Published online Mar 6, 2020. doi: 10.12998/wjcc.v8.i5.912
Peer-review started: November 25, 2019
First decision: January 7, 2020
Revised: January 8, 2020
Accepted: February 15, 2020
Article in press: February 15, 2020
Published online: March 6, 2020
Processing time: 101 Days and 20.4 Hours
Allograft artery mycotic aneurysm (MA) represents a rare but life-threatening complication of kidney transplantation. Graftectomy is widely considered the safest option. Due to the rarity of the disease and the substantial risk of fatal consequences, experience with conservative strategies is limited. To date, only a few reports on surgical repair have been published. We describe a case of true MA successfully managed by aneurysm resection and arterial re-anastomosis.
An 18-year-old gentleman, on post-operative day 70 after deceased donor kidney transplantation, presented with malaise, low urinary output, and worsening renal function. Screening organ preservation fluid cultures, collected at the time of surgery, were positive for Candida albicans. Doppler ultrasound and contrast-enhanced computer tomography showed a 4-cm-sized, saccular aneurysm of the iuxta-anastomotic segment of the allograft artery, suspicious for MA. The lesion was wide-necked and extended to the distal bifurcation of the main arterial branch, thus preventing endovascular stenting and embolization. After multidisciplinary discussion, the patient underwent surgical exploration, aneurysm excision, and re-anastomosis between the stump of the allograft artery and the internal iliac artery. The procedure was uneventful. Histology and microbiology evaluation of the surgical specimen confirmed the diagnosis of MA caused by Candida infection. Three years after the operation, the patient is doing very well with excellent allograft function and no signs of recurrent disease.
Surgical repair represents a feasible option in carefully selected patients with allograft artery MA. Anti-fungal prophylaxis is advised when preservation fluid cultures are positive.
Core tip: Fungal infections transmitted by contaminated organs represent an important source of complications after kidney transplantation. Among the others, allograft artery mycotic aneurysm (MA) deserves a special consideration as it may cause transplant loss and death. Elective management of non-complicated MA is controversial. For many years, graftectomy has been considered the only reasonable option. However, recent reports have shown that in carefully selected patients, surgical repair can offer excellent results. We herein describe a rare case of true MA of the transplant artery caused by Candida albicans and successfully treated by aneurysm resection and re-anastomosis.
- Citation: Bindi M, Ferraresso M, De Simeis ML, Raison N, Clementoni L, Delbue S, Perego M, Favi E. Allograft artery mycotic aneurysm after kidney transplantation: A case report and review of literature. World J Clin Cases 2020; 8(5): 912-921
- URL: https://www.wjgnet.com/2307-8960/full/v8/i5/912.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i5.912
Kidney transplantation (KTx) is currently considered the treatment of choice for patients with end-stage renal disease[1]. Progressive improvements in surgical technique, intensive care, and immunosuppression have undoubtedly led to a significant reduction of transplant-related morbidity and mortality[2]. However, particularly in the early post-operative phase, the incidence of non-immunological complications remains high[3]. Among others, mycotic aneurysm (MA) involving the allograft artery deserves special consideration as it represents a dreadful condition potentially causing transplant loss and death[4]. Graftectomy is widely considered the safest therapeutic option. Due to the rarity of the disease and the substantial risk of fatal consequences, overall experience with conservative strategies such as active surveillance or endovascular treatment is limited[5-8]. To date, only a few reports on surgical repair have been published[7,9-13]. We herein describe the long-term outcome of a case of true renal allograft artery MA successfully managed by aneurysm resection and arterial re-anastomosis.
An 18-year-old Caucasian gentleman, on post-operative day 70 after KTx, presented to our rapid assessment unit with malaise and low urinary output.
The patient was first admitted to our institution for his primary deceased donor KTx in April 2016. The donor was a 19-year-old woman with recreational cocaine abuse who died from post-traumatic intracranial hemorrhage after 7 d in the intensive care unit. At the time of organ retrieval, there were no signs of infection and routine microbiological tests were negative. Macroscopically, the kidney looked normal. It was medium-sized, had one artery with an early bifurcation on a Carrel patch, one vein, and one ureter. Cold ischemia time on static cold storage was 24 h. Donor and recipient were blood group compatible (O Rh negative) and had a 4 human leukocyte antigen mismatch. Pre-transplant panel-reactive antibody test and T-cell cross-match were negative. No donor-specific antibodies could be detected. Surgical prophylaxis consisted of intravenous (IV) cefazolin (Cefazolin, Teva Pharmaceutical Industries Ltd, Petach Tikva, Israel) 2 g one shot. The allograft was implanted in the retro-peritoneum via a para-rectal approach. The renal artery was anastomosed side-to-end to the external iliac artery while the renal vein was anastomosed end-to-side to the external iliac vein using 6/0 polypropylene (Prolene, Ethicon Inc, Bridgewater, New Jersey) running sutures. Warm ischemia time was 35 min. The ureter was anastomosed to the bladder according to the Lich-Gregoir technique using 6/0 polydioxanone (PDS, Ethicon Inc, Bridgewater, New Jersey) running sutures. After declamping, the kidney perfused homogeneously with immediate urinary output. The procedure took 135 min with minimal blood loss and no intra-operative complications. Immunosuppression consisted of basiliximab (Simulect, Novartis Pharmaceuticals Corporation, Basel, Switzerland), tacrolimus (Prograf, Astellas Pharma, Deerfield, Illinois), mycophenolate mofetil (Myfenax, Teva Pharmaceutical Industries Ltd, Petach Tikva, Israel), and steroids. Early post-operative course was uneventful. Screening preservation fluid cultures (available 72 h after surgery) were positive for Candida albicans. Since there was no clinical (fever, malaise, abdominal pain, wound discharge, tachycardia, hypotension, tachypnoea, oxygen desaturation), laboratory (white blood cell count, C-reactive protein, procalcitonin, galactomannan, urinalysis), microbiological (blood culture, urine culture) or radiological (chest X-ray, abdominal ultrasound) findings suggestive of localized or systemic infection, we opted for active surveillance rather than anti-fungal prophylaxis. The recipient was discharged with satisfactory allograft function (serum creatinine concentration, 2.3 mg/dL), negative microbiology screening, normal inflammatory markers, and normal color Doppler ultrasound scan 10 d after the operation. As per standard practice, he was given prophylaxis for Pneumocystis jirovecii with oral trimethoprim/ sulphamethoxazole (Bactrim, F. Hoffmann - La Roche Ltd, Basel, Switzerland) 80/400 mg every other day for 6 mo. Clinical examination, laboratory tests, microbiological cultures, and imaging of the kidney were repeated as an outpatient three times a week during the first month and weekly thereafter. During early post-transplant follow-up, serum creatinine concentration decreased to 1.3 mg/dL.
Past medical history included end-stage renal disease due to bilateral cystic dysplasia, hypertension, secondary hyperparathyroidism, and cavernous angioma of the spinal cord.
Personal and family history were unremarkable.
Body temperature, blood pressure, heart rate, respiratory rate, oxygen saturation, chest examination, and abdominal examination were normal.
Laboratory tests showed elevated neutrophil count (8.1 cell × 109/L) and impaired renal function (serum creatinine concentration, 2.4 mg/dL). Liver function tests, C-reactive protein, procalcitonin, galactomannan, urinalysis, and microbiology screening (cytomegalovirus and Epstein-Barr virus polymerized chain reaction, urine and blood culture) were normal.
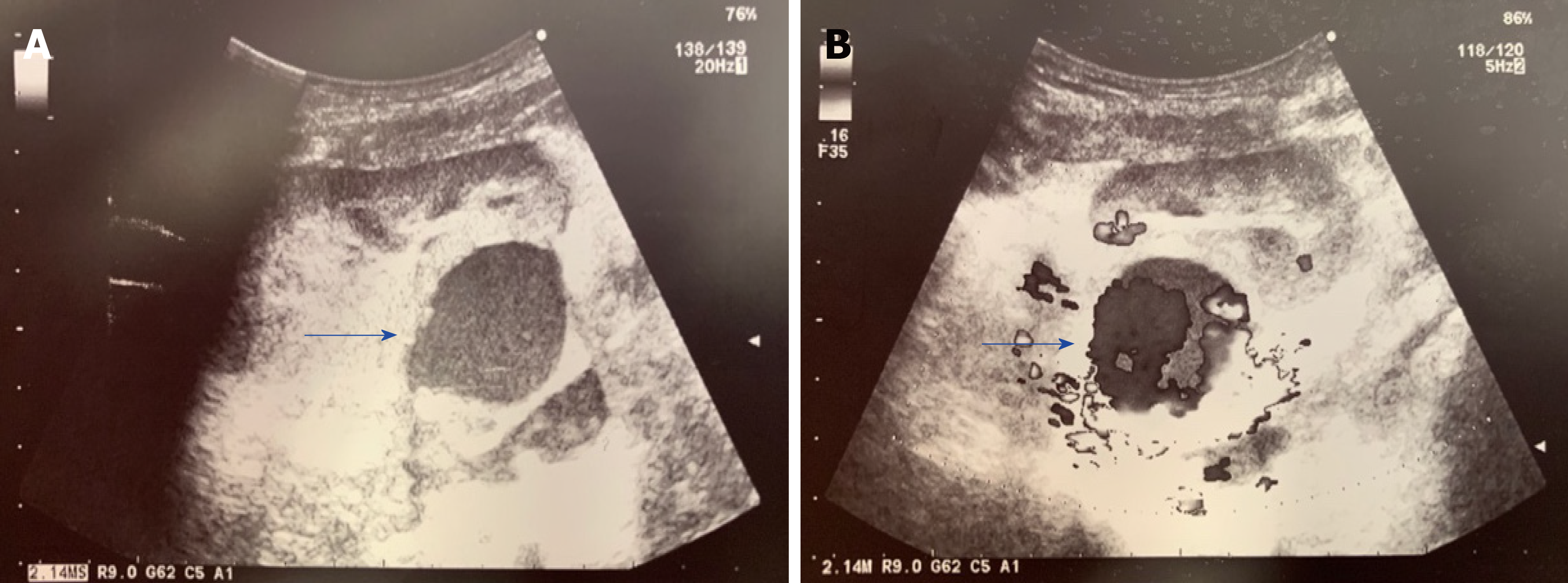
Urgent color Doppler ultrasound scan revealed a rounded, 3.8 cm-sized, hypoechoic mass with turbulent intra-lesional flow (yin-yang sign) along the renal allograft artery, suspicious for pseudoaneurysm (Figure 1).
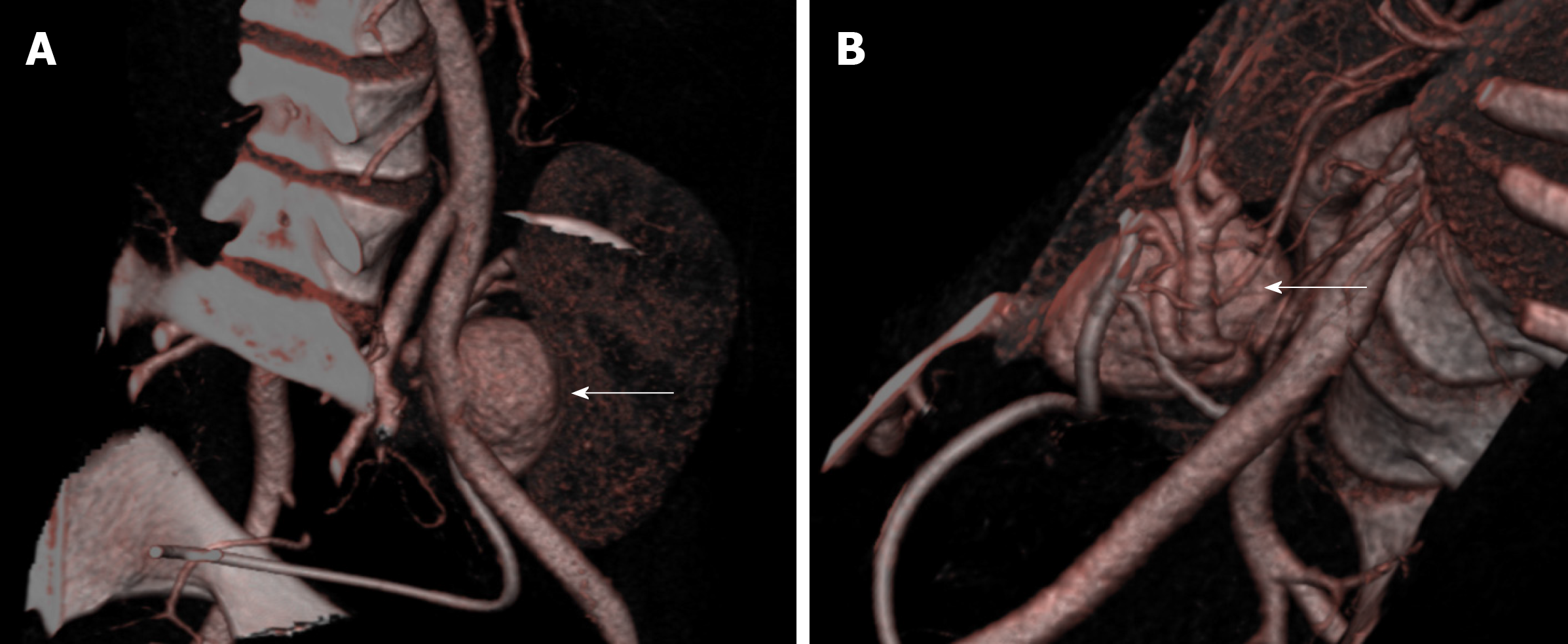
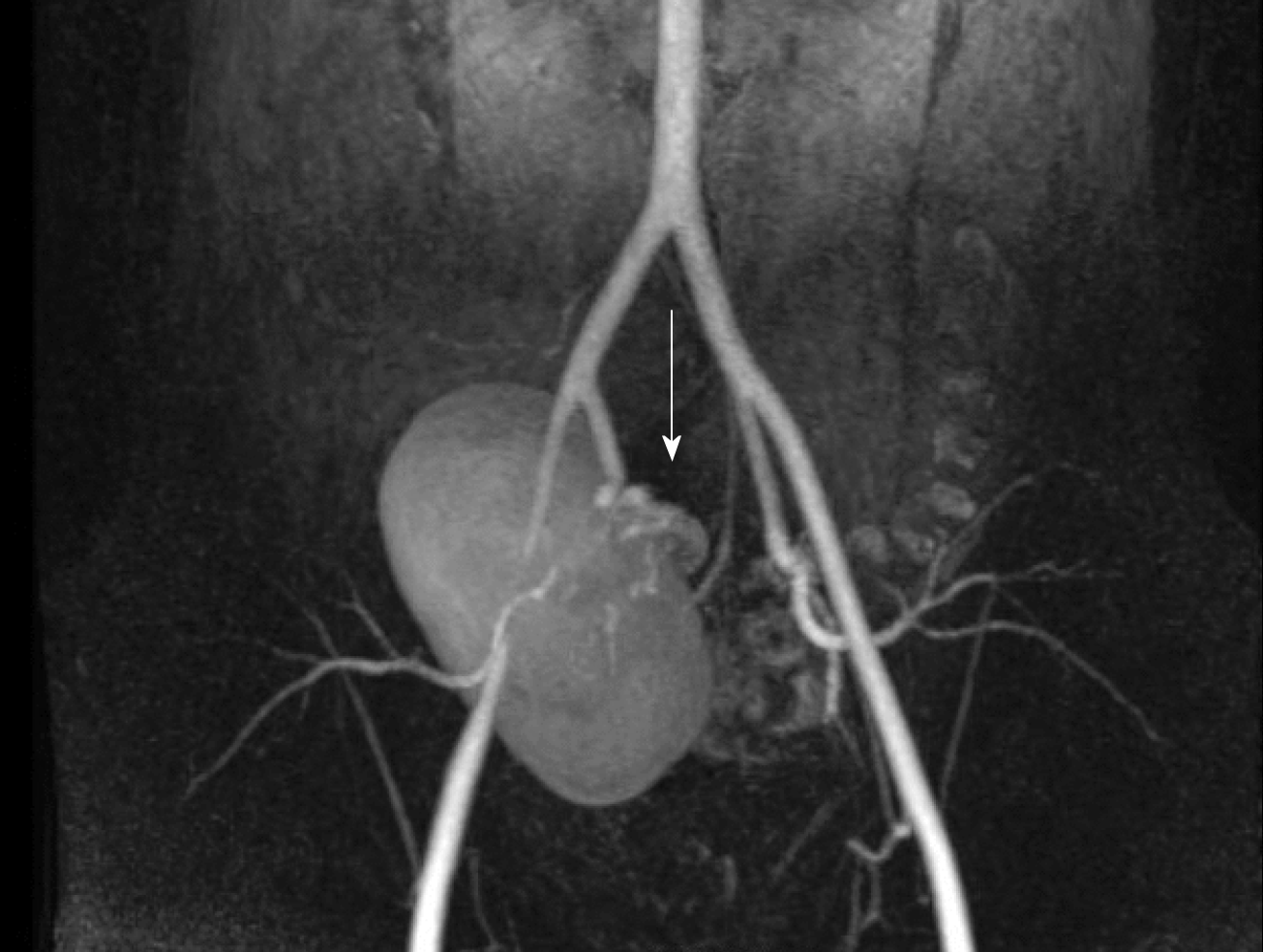
Subsequent contrast-enhanced computed tomography (CT) scan showed a saccular dilation of the iuxta-anastomotic segment of the renal artery measuring 3.2 cm × 3.5 cm × 4.0 cm and causing parenchymal compression (Figure 2). The risk of rupture was deemed significant and the case was discussed in a multidisciplinary meeting with nephrologists, infectious disease specialists, interventional radiologists, vascular surgeons, and transplant surgeons. Considering the quality of the kidney, the age of the recipient, and the low chance of receiving another transplant due to difficult blood group and sensitization, a plan for allograft salvage was made. The lesion was saccular, wide-necked, and located at the artery bifurcation (Figure 2) thus preventing endovascular stenting or embolization. Therefore, we opted for open aneury-smectomy and in-vivo repair.
Pathology report described a true aneurysm of the renal allograft artery with signs of chronic inflammation and no evidence of infection whereas microbiological cultures of the surgical specimen were positive for C. albicans.
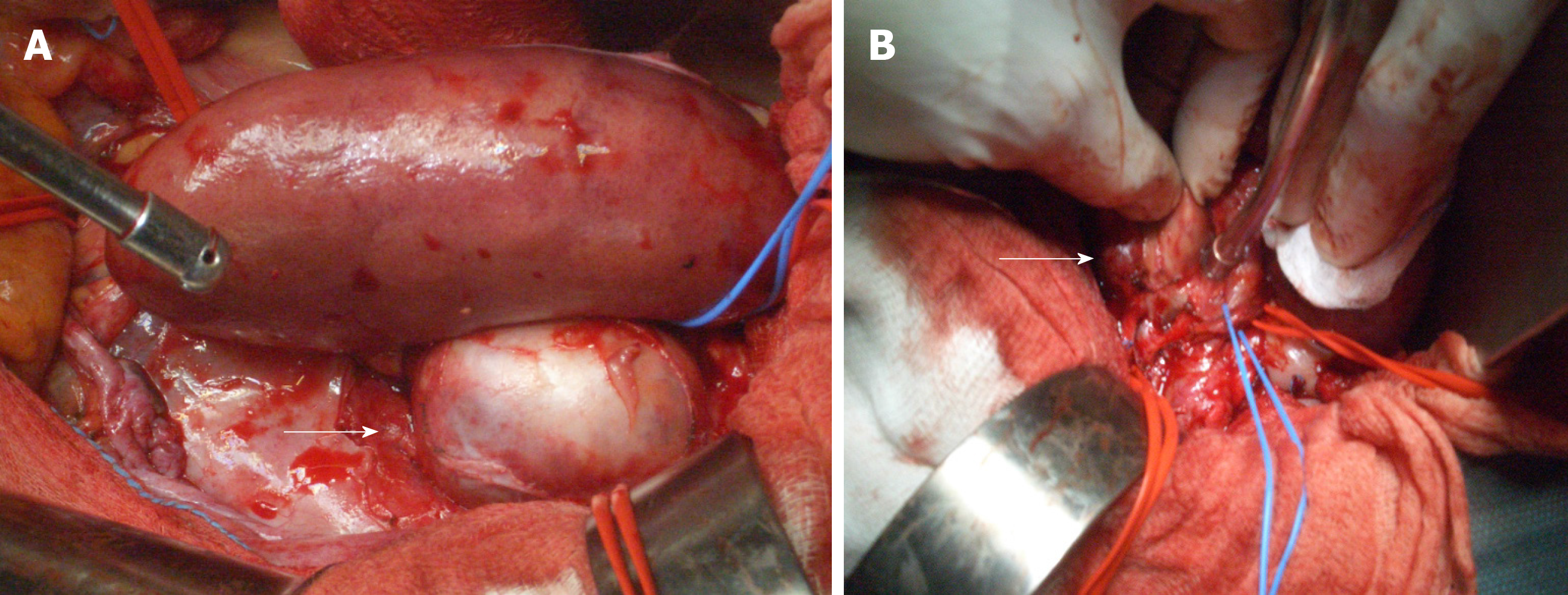
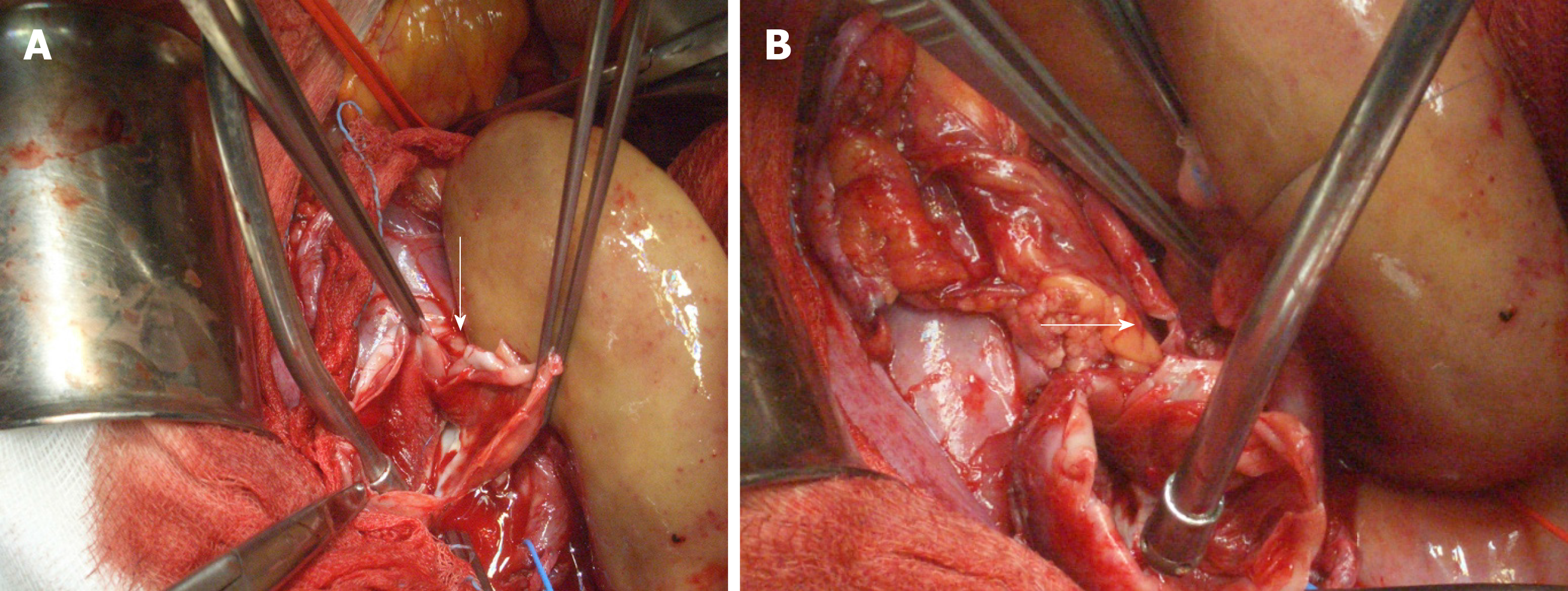
The patient was placed in supine position. As antibiotic prophylaxis, we used a single dose of IV cefazolin 2 g. A midline incision was performed, and the peritoneal cavity was entered. The kidney looked normal and well perfused. A 4-cm in maximal diameter saccular aneurysm was identified along the main branch of the renal artery (Figure 3). Both the external iliac vein and artery were extensively dissected and clamped. In order to reduce the risk of ischemia-reperfusion injury, a bolus of IV N-acetyl cysteine (Acetadote, Cumberland Pharmaceuticals Inc., Nashville, Tennessee) 600 mg was given. The allograft artery was resected 1.5 cm above the neck of the lesion whereas a small longitudinal incision was made in the external iliac vein. The transplant was flushed with 1 L of cold organ preservation fluid (Servator C, SALF SpA, Bergamo, Italy) and covered with sterile crushed ice. The aneurysm was completely excised (Figure 4), the anastomotic stump of the artery was removed, and the specimens were sent for histology and microbiological evaluation. Cold ischemia time was 20 min. Given the short length and the small size of the allograft artery, we decided to perform an end-to-end anastomosis with the internal iliac artery using 7/0 polypropylene running sutures (Figure 4). The external iliac vessels were repaired with 6/0 polypropylene sutures and unclamped. Warm ischemia time was 25 min. The allograft re-perfused slowly but effectively. The surgical field was copiously washed with warm normal saline and the abdomen closed. The length of the operation was 290 min with an estimated blood loss of 500 mL, no peri-operative complications, and maintained urinary output.
After the procedure, the recipient spent 24 h in intensive care unit for close monitoring and then he was stepped down to the ward. Apart from a transient impairment (peak serum creatinine concentration, 3.3 mg/dL), allograft function remained stable and post-operative course was normal. In agreement with nephrologists and infectious disease specialists, mycophenolate mofetil was stopped and antifungal therapy started. More in details, the patient was given IV caspofungin (Cancidas, Merck Sharp and Dohme Inc, Readington, New Jersey) 50 mg a day for 2 wk and IV anidulafungin (Ecalta, Pfizer Inc, New York City, New York) 100 mg a day for 8 wk. Length of hospital stay was 21 d. After discharge, the recipient was aggressively monitored in our outpatient clinic. Physical examination, laboratory tests, microbiological cultures, and color Doppler ultrasound scan were repeated three times a week during the first month, two times a week during the second month, and weekly during the third month. As a part of the follow-up, the patient also underwent abdominal contrast-enhanced CT scan (post-operative day 180) and magnetic resonance imaging (post-operative day 360). Three years later, he is doing very well, with excellent renal function (serum creatinine concentration, 1.1 mg/dL), and no recurrent disease (Figure 5).
MA is a term first used by Osler in 1855 to describe a case of arterial wall degeneration and dilation caused by a septic embolus in a patient with bacterial endocarditis[14]. In current practice, it more precisely refers to aneurysms associated to fungal infections. Reported incidence after KTx is about 1%[8]. This complication usually occurs within 90 d of surgery. Nevertheless, time interval between engrafting and diagnosis can be extremely variable (Table 1). Several classifications have been proposed: by type (true vs false), morphology (saccular vs fusiform), neck diameter (wide vs narrow), and location (anastomotic vs extra-anastomotic). MA may be due to infections transmitted by the donor, recipient-derived infections or more frequently allografts contaminated during organ retrieval, storage or handling[15]. Candida and Aspergillus species are the most important pathogens responsible for the disease but many other microorganisms have been identified[4,15-17].
| Ref. | Cases, n | Post-Tx, d | FU max, d | Graftectomy, n | IR, n | Repair, n | Death, n | Tx loss, n |
| Potti et al[24], 1998 | 1 | 42 | 45 | 1 | 0 | 0 | 0 | 1 |
| Laouad et al[16], 2005 | 4 | 9-90 | NA | 3 | 0 | 0 | 1 | 3 |
| Fujikata et al[5], 2006 | 1 | 55 | 2040 | 0 | 0 | 0 | 0 | 0 |
| Henderson et al[25], 2007 | 1 | 120 | 360 | 1 | 0 | 0 | 0 | 1 |
| Taksin et al[26], 2009 | 1 | 21 | NA | 1 | 0 | 0 | 0 | 1 |
| Albano et al[15], 2009 | 13 | 3-154 | 2920 | 8 | 0 | 2 | 3 | 8 |
| Osmán et al[6], 2009 | 1 | 36 | NA | 0 | 1 | 0 | 0 | 1 |
| Wang et al[27], 2009 | 4 | 10-60 | 240 | 4 | 0 | 0 | 0 | 4 |
| Minz et al[17], 2010 | 2 | 30-120 | 1440 | 2 | 0 | 0 | 1 | 2 |
| Bracale et al[7], 2010 | 6 | NA | 1830 | 4 | 1 | 1 | 1 | 5 |
| Fadhil et al[28], 2011 | 1 | 18 | NA | 1 | 0 | 0 | 0 | 1 |
| Kountidou et al[9], 2012 | 1 | 90 | 180 | 0 | 0 | 1 | 0 | 0 |
| Leonardou et al[8], 2012 | 5 | 90-450 | 2880 | 0 | 5 | 0 | 0 | 4 |
| Ram et al[29], 2012 | 2 | 21-150 | 1080 | 2 | 0 | 0 | 0 | 2 |
| Srivastava et al[30], 2013 | 2 | 14-21 | NA | 2 | 0 | 0 | 0 | 2 |
| Chandak et al[10], 2014 | 1 | 180 | NA | 0 | 0 | 1 | 0 | 0 |
| Patrono et al[11], 2015 | 3 | 12-24 | 1080 | 2 | 0 | 1 | 0 | 2 |
| Dębska-Ślizień et al[31], 2015 | 2 | 10-22 | NA | 2 | 0 | 0 | 2 | 2 |
| Ministro et al[12], 2017 | 2 | 60-150 | 180 | 0 | 0 | 2 | 0 | 0 |
| Lazareth et al[32], 2017 | 1 | 15 | NA | 1 | 0 | 0 | 0 | 1 |
| Madhav et al[13], 2019 | 1 | 25 | NA | 0 | 0 | 1 | 0 | 0 |
| Asif et al[4], 2019 | 1 | 81 | NA | 1 | 0 | 0 | 1 | 1 |
| Overall | 56 | 3-450 | 2920 | 35 | 7 | 9 | 9 | 41 |
Renal allograft artery MA are often asymptomatic and incidentally detected during routine surveillance imaging studies or diagnostic work-up performed for other reasons. Some patients complain of non-specific symptoms such as fever, abdominal discomfort, claudication, hypertension, or hematuria. Occasionally, a tender pulsating mass can be palpated over the transplanted kidney. Large aneurysms may also cause pressure effects on the surrounding structures thus leading to hydronephrosis, venous obstruction or bladder dysfunction[18]. Rupture represents a catastrophic event with high morbidity and mortality rates. Clinical presentation is typical with sudden-onset pain in the iliac fossa, hypotension, reduced urinary output, and shock due to massive bleeding and acute transplant failure. Early diagnosis is a key factor for optimal patient management and requires a high index of suspicion.
Color Doppler ultrasound is a fast, safe, and reliable imaging diagnostic modality in both elective and emergency settings. Contrast-enhanced CT scan, magnetic resonance imaging or selective angiography are routinely performed for treatment planning[18,19]. Due to the high risk of transplant loss and death, the general consensus is that virtually all MA should be treated in a timely fashion. In case of acute hemorrhage with hemodynamic instability, graftectomy with or without iliac artery reconstruction is universally considered the safest choice. On the contrary, elective treatment of non-complicated MA involving the allograft artery is still controversial.
Prolonged anti-fungal administration with strict imaging-based surveillance of a small extra-anastomotic lesion has been described but it remains anecdotal[5]. Endovascular procedures such as arterial covered-stent placement and trans-catheter coil embolization have been also proposed[6-8]. Theoretical advantages are low invasiveness and morbidity. However, indication is strict (small, saccular, narrow-necked, extra-anastomotic aneurysms involving the main branch and distant from the hylum) and long-term results disappointing (Table 1). Experience with ultrasound-guided percutaneous needle injection of thrombin is limited to a single procedure in a patient with a relatively small iuxta-anastomotic pseudoaneurysm and does not represent a suitable option for true MA[18].
Given the poor results achieved by conservative strategies, for many years transplant nephrectomy has been widely considered the most reasonable option (Table 1). However, recent reports have shown that surgical repair can offer excellent long-term outcomes in carefully selected cases (Table 1). They also demonstrate that the risk of life-threatening complications related to graft salvage interventions is not necessarily higher than the risk of death arising from transplantectomy and return to chronic dialysis[20]. True extra-anastomotic aneurysm repair involves suture of the neck of the aneurysm or excision of the lesion with reconstruction of the artery. In patients with anastomotic disease, partial resection of the iliac artery with re-anastomosis or by-pass grafting is often needed[7,9-12].
In order to reduce intra-operative bleeding and allow optimal manipulation of the allograft, these procedures generally require temporary interruption of the transplant blood flow with possible development of ischemia-reperfusion injury. In such a context, IV administration of N-acetyl cysteine, cold perfusion of the kidney with organ preservation solutions, and surface cooling with sterile ice can reduce the risk of acute tubular necrosis and allograft dysfunction[21,22]. Our patient presented about 2 mo after KTx with non-specific symptoms and impaired renal function. As per standard practice at our center, he underwent a thorough evaluation with blood tests, urinalysis, microbiology screening, and color Doppler ultrasound scan. Preliminary diagnosis was pseudoaneurysm of the transplant artery but subsequent contrast-enhanced CT scan showed a true saccular dilation of the iuxta-anastomotic segment of the vessel involving the main branch and extending to the level of its distal bifurcation.
Due to the high index of suspicion for infectious etiology (positive preservation fluid cultures) a plan for immediate intervention was made. Active surveillance with antifungal therapy was not an option as the rapid growth of the lesion suggested an elevated risk of rupture. Endovascular treatments (stenting or embolization) were also not feasible because the aneurysm was large, wide-necked, and close to the distal bifurcation. Graftectomy would have probably been the easiest and safest strategy but considering the age of the recipient, his excellent performance status and strong will to avoid dialysis, the optimal quality of the transplanted kidney, the lack of signs of systemic infection, and our previous experience with complex renal vascular reconstructions[22,23], we opted for surgical repair. To minimize the risk of recurrence, the aneurysm was completely excised, and the distal stump of the allograft artery was anastomosed to the internal iliac artery.
Severe acute tubular necrosis was avoided administering IV N-acetyl cysteine before vascular clamping and perfusing the kidney with a cold preservation solution. Mycotic infections transmitted by contaminated allografts are not uncommon and screening preservation fluid cultures have been increasingly recognized as a valuable tool for early detection of potentially virulent pathogens[7,15]. Nevertheless, to treat or not to treat an asymptomatic KTx recipient with positive screening cultures remains a difficult choice.
In this particular group of patients, the benefit arising from systematic anti-fungal prophylaxis should be balanced against the risk of significant drug-related adverse events. Our personal experience clearly suggests administering prolonged anti-fungal medications in every case of mycotic contamination of the preservation fluid but further evidence is needed to address this unresolved issue.
True allograft artery MA is a rare but life-threatening complication of KTx. Fungal infections transmitted by contaminated organs represent the primary source of the disease. In case of rupture, transplant nephrectomy is the safest option. However, in carefully selected patients with excellent performance status and no signs of active infection, a surgical repair should be always attempted because of the high risk of death arising from chronic dialysis and the low chances of receiving another organ. Prophylactic anti-fungal therapy is strongly advised for all recipients with positive screening preservation fluid cultures.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gao BL S-Editor: Dou Y L-Editor: Filipodia E-Editor: Xing YX
| 1. | Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3684] [Cited by in RCA: 3856] [Article Influence: 148.3] [Reference Citation Analysis (1)] |
| 2. | Favi E, Salerno MP, Romagnoli J, Castagneto M, Citterio F. Significant improvement in patient survival after renal transplantation in the last decade. Transplant Proc. 2011;43:285-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Cohen-Bucay A, Gordon CE, Francis JM. Non-immunological complications following kidney transplantation. F1000Res. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Asif S, Bennett J, Pauly RR. A Rare Case of an Infectious Pseudoaneurysm due to Aspergillus flavus in the Setting of Renal Transplant. Cureus. 2019;11:e4208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Fujikata S, Tanji N, Iseda T, Ohoka H, Yokoyama M. Mycotic aneurysm of the renal transplant artery. Int J Urol. 2006;13:820-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Osmán I, Barrero R, León E, Medina R, Torrubia F. Mycotic pseudoaneurysm following a kidney transplant: a case report and review of the literature. Pediatr Transplant. 2009;13:615-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Bracale UM, Santangelo M, Carbone F, Del Guercio L, Maurea S, Porcellini M, Bracale G. Anastomotic pseudoaneurysm complicating renal transplantation: treatment options. Eur J Vasc Endovasc Surg. 2010;39:565-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Leonardou P, Gioldasi S, Zavos G, Pappas P. Mycotic pseudoaneurysms complicating renal transplantation: a case series and review of literature. J Med Case Rep. 2012;6:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Kountidou CS, Stier K, Niehues SM, Lingnau A, Schostak M, Fuller TF, Lützenberg R. Successful repair of post-transplant mycotic aneurysm of iliac artery with renal graft preservation: a case report. Urology. 2012;80:1151-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Chandak P, Kessaris N, Uwechue RU, Abboudi H, Hossain M, Harris F, Jones K, Fronek J. Successful excision of a suspected mycotic transplant renal artery patch aneurysm with renal allograft autotransplantation. Transplantation. 2014;97:e25-e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 11. | Patrono D, Verhelst R, Buemi A, Darius T, Godefroid N, Mourad M. Presentation and management of mycotic pseudoaneurysm after kidney transplantation. Transpl Infect Dis. 2015;17:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Ministro A, Ferreira T, Batista L, Santana A, Alves N, Guerra J, Fernandes E Fernandes J. Mycotic Pseudoaneurysm After Kidney Transplantation: Two Case Reports. Transplant Proc. 2017;49:906-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Madhav D, Kumar P, Mohan C, Vijay, Mahesh U, Anusha, Suneetha, Suryaprakash. Candida-associated pseudo-aneurysm of the transplant renal artery presenting as malignant hypertension and managed successfully without nephrectomy. Saudi J Kidney Dis Transpl. 2015;26:1000-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Osler W. The Gulstonian Lectures, on Malignant Endocarditis. Br Med J. 1885;1:467-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 438] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 15. | Albano L, Bretagne S, Mamzer-Bruneel MF, Kacso I, Desnos-Ollivier M, Guerrini P, Le Luong T, Cassuto E, Dromer F, Lortholary O; French Mycosis Study Group. Evidence that graft-site candidiasis after kidney transplantation is acquired during organ recovery: a multicenter study in France. Clin Infect Dis. 2009;48:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Laouad I, Buchler M, Noel C, Sadek T, Maazouz H, Westeel PF, Lebranchu Y. Renal artery aneurysm secondary to Candida albicans in four kidney allograft recipients. Transplant Proc. 2005;37:2834-2836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Minz M, Sharma A, Kumar S, Singh S, Shivaprakash MR, Bag S. Use of autogenous internal iliac artery for bridging the external iliac artery after excision of Aspergillus mycotic aneurysm in renal transplant recipients. J Vasc Surg. 2011;53:802-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Siu YP, Tong MK, Leung KT, Kwan TH, Au TC, Cheung YK, Luk SH. Renal artery pseudoaneurysm following renal transplantation and treatment by percutaneous thrombin injection. Hong Kong Med J. 2006;12:80-81. [PubMed] |
| 19. | Piacentino F, Fontana F, Micieli C, Angeretti MG, Cardim LN, Coppola A, Molinelli V, Piffaretti G, Novario R, Fugazzola C. Nonenhanced MRI Planning for Endovascular Repair of Abdominal Aortic Aneurysms: Comparison With Contrast-Enhanced CT Angiography. Vasc Endovascular Surg. 2018;52:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Rao PS, Schaubel DE, Jia X, Li S, Port FK, Saran R. Survival on dialysis post-kidney transplant failure: results from the Scientific Registry of Transplant Recipients. Am J Kidney Dis. 2007;49:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Orban JC, Quintard H, Cassuto E, Jambou P, Samat-Long C, Ichai C. Effect of N-acetylcysteine pretreatment of deceased organ donors on renal allograft function: a randomized controlled trial. Transplantation. 2015;99:746-753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Favi E, Citterio F, Tondolo V, Chirico A, Brescia A, Romagnoli J, Castagneto M. Abdominal aortic aneurysm in renal transplant recipients. Transplant Proc. 2005;37:2488-2490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Favi E, Cacciola R, Muthuppalaniappan VM, Thuraisingham R, Ferraresso M, Puliatti C. Multidisciplinary management of complicated bilateral renal artery aneurysm in a woman of childbearing age. J Surg Case Rep. 2018;2018:rjy147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Potti A, Danielson B, Sen K. "True" mycotic aneurysm of a renal artery allograft. Am J Kidney Dis. 1998;31:E3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Henderson A, Pall AA, Chakraverty S. Unsuspected mycotic aneurysm of renal transplant artery. Kidney Int. 2007;72:775-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Taksin L, Mallick S, Frachet O, Julien M, Lepennec V, Ficheux M, Bensadoun H. [Mycotic aneurysm and renal transplant. A case report]. Prog Urol. 2009;19:149-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Wang R, Wu J, Wang Y, Huang H, He Q, Chen J. Aspergillus infection limited to the anastomosed artery following renal transplantation: a report of 4 cases. Transpl Infect Dis. 2009;11:363-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Fadhil RA, Al-Thani H, Al-Maslamani Y, Ali O. Trichosporon fungal arteritis causing rupture of vascular anastamosis after commercial kidney transplantation: a case report and review of literature. Transplant Proc. 2011;43:657-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Ram Reddy C, Ram R, Swarnalatha G, Krishna LS, Prayaga A, Murthy PV, Dakshinamurty KV. "True" mycotic aneurysm of the anastomotic site of the renal allograft artery. Exp Clin Transplant. 2012;10:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Srivastava A, Kumar J, Sharma S, Abhishek, Ansari MS, Kapoor R. Vascular complication in live related renal transplant: An experience of 1945 cases. Indian J Urol. 2013;29:42-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Dębska- Ślizień A, Chrobak Ł, Bzoma B, Perkowska A, Zadrożny D, Chamienia A, Kostro J, Milecka A, Bronk M, Śledziński Z, Rutkowski B. Candida arteritis in kidney transplant recipients: case report and review of the literature. Transpl Infect Dis. 2015;17:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Lazareth H, Burbach M, Gosset C, Lefaucheur C, Pashootan P, Zagdanski AM, Denis B. Renal Arterial Mycotic Aneurysm After Kidney Transplantation. Urology. 2017;106:e7-e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |