | 137.0 (99.0, 179.0) | 1.000 (0.998-1.001) | 0.721 | ||
| GGT (IU/L) | 49.0 (35.8, 78.8) | 56.0 (33.0, 78.0) | 0.999 (0.995-1.004) | 0.821 | ||
| Cr (μmol/L) | 98.5 (79.5, 126.3) | 122.0 (87.0, 196.0) | 1.002 (1.001-1.003) | 0.001 | ||
| Na (mmol/L) | 133.0 (130.0, 136.0) | 132.0 (128.0, 136.0) | 0.996 (0.962-1.031) | 0.830 | ||
| INR | 1.9 (1.7, 2.3) | 2.6 (2.1, 3.3) | 1.762 (1.487-2.089) | < 0.001 | ||
| PTA (%) | 33.2 ± 11.1 | 23.3 ± 9.9 | 0.937 (0.917-0.957) | < 0.001 | 0.967 (0.941-0.993) | 0.015 |
| CRP (mg/L) | 20.5 (13.2, 43.4) | 16.2 (8.9, 39.6) | 0.998 (0.991-1.005) | 0.530 | ||
| PCT (ng/mL) | 1.2 (0.8, 1.8) | 1.2 (0.6, 2.9) | 1.017 (1.002-1.033) | 0.025 | ||
| Ascites (%) | 57 (98.3) | 96 (97.0) | 0.578 (0.183-1.828) | 0.351 | ||
| AKI (%) | 24 (41.4) | 61 (61.6) | 2.777 (1.734-4.449) | < 0.001 | 2.187 (1.259-3.799) | 0.005 |
| HE (%) | 23 (39.7) | 76 (76.8) | 1.635 (1.089-2.454) | 0.018 | ||
| AVB (%) | 8 (13.8) | 26 (26.3) | 1.476 (0.942-2.312) | 0.089 | ||
| Pneumonia (%) | 20 (34.5) | 59 (59.6) | 1.831 (1.222-2.744) | 0.003 | ||
| SBP (%) | 34 (58.6) | 33 (33.3) | 0.553 (0.364-0.842) | 0.006 | ||
| BSI (%) | 6 (10.3) | 20 (20.2) | 1.661 (1.016-2.715) | 0.043 | 2.339 (1.384-3.952) | 0.002 |
| Other BIs (%) | 4 (6.9) | 16 (16.2) | 1.488 (0.870-2.544) | 0.146 | ||
| Multiple sites of BIs (%) | 6 (10.3) | 24 (24.2) | 1.797 (1.132-2.852) | 0.013 | ||
| MDROs (%) | 4 (6.9) | 11 (11.1) | 1.401 (0.728-2.696) | 0.313 | ||
| CA BIs (%) | 4 (6.9) | 13 (13.1) | 1.537 (0.857-2.757) | 0.149 | ||
| HCA BIs (%) | 24 (41.4) | 47 (47.5) | 1.046 (0.705-1.553) | 0.821 | ||
| Nosocomial BIs (%) | 30 (51.7) | 39 (39.4) | 0.804 (0.537-1.204) | 0.290 | ||
| COSSH-ACLF Scores | 6.1 (5.8, 6.7) | 7.4 (6.6, 8.7) | 1.704 (1.498, 1.937) | < 0.001 | 1.371 (1.127-1.666) | 0.002 |
This study is unique, because it assessed the clinical characteristics, bacterial identification, and outcomes in patients with HBV-ACLF (as defined by COSSH) combined with first BIs in China. The first main finding of our study is that the majority of BIs belong to HCA and nosocomial BIs. Pneumonia, not SBP, was the most common form of BI for patients with HBV-ACLF. Second, E. coli and K. pneumoniae were the most common cultured bacteria, and the proportion of MDROs was 29.5%. Third, some important clinical indicators, including the COSSH-ACLF scores, can serve as independent predictors of the 28-d outcomes for those patients.
For patients with HBV-ACLF combined with BIs, CA BIs accounted for 13.4%, HCA BIs accounted for 46.4%, and nosocomial BIs accounted for 40.2%. HCA and nosocomial BIs constituted the main types of BIs in those patients, and the results were similar to those of previous studies in Eastern countries[22,23]. However, in Western countries, CA BIs account for 25.0%-52.1%[6,7,24], which is significantly higher than the proportion in Eastern countries (15.7%-18.7%)[22,23] and in our study (13.4%). The large number of HCA and nosocomial BIs observed in ACLF patients may be attributed to the use of invasive procedures and ICU admissions in patients before they are transferred to a tertiary referral hospital in China[22]. Hence, patients with ACLF, who have been treated in a hospital over the past 180 d, should be screened for BIs after admission.
In our study, pneumonia (40.7%), SBP (34.5%), and BSI (13.4%) were the three most common forms of first BIs in patients with HBV-ACLF. Our findings are consistent with some studies in China and India in which pneumonia (45.0%-49.4%) is the most common form of BI[22,23,25], but studies in Western countries show that SBP (25.0%-32.4%) is the most common form of BI in patients with ACLF[6,8,24]. Moreover, pneumonia is the most common form in patients with ACLF-2 and ACLF-3, and SBP is most common form in patients with AD and ACLF-1. As the ACLF grade increased, the incidence of SBP decreased. A correlation was observed between the occurrence of pneumonia and the severity of ACLF. Patients with end-stage liver disease combined with pneumonia had higher mortality than other infections[12], and poor air quality is related to the high prevalence of pneumonia[26], which may be related to this phenomenon in China. Another interesting phenomenon is that the proportion of urinary tract infection (2.1%) in our study was significantly lower than that in previous studies (>10%)[7,25]. The likely reason is that patients in the present study were recruited from non-ICU and in a single hospital, and the rate of urinary catheter placement should be lower than that of patients from ICU.
Similar to previous research, the present study showed that Gram-negative bacteria (83.6%) comprised the majority of cultured bacteria, and K. pneumoniae (44.3%) and E. coli (23.0%) were the most common. However, the proportion of Gram-positive bacteria in cultured bacteria was low (16.4%), and the MDRO rate was 80.0%. Previous studies reported that more than half of the cultured bacteria were Gram positive[6,22]. This finding is inconsistent with our present study possibly because the subjects selected were non-ICU patients and that the bacterial spectrum differs in each region. Moreover, Gram-positive bacteria have a higher rate of MDROs, and the empirical treatment of antibiotics should be adjusted in time according to the results of bacterial susceptibility testing.
Similar to previous studies[24,25], the prevalence of MDRO strains (29.5%) was high in patients with ACLF in the present study. This condition might be related to the large numbers of diagnosed HCA (44.3%) and nosocomial BIs (39.3%). HCA and nosocomial BIs are caused more frequently by antibiotic-resistant bacteria and associated with worse clinical outcome than CA BIs[27]. Selective intestinal decontamination with non-absorbable antibiotics is often used to prevent the occurrence of SBP and HE in patients with ACLF, but it could also promote the development of MDROs[28].
We observed significantly higher mortality rate and shorter probability of survival in patients with ACLF combined with BIs than in patients with ACLF without BIs through comparison with previous studies[7,22,29]. As the ACLF grade increased, the 28-d transplant-free survival rates decreased. Hence, BIs exert a major effect on the prognosis of patients with ACLF. This finding is supported by the finding of previous studies that BIs are an independent predictor of mortality in patients with ACLF. BIs can aggravate the extent of liver failure, and multiple extra-hepatic organ failures can also occur in the presence of organ damage from exaggerated inflammation[30]. In comparison with patients with cirrhosis, monocytes from patients with ACLF featured elevated frequencies of interleukin-10 producing cells, reduced human leucocyte antigen DR isotype expression, and impaired phagocytic and oxidative burst capacity[31]. Therefore, the 28-d transplant-free survival rate of patients with AD combined with BIs was significantly higher than that of patients with ACLF combined with BIs.
In this study, we identified five independent factors that can predict the 28-d outcomes of patients with HBV-ACLF combined with BIs. Regardless of the presence of BIs in patients with ACLF, PTA and AKI are important risk factors for prognosis[32,33]. The COSSH-ACLF scores for severity and short-term mortality of patients with HBV-ACLF were superior or comparable with other scores obtained in previous studies[5,34]. In our study, the COSSH-ACLF score has also been shown to be a very important prognostic factor for patients with HBV-ACLF combined with BIs. Notably, BSI and invasive catheterization were independent predictors of the 28-d outcomes of patients with HBV-ACLF combined with BIs. MDROs accounted for 31% of BSI in cirrhotic patients (30.8% in our study), and its occurrence was found to be related to previous antimicrobial exposure and invasive procedures. This condition often results in delayed or inadequate empirical antimicrobial therapy and increased mortality rates[35]. Continuous/extended infusion beta-lactams or carbapenems, as adequate empiric treatment in cirrhotic patients with BSI, may deal with MDROs and improve the outcomes compared with intermittent bolus infusion[36]. We believe that the unreasonable and prolonged placement of invasive catheters may increase the incidence of HCA and nosocomial BIs and MDROs, which may affect the outcomes of patients with ACLF[35,37]. Hence, disinfection and care of invasive catheters should be beneficial to these patients.
Our study has some limitations. First, the history of the use of steroids, antibiotics, and proton pump inhibitors and the phenomenon of HBV reactivation of patients before BIs were unclear in our electronic database. Such information may be related to the clinical manifestations and prognosis of patients. Second, considering the first BIs of the patients, fungal infections and second BIs were not evaluated in the present cohort, which may have a certain impact on the true prognosis. Third, BIs can be as acute insult in triggering ACLF. But this was a retrospective study and many patients were transferred from other lower-level hospitals, so the direct contribution of BIs was unclear. Fourth, because some patients were transferred to other hospitals during treatment, the specific cause of death was not completely clear. Fifth, this single-center retrospective study had a small sample size. Hence, additional prospective randomized studies should be conducted in the future.
In conclusion, HCA and nosocomial BIs are the most common types for patients with HBV-ACLF combined with BIs. Pneumonia is the most common form of BI in patients with ACLF-2 and ACLF-3, and SBP is the most common form of BI in patients with AD and ACLF-1. Gram-negative bacteria account for the majority of cultured bacteria, and MDROs are common. The 28-d transplant-free survival rate of patients is very low and decreases with increasing ACLF grade. The independent predictors of the 28-d outcomes of these patients are COSSH-ACLF scores, AKI, BSI, PTA, and invasive catheterization.
Hepatitis B virus (HBV) related acute-on-chronic liver failure (ACLF) is a complicated syndrome with a high short-term mortality rate that develops in patients with HBV related chronic liver disease (CLD) regardless of the presence of cirrhosis and is characterized by acute deterioration of liver function and hepatic and/or extrahepatic organ failure. Bacterial infections (BIs) trigger ACLF and play pivotal roles in the deterioration of clinical course.
The Chinese Group on the Study of Severe Hepatitis B (COSSH) has recently developed a new criterion for HBV-ACLF. However, the clinical characteristics, survival rates, and prognostic effects of BIs in the COSSH definition for patients with HBV-ACLF are unclear. Patients with COSSH-HBV-ACLF combined with first BIs were selected for this study.
This study aimed to investigate the clinical characteristics, the site of BIs, bacterial detection, 28-d outcomes, and independent predictors of outcomes of first BIs either at admission or during hospitalization in patients with HBV-ACLF as defined by the COSSH.
A total of 159 patients with HBV-ACLF and 40 patients with acute decompensation of HBV-CLD combined with first BIs were selected for a retrospective analysis between October 2014 and March 2016. The characteristics of BIs, the site of BIs, and bacterial detection were evaluated. Cumulative survival probability curves of the 28-d transplant-free survival rates were calculated by Kaplan–Meier method and compared by log-rank test. COX proportional hazard regression analysis was used to screen the independent predictors of 28-d outcomes.
A total of 194 episodes of BIs occurred in 159 patients with HBV-ACLF. Among the episodes, 13.4% were community-acquired, 46.4% were healthcare-associated, and 40.2% belonged to nosocomial BIs. Pneumonia (40.7%), spontaneous bacterial peritonitis (SBP) (34.5%), and bloodstream infection (BSI) (13.4%) were the most prevalent. As the ACLF grade increased, the incidence of SBP showed a downward trend (P = 0.021). Sixty-one strains of bacteria, including 83.6% of Gram-negative bacteria and 29.5% of multidrug-resistant organisms (MDROs), were cultivated from 50 patients with ACLF. E. coli (44.3%) and K. pneumoniae (23.0%) were the most common bacteria. As the ACLF grade increased, the 28-d transplant-free survival rates showed a downward trend (ACLF-1, 55.7%; ACLF-2, 29.3%; ACLF-3, 5.4%; P < 0.001). The independent predictors of the 28-d outcomes of patients with HBV-ACLF were COSSH-ACLF score (hazard ratio [HR] = 1.371), acute kidney injury (AKI) (HR = 2.187), BSI (HR = 2.339), prothrombin activity (PTA) (HR = 0.967), and invasive catheterization (HR = 2.173).
For patients with COSSH-HBV-ACLF combined with first BIs, pneumonia is the most common, and the incidence of SBP decreases with increasing ACLF grade. Gram-negative bacteria account for the majority of cultured bacteria, and MDROs are common. The 28-d transplant-free survival rate of patients is very low and decreases with increasing ACLF grade. The independent predictors of the 28-d outcomes are COSSH-ACLF score, AKI, BSI, PTA, and invasive catheterization.
The clinical characteristics, the site of BIs, bacterial detection, 28-d outcomes and independent predictors of outcomes of first BIs in patients with COSSH-HBV-ACLF were described in detail in this study. However, this single-center retrospective study had a small sample size. Hence, additional multi-center prospective randomized study studies should be conducted to reveal the role of BIs in the deterioration of clinical course in patients with COSSH-HBV-ACLF in the future.
The authors would like to thank Dr. Xiang Xu, Dr. Yu-Hui Peng, Dr. Li-Long Yan, and Dr. Hui Li from Liver Failure Treatment and Research Center, The Fifth Medical Center of Chinese PLA General Hospital for their help in collecting the data of patients and Dr. Jing-Feng Bi from Department of Epidemiology and Public Health, The Fifth Medical Center of Chinese PLA General Hospital for his help with statistical analysis.
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bouare N, Shimizu Y, Tajiri K S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Liu MY
| 1. | Hernaez R, Solà E, Moreau R, Ginès P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 441] [Article Influence: 55.1] [Reference Citation Analysis (1)] |
| 2. | Blasco-Algora S, Masegosa-Ataz J, Gutiérrez-García ML, Alonso-López S, Fernández-Rodríguez CM. Acute-on-chronic liver failure: Pathogenesis, prognostic factors and management. World J Gastroenterol. 2015;21:12125-12140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (2)] |
| 3. | Bajaj JS, O'Leary JG, Reddy KR, Wong F, Biggins SW, Patton H, Fallon MB, Garcia-Tsao G, Maliakkal B, Malik R, Subramanian RM, Thacker LR, Kamath PS; North American Consortium For The Study Of End-Stage Liver Disease (NACSELD). Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 436] [Article Influence: 39.6] [Reference Citation Analysis (1)] |
| 4. | Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H, Kim DJ, Dhiman RK, Duseja A, Taneja S, Eapen CE, Goel A, Ning Q, Chen T, Ma K, Duan Z, Yu C, Treeprasertsuk S, Hamid SS, Butt AS, Jafri W, Shukla A, Saraswat V, Tan SS, Sood A, Midha V, Goyal O, Ghazinyan H, Arora A, Hu J, Sahu M, Rao PN, Lee GH, Lim SG, Lesmana LA, Lesmana CR, Shah S, Prasad VGM, Payawal DA, Abbas Z, Dokmeci AK, Sollano JD, Carpio G, Shresta A, Lau GK, Fazal Karim M, Shiha G, Gani R, Kalista KF, Yuen MF, Alam S, Khanna R, Sood V, Lal BB, Pamecha V, Jindal A, Rajan V, Arora V, Yokosuka O, Niriella MA, Li H, Qi X, Tanaka A, Mochida S, Chaudhuri DR, Gane E, Win KM, Chen WT, Rela M, Kapoor D, Rastogi A, Kale P, Rastogi A, Sharma CB, Bajpai M, Singh V, Premkumar M, Maharashi S, Olithselvan A, Philips CA, Srivastava A, Yachha SK, Wani ZA, Thapa BR, Saraya A, Shalimar, Kumar A, Wadhawan M, Gupta S, Madan K, Sakhuja P, Vij V, Sharma BC, Garg H, Garg V, Kalal C, Anand L, Vyas T, Mathur RP, Kumar G, Jain P, Pasupuleti SSR, Chawla YK, Chowdhury A, Alam S, Song DS, Yang JM, Yoon EL, APASL ACLF Research Consortium (AARC) for APASL ACLF working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 614] [Cited by in RCA: 590] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 5. | Wu T, Li J, Shao L, Xin J, Jiang L, Zhou Q, Shi D, Jiang J, Sun S, Jin L, Ye P, Yang L, Lu Y, Li T, Huang J, Xu X, Chen J, Hao S, Chen Y, Xin S, Gao Z, Duan Z, Han T, Wang Y, Gan J, Feng T, Pan C, Chen Y, Li H, Huang Y, Xie Q, Lin S, Li L, Li J; Chinese Group on the Study of Severe Hepatitis B (COSSH). Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67:2181-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 298] [Article Influence: 42.6] [Reference Citation Analysis (2)] |
| 6. | Mücke MM, Rumyantseva T, Mücke VT, Schwarzkopf K, Joshi S, Kempf VAJ, Welsch C, Zeuzem S, Lange CM. Bacterial infection-triggered acute-on-chronic liver failure is associated with increased mortality. Liver Int. 2018;38:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | Fernández J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C, Reverter E, Martínez J, Saliba F, Jalan R, Welzel T, Pavesi M, Hernández-Tejero M, Ginès P, Arroyo V; European Foundation for the Study of Chronic Liver Failure. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2018;67:1870-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 398] [Article Influence: 56.9] [Reference Citation Analysis (2)] |
| 8. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1-1437.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2171] [Article Influence: 180.9] [Reference Citation Analysis (5)] |
| 9. | Riordan SM, Williams R. Treatment of hepatic encephalopathy. N Engl J Med. 1997;337:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 257] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | de Franchis R, Baveno V Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1031] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 11. | Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, Jalan R, Sarin SK, Piano S, Moore K, Lee SS, Durand F, Salerno F, Caraceni P, Kim WR, Arroyo V, Garcia-Tsao G; International Club of Ascites. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 387] [Article Influence: 38.7] [Reference Citation Analysis (1)] |
| 12. | European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1132] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 13. | Mandell LA, Wunderink RG, Waterer GW. Community-acquired pneumonia. N Engl J Med. 2015;372:293-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4031] [Cited by in RCA: 4732] [Article Influence: 278.4] [Reference Citation Analysis (0)] |
| 15. | Fernández J, Acevedo J, Prado V, Mercado M, Castro M, Pavesi M, Arteaga M, Sastre L, Juanola A, Ginès P, Arroyo V. Clinical course and short-term mortality of cirrhotic patients with infections other than spontaneous bacterial peritonitis. Liver Int. 2017;37:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Nahon P, Lescat M, Layese R, Bourcier V, Talmat N, Allam S, Marcellin P, Guyader D, Pol S, Larrey D, De Lédinghen V, Ouzan D, Zoulim F, Roulot D, Tran A, Bronowicki JP, Zarski JP, Goria O, Calès P, Péron JM, Alric L, Bourlière M, Mathurin P, Blanc JF, Abergel A, Serfaty L, Mallat A, Grangé JD, Attali P, Bacq Y, Wartelle C, Dao T, Benhamou Y, Pilette C, Silvain C, Christidis C, Capron D, Bernard-Chabert B, Hillaire S, Di Martino V, Trinchet JC, Moreau R, Roudot-Thoraval F; ANRS CO12 CirVir and Microcir Groups. Bacterial infection in compensated viral cirrhosis impairs 5-year survival (ANRS CO12 CirVir prospective cohort). Gut. 2017;66:330-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Swartz MN. Clinical practice. Cellulitis. N Engl J Med. 2004;350:904-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 241] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 18. | Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6211] [Cited by in RCA: 6524] [Article Influence: 197.7] [Reference Citation Analysis (0)] |
| 19. | Bajaj JS, O'Leary JG, Wong F, Reddy KR, Kamath PS. Bacterial infections in end-stage liver disease: current challenges and future directions. Gut. 2012;61:1219-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Merli M, Lucidi C, Giannelli V, Giusto M, Riggio O, Falcone M, Ridola L, Attili AF, Venditti M. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 231] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 21. | Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6072] [Cited by in RCA: 8772] [Article Influence: 626.6] [Reference Citation Analysis (0)] |
| 22. | Cai J, Zhang M, Han T, Jiang HQ. Characteristics of infection and its impact on short-term outcome in patients with acute-on-chronic liver failure. Medicine (Baltimore). 2017;96:e8057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Cao Z, Liu Y, Wang S, Lu X, Yin S, Jiang S, Chen L, Cai M, Zeng B, Yao Y, Tang W, Zhao G, Xiang X, Wang H, Cai W, Zhu C, Li H, Xie Q. The impact of HBV flare on the outcome of HBV-related decompensated cirrhosis patients with bacterial infection. Liver Int. 2019;39:1943-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Fernández J, Prado V, Trebicka J, Amoros A, Gustot T, Wiest R, Deulofeu C, Garcia E, Acevedo J, Fuhrmann V, Durand F, Sánchez C, Papp M, Caraceni P, Vargas V, Bañares R, Piano S, Janicko M, Albillos A, Alessandria C, Soriano G, Welzel TM, Laleman W, Gerbes A, De Gottardi A, Merli M, Coenraad M, Saliba F, Pavesi M, Jalan R, Ginès P, Angeli P, Arroyo V; European Foundation for the Study of Chronic Liver Failure (EF-Clif). Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J Hepatol. 2019;70:398-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 248] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 25. | Shalimar, Rout G, Jadaun SS, Ranjan G, Kedia S, Gunjan D, Nayak B, Acharya SK, Kumar A, Kapil A. Prevalence, predictors and impact of bacterial infection in acute on chronic liver failure patients. Dig Liver Dis. 2018;50:1225-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Song C, He J, Wu L, Jin T, Chen X, Li R, Ren P, Zhang L, Mao H. Health burden attributable to ambient PM2.5 in China. Environ Pollut. 2017;223:575-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 273] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 27. | Sargenti K, Prytz H, Strand A, Nilsson E, Kalaitzakis E. Healthcare-associated and nosocomial bacterial infections in cirrhosis: predictors and impact on outcome. Liver Int. 2015;35:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Daneman N, Sarwar S, Fowler RA, Cuthbertson BH; SuDDICU Canadian Study Group. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:328-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 29. | Wang C, Ma DQ, Luo S, Wang CM, Ding DP, Tian YY, Ao KJ, Zhang YH, Chen Y, Meng ZJ. Incidence of infectious complications is associated with a high mortality in patients with hepatitis B virus-related acute-on-chronic liver failure. World J Clin Cases. 2019;7:2204-2216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Wu W, Yan H, Zhao H, Sun W, Yang Q, Sheng J, Shi Y. Characteristics of systemic inflammation in hepatitis B-precipitated ACLF: Differentiate it from No-ACLF. Liver Int. 2018;38:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 31. | Korf H, du Plessis J, van Pelt J, De Groote S, Cassiman D, Verbeke L, Ghesquière B, Fendt SM, Bird MJ, Talebi A, Van Haele M, Feio-Azevedo R, Meelberghs L, Roskams T, Mookerjee RP, Mehta G, Jalan R, Gustot T, Laleman W, Nevens F, van der Merwe SW. Inhibition of glutamine synthetase in monocytes from patients with acute-on-chronic liver failure resuscitates their antibacterial and inflammatory capacity. Gut. 2019;68:1872-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 32. | Sun QF, Ding JG, Xu DZ, Chen YP, Hong L, Ye ZY, Zheng MH, Fu RQ, Wu JG, Du QW, Chen W, Wang XF, Sheng JF. Prediction of the prognosis of patients with acute-on-chronic hepatitis B liver failure using the model for end-stage liver disease scoring system and a novel logistic regression model. J Viral Hepat. 2009;16:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Shi X, Zhu P, Yan G, Liu C, Zhang C, Huang G, Zhang Y, Yan Z, Wang Y. Clinical characteristics and long-term outcome of acute kidney injury in patients with HBV-related acute-on-chronic liver failure. J Viral Hepat. 2016;23:920-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Gao F, Zhang Q, Liu Y, Gong G, Mao D, Gong Z, Li J, Luo X, Li X, Chen G, Li Y, Zhao W, Wan G, Li H, Sun K, Wang X. Nomogram prediction of individual prognosis of patients with acute-on-chronic hepatitis B liver failure. Dig Liver Dis. 2019;51:425-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Bartoletti M, Giannella M, Lewis R, Caraceni P, Tedeschi S, Paul M, Schramm C, Bruns T, Merli M, Cobos-Trigueros N, Seminari E, Retamar P, Muñoz P, Tumbarello M, Burra P, Torrani Cerenzia M, Barsic B, Calbo E, Maraolo AE, Petrosillo N, Galan-Ladero MA, D'Offizi G, Bar Sinai N, Rodríguez-Baño J, Verucchi G, Bernardi M, Viale P; ESGBIS/BICHROME Study Group. A prospective multicentre study of the epidemiology and outcomes of bloodstream infection in cirrhotic patients. Clin Microbiol Infect. 2018;24:546.e1-546.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 36. | Bartoletti M, Giannella M, Lewis RE, Caraceni P, Tedeschi S, Paul M, Schramm C, Bruns T, Merli M, Cobos-Trigueros N, Seminari E, Retamar P, Muñoz P, Tumbarello M, Burra P, Torrani Cerenzia M, Barsic B, Calbo E, Maraolo AE, Petrosillo N, Galan-Ladero MA, D'Offizi G, Zak-Doron Y, Rodriguez-Baño J, Baldassarre M, Verucchi G, Domenicali M, Bernardi M, Viale P; ESGBIS/BICHROME study group. Extended Infusion of β-Lactams for Bloodstream Infection in Patients With Liver Cirrhosis: An Observational Multicenter Study. Clin Infect Dis. 2019;69:1731-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 37. | Lopes-Secundo TM, Sevá-Pereira T, Correa BR, Silva NCM, Imbrizi MR, Cunha-Silva M, Soares EC, Almeida JRS. Serum sodium, model for end-stage liver disease, and a recent invasive procedure are risk factors for severe acute-on-chronic liver failure and death in cirrhotic patients hospitalized with bacterial infection. Eur J Gastroenterol Hepatol. 2018;30:1055-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |