Published online Feb 26, 2020. doi: 10.12998/wjcc.v8.i4.771
Peer-review started: October 20, 2019
First decision: December 4, 2019
Revised: December 11, 2019
Accepted: December 22, 2019
Article in press: December 22, 2019
Published online: February 26, 2020
Processing time: 129 Days and 3.6 Hours
Lymphoepithelioma-like carcinomas (LELCs) are rare, malignant epithelial tumors, generally considered a subtype of squamous cell carcinoma. LELCs are undifferentiated and can occur in multiple tissues, although LELCs in the urinary tract are extremely rare. As such, evidence does not provide clinicians with guidelines for the best practices. Even though this is a rare disease, it is associated with high morbidity and mortality. Therefore, we must learn to differentiate LELC types and identify risk factors for early identification.
To develop an evidence base to guide clinicians treating primary LELCs of the upper urinary tract (UUT-LELC).
We performed a systematic review of all reports on UUT-LELC from the first published case in 1998 until October 2019, according to the PRISMA. A database was then developed by extracting data from previously published reports in order to analyze interactions between clinical characteristics, pathological features, interventions and outcomes. Survival was analyzed using Kaplan–Meier estimates, which were compared using log rank tests.
A total of 28 previously published cases were identified for inclusion. The median age was 72 years with a male to female ratio of 4:3. Pure type LELCs were most common with 48.3% (n = 14), followed by 37.9% (n = 11) predominant LELCs and 3.4% (n = 1) focal LELCs. Epstein-Barr virus testing was negative in all cases. Fourteen patients received radical nephroureterectomy (RNU)-based intervention. Twenty-three patients survived with no evidence of further metastasis, although six died before the median 18 mo follow-up point. Survival analysis suggests pure histological subtypes, and patients who receive complete tumor resection have more favorable prognoses. As always in cancer care, early identification generally increases the probability of interventional success.
The most effective treatment for UUT-LELC is RNU-based therapy. Since cases are few in number, case reporting must be enhanced and publishing encouraged to both save and prolong lives.
Core tip: Lymphoepithelioma-like carcinomas in the urinary tract are extremely rare, hence there is little evidence to provide clinical guidelines. Of the 28 participants included in this systematic review of case reports, 23 patients survived with no evidence of further metastasis. Survival analysis suggests pure histological subtypes, and patients who receive complete tumor resection have more favorable prognoses. As always in cancer care, early identification generally increases the probability of interventional success, although this evidence base must be developed with more rigorous testing and case reporting.
- Citation: Lai SC, Seery S, Zhang W, Liu M, Zhang G, Wang JY. Lymphoepithelioma-like carcinoma of the upper urinary tract: A systematic review of case reports. World J Clin Cases 2020; 8(4): 771-781
- URL: https://www.wjgnet.com/2307-8960/full/v8/i4/771.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i4.771
Lymphoepithelioma-like carcinomas (LELC) are rare, malignant epithelial tumors that are generally considered a subtype of squamous cell carcinoma. LELCs are undifferentiated, with histological features that bear a striking morphological resemblance to non-keratinizing nasopharyngeal carcinomas[1]. LELCs occur in multiple tissues across the body, including salivary glands, thymus, lung, stomach, ovary, uterine cervix and breast[2-4]. However, LELCs in the urinary tract are extremely rare and, to the best of our knowledge, most previously described urinary LELCs have been reported to occur in the bladder[1,5]. Rationally speaking, there are likely to be distinctions between subtypes related to the exact location, as well as specific risk factors associated with these carcinomas.
Current evidence does not provide clinicians with clear guidelines, due to the relative rareness of these carcinomas. Even within the most common LELC locations, there remains some controversy. Several studies have identified an association between Epstein-Barr virus (EBV) and nasopharyngeal, stomach, and lung LELCs[6], although this association is not apparent in other locations. For example, a systematic review of 142 patients[7] diagnosed with bladder LELCs, as well as several case reports focusing on renal LELCs[8], suggests that these types are not associated with the presence of EBV. Clearly, there is a need to differentiate all LELC types, and to identify risk factors for early identification. At present, little is known about upper urinary tract LELCs (UUT-LELCs) due to the lack of evidence and the limited number of publications.
Consequently, we performed a systematic review of all available English literature to analyze and differentiate associations with this LELC type. The primary goal was to develop this evidence base for practitioners and clinicians. As such, we investigated disease management techniques, with a view to quantifying risks and identifying prognostic factors associated with this rare malignant neoplasm.
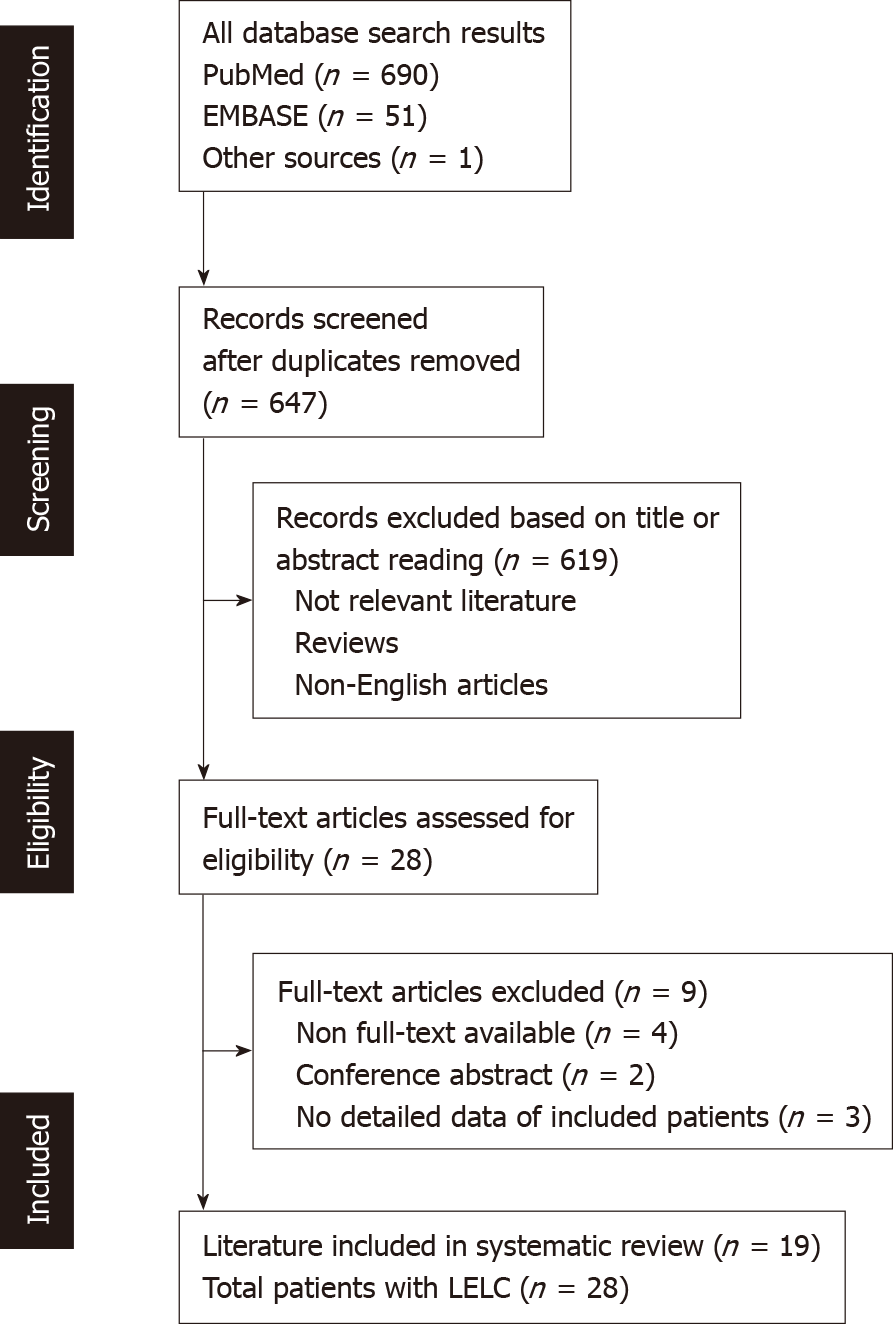
A systematic search of PubMed, EMBASE, and other databases was conducted for all reports on UUT-LELC up until October, 2019. The search strategy was conducted for all types of UUT-LELC studies up until March 8th, 2019. The following key terms were defined to source pertinent studies; LELC, urinary, UUT, pelvis, and ureter. In addition, manual searches of references and citations were conducted to ensure that all necessary evidence was identified. This search and selection strategy was adapted from the PRISMA. Literature searching, selection and data extraction were independently undertaken by two reviewers (Lai SC and Seery S), which were then cross-checked, and any discrepancies were resolved through discussion. A flowchart representing the search and selection process is presented in Figure 1. Ethical approval was unnecessary because all data were carefully extracted from existing published case reports.
A database was then designed to extract information that included patient age, gender, chief complaint(s), tumor laterality, location, LELC type, immuno-histochemical staining results, EBV status, TNM staging, treatment strategies, follow-up time, and survival. Detailed demographic characteristics of this sample are summarized in Table 1. We adopted a simple descriptive analytical approach, and then utilized the Kaplan-Meier method to analyze survival. Survival rates between subgroups were compared using the log-rank test to analyze prognostic factors. P values of less than 0.05 were considered significant. All statistical analysis was conducted using SPSS Software v.19.0 (SPSS Inc., Chicago, IL, United States).
| Case No. | Age/gender | Region | Presentation | Side | Location | Tumor stage | HC | CK antigen | EBV-status | Treatment | Follow-up in mo | Outcome | Ref. |
| 1 | 73/F | Asia (Chinese Taipei) | Hematuria | Right | Renal pelvis | pT2N0M0 | Pure | 7 | NM | RNU | 24 | NED | Chen et al[16], 2018 |
| 2 | 54/M | 4 Europe (Spain); The remaining 6 unknown | Hematuria | NM | Ureter | pT3N1Mx | Predominant | 7;20;CKAE1/AE3 | Negative | RN + Ch | 39 | DWD | Lopez-Beltran et al[15], 2017 |
| 3 | 62/M | Hematuria | NM | Ureter | pT1N0Mx | Pure | 7;20;CKAE1/AE3 | Negative | RNU | 55 | NED | Lopez-Beltran et al[15], 2017 | |
| 4 | 62/F | Hematuria | NM | Ureter | pT2N0Mx | Predominant | 7;20;CKAE1/AE3 | Negative | U | 18 | NED | Lopez-Beltran et al[15], 2017 | |
| 5 | 80/F | Hematuria | NM | Ureter | pT3N0Mx | Pure | 7;20;CKAE1/AE3 | Negative | RNU | 39 | NED | Lopez-Beltran et al[15], 2017 | |
| 6 | 76/M | Hematuria | NM | Ureter | pT3N1M0 | Predominant | 7;20;CKAE1/AE3 | Negative | RNU + Ch | 29 | NED | Lopez-Beltran et al[15], 2017 | |
| 7 | 61/M | Hematuria | NM | Ureter | pT1N0Mx | Pure | 7;20;CKAE1/AE3 | Negative | U | 35 | NED | Lopez-Beltran et al[15], 2017 | |
| 8 | 64/M | Hematuria | NM | Renal pelvis | pT2N0Mx | Predominant | 7;20;CKAE1/AE3 | Negative | RN | 58 | NED | Lopez-Beltran et al[15], 2017 | |
| 9 | 81/M | Hematuria | NM | Renal pelvis | pT3N1Mx | Predominant | 7;20;CKAE1/AE3 | Negative | RN+ Ch | 7 | DWD | Lopez-Beltran et al[15], 2017 | |
| 10 | 85/M | Hematuria | NM | Renal pelvis | pT3N1Mx | Predominant | 7;20;CKAE1/AE3 | Negative | RN + Ch | 4 | DWD | Lopez-Beltran et al[15], 2017 | |
| 11 | 81/M | Hematuria | NM | Renal pelvis | pT3N1M0 | Predominant | 7;20;CKAE1/AE3 | Negative | RN + Ch | 10 | DWD | Lopez-Beltran et al[15], 2017 | |
| 12 | 65/F | Asia (Korea) | Hematuria | Right | Renal pelvis | T3N0M0 | Predominant | Cytokeratin | Negative | RN | 6 | NED | Ahn et al[17], 2014 |
| 13 | 61/M | Asia (China) | Flank pain; Hematuria | Left | Renal pelvis | T4N2M0 | NM | 7 | Negative | RN | 3 | DWD | Liu et al[20], 2014 |
| 14 | 75/F | Europe (UK) | Flank pain; Hematuria | Right | Renal pelvis | T3N1M0 | Predominant | 20;AE1/AE3 | NM | RNU | 6 | NED | Modi et al[14], 2013 |
| 15 | 64/M | Asia (Chinese Taipei) | Nausea | Right | Ureter | pT3N0M0 | Pure | 7 | Negative | RNU | 6 | NED | Wen et al[25], 2012 |
| 16 | 76/F | Europe (Spain) | Recurrent UTI | Left | Ureter | T2N0M0 | Pure | 7;20;CKAE1/AE3 | Negative | PN+U | 5 | NED | Val-Bernal et al[27], 2011 |
| 17 | 71/M | America (United States) | Hematuria | Right | Ureter | T2NxMx | Pure | 7;20;CKAE1/AE3 | Negative | RNU | 5 | NED | Allend et al[18], 2010 |
| 18 | 64/F | America (United States) | Hematuria | Left | Ureter | T2NxMx | Pure | 7;CKAE1/AE3 | Negative | U | 24 | NED | Ma et al[21], 2008 |
| 19 | 75/F | Asia (Japan) | Flank pain; Hematuria | Left | Renal pelvis | T1N1M0 | Pure | Unknown | Negative | RNU | 36 | NED | Haga et al[26], 2007 |
| 20 | 75/F | Asia (Japan) | Flank pain; Hematuria | Left | Renal pelvis | T3N0M0 | NM | 15 | Negative | RN | 6 | NED | Yamada et al[11], 2007 |
| 21 | 72/F | Europe or America | Incidental finding | NM | Renal pelvis | T3 | Predominant | AE1/AE3 | Negative | RN | 3 | DWD | Perez-Montiel et al[13], 2006 |
| 22 | 68/M | Europe or America | Incidental finding | NM | Renal pelvis | T3 | Focal | AE1/AE3 | Negative | RN | 12 | DWD | Perez-Montiel et al[13], 2006 |
| 23 | 73/M | Asia (Japan) | Hematuria | Right | Ureter | T2N0Mx | Pure | CKAE1/AE3 | Negative | RNU | 30 | NED | Terai et al[24], 2005 |
| 24 | 58/M | Europe (Spain) | Hematuria | Left | Ureter | T3N0M0 | Pure | CKAE1/AE3 | Negative | RNU | 18 | NED | Roig et al[23], 2001 |
| 25 | 62/F | Asia (Chinese Taipei) | Hematuria | Right | Ureter | T2N0M0 | Pure | CKAE1/AE 3 | Negative | RNU | 18 | NED | Ng et al[22], 1999 |
| 26 | 79/F | Australia | Flank pain; Hematuria | Right | Renal pelvis | T3N0M0 | NM | AE1/AE3 | Negative | RNU | 6 | NED | Cohen et al[10], 1999 |
| 27 | 70/M | Asia (Japan) | Flank pain; Hematuria | NM | Renal pelvis | T1N0M0 | Pure | 7;20 | Negative | RNU + RT | 72 | NED | Fukunaga et al[9], 1998 |
| 28 | 75/M | America (United States) | Hematuria | Right | Ureter | T3N0M0 | Pure | 7;20 | Negative | U | 12 | NED | Chalik et al[19], 1998 |
In total, 28 UUT-LELC cases were identified from 1998 up until October 2019[9-27]. Among this sample, 14 cases reported LELCs located in the renal pelvis, with the remaining 14 located in the ureter. The median age of these patients was 72 years, ranging from 54 to 85 years. Of the patients, 57.1% were male (n = 16) (Table 2), and 32.1% were of Asian descent. The most common primary presentations at diagnosis were gross hematuria (67.9%), combined hematuria and flank pain (10.7%), urinary infection (3.6%) and nausea (3.6%). The remaining two cases were incidentally detected. Histological subtypes were described in all but three cases (10.7%). Pure LELC occurs most frequently at 50% (n = 14), followed by predominant LELC at 35.7% (n = 10) and focal LELC the least frequently at 3.6% (n = 1). EBV testing was conducted in 93.1% of cases, all of which were negative.
| Variable | n (%), median (range) |
| Age in yr | 72 (54-85) |
| Gender | |
| Female | 12 (42.9) |
| Male | 16 (57.1) |
| Presentation | |
| Hematuria | 19 (67.9) |
| Pain | 2 (7.1) |
| Hematuria and flank pain | 3 (10.7) |
| Nausea | 1 (3.6) |
| Recurrent UTI | 1 (3.6) |
| Incidental finding | 2 (7.1) |
| Side | |
| Left | 6 (21.4) |
| Right | 9 (32.2) |
| NM | 13 (46.4) |
| Location | |
| Renal pelvis | 14 (51) |
| Ureter | 14 (50) |
| HC | |
| Pure | 14 (50) |
| Predominant | 10 (35.7) |
| Focal | 1 (3.6) |
| Unknown | 3 (10.7) |
| Tumor stage | |
| T1 | 4 (14.2) |
| T2 | 8 (28.6) |
| T3 | 15 (53.6) |
| T4 | 1 (3.6) |
| Treatment strategy | |
| RNU-based therapy | 13 (46.4) |
| Non RNU-based therapy | 15 (53.6) |
| Follow-up in mo | 15 (3-72) |
| Outcome | |
| NED | 21 (75) |
| DWD | 7 (25) |
Cases related to each pathological tumor stage appeared as follows; pT1 in 14.2% (n = 4), pT2 in 28.6% (n = 8), pT3 in 53.6% (n = 15) and pT4 in 3.6% (n = 1). Lymph node metastasis was reported in 27.6% of cases, although no distant metastasis was noted at the time of diagnosis. Surgery was the first-line therapy for all patients. Of the cases, 85.2% utilized a single treatment modality, and in combination with “radical nephroureterectomy” (RNU), which appeared to be the most frequently administered (50%), followed by “radical nephrectomy” (RN) alone (27.3%), ureterectomy (18.2%), and partial nephrectomy plus ureterectomy (4.5%).
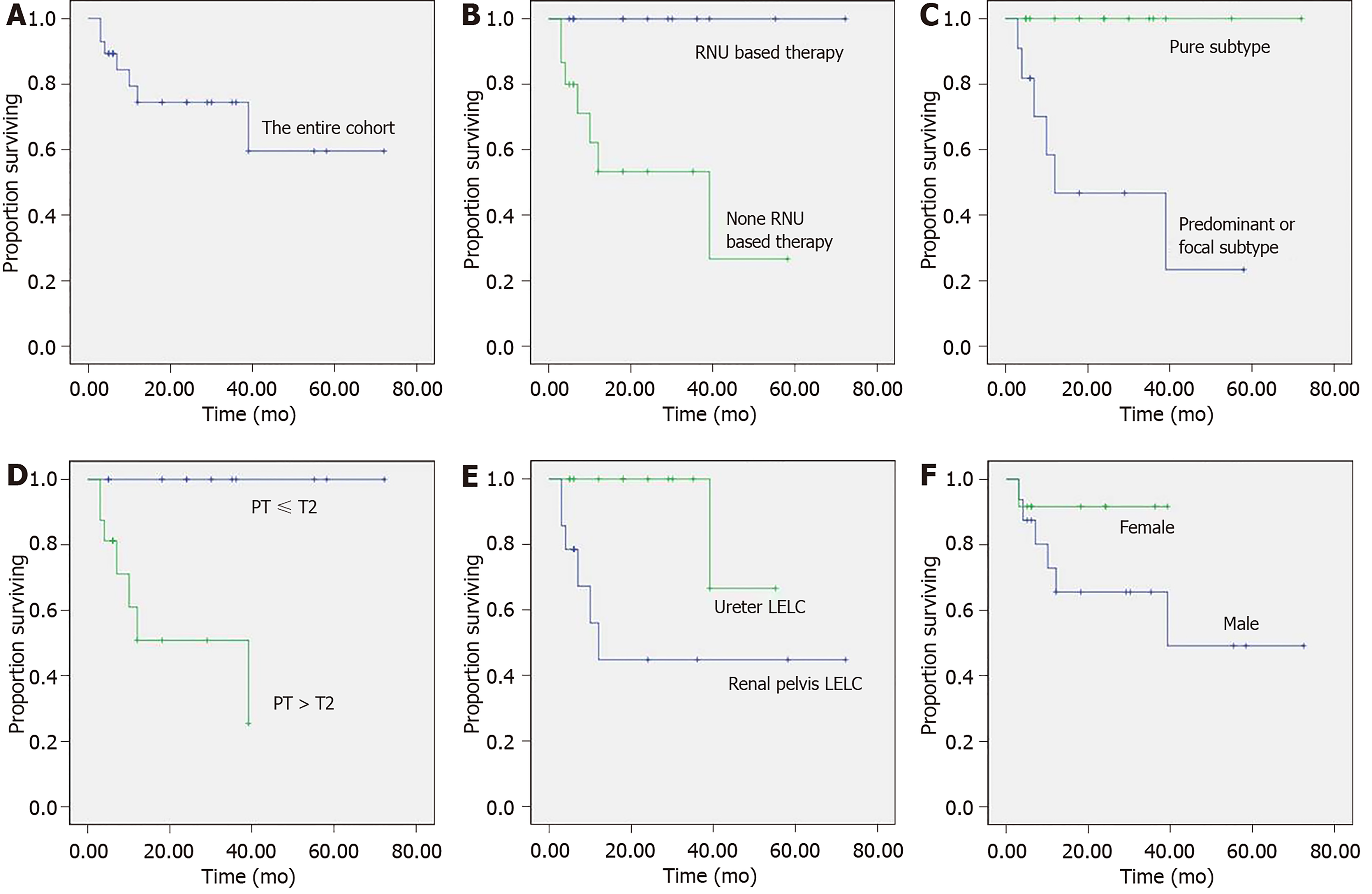
There was no evidence of further metastasis in 87% of these cases; however, 13% died of the disease post-surgery. Of the multi-modal treatments, primary treatments included RN (66.7%) and RNU (33.3%). Adjuvant treatments involved either chemotherapy (83.3%) or radiotherapy (16.7%). Outcomes for those receiving multi-modal treatments included no evidence of further metastasis (33.3%), although 66.7% died postoperatively. Overall, 21 patients were alive with no evidence of disease recurrence. Seven died at the median follow-up point of 18 mo, and cancer-specific survival curves of the entire cohort are presented in Figure 2A.
A non-parametric log-rank test was implemented to compare survival curves and explore prognostic factors. With univariate analysis, the following clinicopathological parameters were significantly associated with survival; treatment modality (P = 0.006, Figure 2B), histological subtypes (P = 0.002, Figure 2C), pathological stage (P = 0.004, Figure 2D) and tumor location (P = 0.016, Figure 2E). RNU-based therapy significantly improved the cancer-specific survival rate compared with Non-RNU-based therapy (P < 0.01). The pure histological subtype also appears to have better prognosis compared with the non-pure subtypes, including predominant and focal subtypes (P < 0.01). Higher tumor stage (i.e., pT3–pT4) corresponds with a higher mortality rate, and location within the urinary tract also appears to have a significant impact on outcomes. For example, renal pelvis LELC seems to have poorer outcomes. There also appears to be a difference between men and women, although this was not statistically significant (P = 0.217, Figure 2F).
More than 95% of all malignant neoplasms arising within the urinary tract are pure type urothelial carcinomas, with an estimated incidence of 1-4 cases per 100000 individuals per year[28,29]. Several “variant” morphologies (i.e., small cell, lympho-epithelioma-like, clear cell, nested, plasmacytoid, micropapillary, sarcomatoid variants, etc.) have been reported, and most have been classified according to the World Health Organization Classification of Tumors in the Urinary System[30] since 2016. Even though these malignancies are rare, awareness of morphological variants has a multitude of clinical implications, such as avoidable misdiagnoses, prognostics based upon patient risk stratification, stress management, and of course administering the most appropriate and beneficial interventions. Clinical guidelines are not yet available and, therefore, the aim of this study was to report and develop the existing evidence base for clinicians treating this rare malignant disease.
Only 28 cases of UUT-LELC (14 renal pelvis and 14 ureter) have been described within the English language literature since it was first reported by Fukunaga et al[9] in 1998. The complete data suggests that men are more likely to develop UUT-LELC, with a male to female ratio of 4:3. This finding is dissimilar to the general UUT-UC population, which has a median age of 65 years and a gender ratio of 2-3 male: 1 female[28]. Previous studies have also suggested there may be an increased prevalence within Asian communities; however, across this larger sample, we found that 32.1% of all cases were of Asian descent (n = 9). This may be used to differentiate LELCs from small cell carcinomas in the UUT. In a recent case report combined with a review of UUT-SCC involving 39 cases, Ouzzane et al[28] found that 59% of all cases were Asian. That said, six case reports included in this study did not report any information about ethnicity, which may have provided necessary basic insights into associated genetic risk factors.
Unnecessary diagnostic testing not only increases healthcare expenditures, but also impacts patient trust and satisfaction in health service provisions. To the best of our knowledge, EBV is positively associated with nasopharyngeal carcinoma and LELC developing in specific anatomical sites, including the salivary glands, esophageal, lungs, and stomach[6]. However, urinary tract LELCs appear uniformly EBV negative. Among all tumors arising within the UUT, no case has been associated with EBV, which suggests that urinary tract LELCs are distinct from gastrointestinal LELCs. Clinicians may be able to use this information when considering which diagnostic approach to administer, thereby alleviating some unnecessary stress and maintaining confidence in practitioners.
Unfortunately, clinical presentation of UUT-LELC may not be distinct from other UUT-UCs, with gross hematuria (78.6% vs 77%) and flank pain (17.8% vs 18%) being the most commonly described symptoms[28]. Consequently, pre-operative diagnosis is currently not possible. Again, from the patient’s perspective, this “not knowing” is likely to increase stress and will impact their psychological well-being. Therefore, clinicians should pay special attention to this aspect of patient-practitioner consultations in order to alleviate unnecessary stress and provide reassurance.
The best available UUT-LELC diagnostic method remains postoperative pathological examination and immunohistochemical staining. Upon histological examination, the epithelial components of LELCs consist of nests and individual cells of undifferentiated carcinoma, which are characterized by indistinct cytoplasmic borders and syncytial growth patterns[14,15]. Most tumor cells display vesicular nuclei, prominent nucleoli, and frequent mitoses, which are indicatively considered[15]. Immunohistochemically, the epithelial component of the tumor typically stains positive for a number of cytokeratin (CK) markers, namely AE1/AE3, CK7, CK8 and CK20, which is then used for diagnostic confirmation[14,15,18].
LELCs are generally classified into three subtypes: Pure (100%), predominant (50-99%), and focal (< 50%)[12,14], and the pathological results of the current study were consistent with the predominant histological subtype. There may also be further distinctions between LELCs. For example, pathological features of urinary tract LELCs may be composed of two or more elements, such as usual infiltrating urothelial carcinoma, squamous carcinoma or adenocarcinoma[12]. Each of these are likely to respond differently to treatments, adding complexity to therapeutic planning and suggesting the need for targeted rather than generic interventions.
Similarly, there may be distinctions between lower and UUT-LELCs. For example, according to the relatively large number of studies in LELCs of the urinary bladder, the prognosis of patients presenting with pure and predominant forms appears more favorable than that in patients presenting with a focal form[5]. However, evidence confirming whether these results are consistent across all other LELC cases in the urinary tract, such as the bladder, remains limited. Our systematically sourced case reports presented in Table 1 consist of six cases with a non-pure histological subtype, all of which had poor outcomes. Most died within one year after surgery, although the remaining 14 patients had pure predominant histological subtypes with no evidence of disease at a long-term follow-up juncture. Again, as this evidence base develops, we should be able to determine which intervention is most beneficial for each histological subtype, although data recording and reporting must be enhanced to understand these subtleties.
Overall, tumor stage had a significant impact on the oncological outcomes, which highlights the need for early identification[15]. In this study, all of those who died with the disease presented at an advanced pathological stage. According to the univariant analysis shown in Figure 2, patients identified at earlier pathological stages have relatively favorable prognoses. In addition, location appears to also be a key factor. For example, renal pelvis LELCs appear to correspond with poor oncologic outcomes, which adds support to the theory that renal pelvis LELC management should be consistent with the standard therapy of renal pelvis carcinoma, requiring radical resection of the entire upper tract of the urothelium. This recommends RNU-based therapy as the first-line management technique for renal pelvis LELC, but does not elaborate on our understanding of UUT-LELC.
Further analysis of these combined cases confirmed that surgical strategies influence prognosis. Importantly, seven of the 15 patients who underwent Non-RNU-based therapies died with the disease. Conversely, all those who received RNU-based treatments survived and had no evidence of disease at the long-term follow-up point. In line with oncological management principles, proper recognition of the disease prognosis and regular follow-up are also essential. During the follow-up period, regular cystoscopy, urine cytology testing and abdominal computer tomography should also be performed at three months, and annually thereafter. Clearly, there is variability within this sample of cases, which may relate to concomitant transitional cell carcinoma admixed with the non-pure subtype LELC, which may add to our prognostic knowledge.
This study was systematically performed to better understand this uncommon variant of urinary carcinoma, although there are some limitations that should be addressed prior to drawing conclusions. Firstly, the evidence around this rare tumor was retrospectively gathered, and included case reports and small case series. However, this evidence base is limited, therefore generalizing it as inappropriate. Additionally, the rigor with which cases are recorded and reported could be improved. Data around smoking and drinking status, family history and co-morbidities might prove useful. Also, follow-up periods in some cases were relatively short, which creates a number of unnecessary unknowns.
In conclusion, these findings suggest that the treatment associated with the highest disease-free survival is an RNU-based intervention. However, this can only be a tentative recommendation given the limitations of this evidence base. We would encourage clinicians to actively communicate findings and publish case reports. UUT-LELC cases may be rare, but there is an opportunity to save and prolong life by sharing knowledge, enhancing the depth and breadth of data recording, and reporting and discussing clinical experiences.
Lymphoepithelioma-like carcinomas (LELC) are rare, malignant epithelial tumors that are generally considered a subtype of squamous cell carcinoma. LELCs are undifferentiated, with histological features bearing a striking morphological resemblance to non-keratinizing nasopharyngeal carcinomas. LELCs occur in multiple tissues across the body, including the salivary glands, thymus, lung, stomach, ovary, uterine cervix and breast. However, LELCs in the urinary tract are extremely rare, and to the best of our knowledge, most previously described urinary LELCs have been reported to occur in the bladder. Rationally speaking, there are likely to be distinctions between subtypes, related to the exact location, as well as specific risk factors associated with these carcinomas. This systematic review is an attempt to expand and synthesize findings to provide clinicians with guidelines for the best practices.
The limited current evidence is not sufficient to provide clinicians with clear guidelines, due to the relative rareness of these carcinomas. Even within the most common LELC locations, there remains some controversy. Several studies have identified an association between Epstein-Barr virus (EBV) and nasopharyngeal, stomach, and lung LELCs, although this association is not apparent in other locations. A previous systematic review of 142 patients diagnosed with bladder LELC, as well as several case reports focusing on renal LELCs, suggests that these types are not associated with the presence of EBV. There are several other controversies that should be investigated using meta-analytical techniques. Therefore, the general motivation was to differentiate all LELC types, and to identify risk factors for early identification, since little is known about upper urinary tract LELC (UUT-LELC).
The fundamental research objective of this study was to develop the evidence base for clinical diagnoses, and to engage patients in shared-decision making for the treatment of primary UUT-LELC.
A systematic search of PubMed, Embase, and other databases was conducted for all reports on UUT-LELC up until October, 2019, according to PRISMA guidelines. A database was then developed by extracting data from previously published reports in order to analyze interactions between clinical characteristics, pathological features, interventions and outcomes. Survival was analyzed using Kaplan–Meier estimates, which were compared using log rank tests.
A total of 28 previously published cases were identified for inclusion. The median age was 72 years with a male to female ratio of 4:3. Pure type LELCs were most common with 48.3% (n = 14), followed by 37.9% (n = 11) predominant LELCs and 3.4% (n = 1) focal LELCs. EBV testing was negative in all cases. Fourteen patients received radical nephroureterectomy (RNU)-based intervention. Twenty-three patients survived with no evidence of further metastasis. Six died before the median 18 mo follow-up point. Survival analysis suggests pure histological subtypes, and patients who receive complete tumor resection have more favorable prognoses. As always in cancer care, early identification generally increases the probability of interventional success.
Findings suggest that the treatment associated with the highest disease-free survival is an RNU-based intervention. However, this can only be a tentative recommendation given the limitations of this evidence base. We would encourage clinicians to actively communicate their findings and publish case reports. UUT-LELC cases may be rare, but there is an opportunity to save and prolong lives by sharing knowledge, enhancing the depth and breadth of data recording, and reporting and discussing clinical experiences.
Case reports are not generally high priority for publication houses, although a lot of very valuable information can be extracted and synthesized. This is an incredibly meaningful approach that we would encourage others to conduct, especially for rare conditions such as UUT-LELC. Clinicians must be guided by the best available evidence to reduce anxiety that may manifest through unnecessary testing and unclear guidelines. Systematic reviews of case reports and case series such as this one can also be used to support best practices, which will reduce the possibility of unnecessary harm.
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rodrigues AT S-Editor: Dou Y L-Editor: Filipodia E-Editor: Liu JH
| 1. | Amin MB. Histological variants of urothelial carcinoma: diagnostic, therapeutic and prognostic implications. Mod Pathol. 2009;22 Suppl 2:S96-S118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 280] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 2. | Butler AE, Colby TV, Weiss L, Lombard C. Lymphoepithelioma-like carcinoma of the lung. Am J Surg Pathol. 1989;13:632-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 116] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Lee S, Park SY, Hong EK, Ro JY. Lymphoepithelioma-like carcinoma of the ovary: a case report and review of the literature. Arch Pathol Lab Med. 2007;131:1715-1718. [PubMed] |
| 4. | Kulka J, Kovalszky I, Svastics E, Berta M, Füle T. Lymphoepithelioma-like carcinoma of the breast: not Epstein-Barr virus-, but human papilloma virus-positive. Hum Pathol. 2008;39:298-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Porcaro AB, Gilioli E, Migliorini F, Antoniolli SZ, Iannucci A, Comunale L. Primary lymphoepithelioma-like carcinoma of the urinary bladder: report of one case with review and update of the literature after a pooled analysis of 43 patients. Int Urol Nephrol. 2003;35:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Iezzoni JC, Gaffey MJ, Weiss LM. The role of Epstein-Barr virus in lymphoepithelioma-like carcinomas. Am J Clin Pathol. 1995;103:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 257] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Rodríguez-Cabello MA, Méndez-Rubio S, Sanz-Miguelañez JL, Saenz-Medina J, Garrido-Abad P, Del-Barrio-Díaz-Aldagalan A, López-Elzaurdia C, Platas-Sancho A. Lymphoepithelioma-Like Bladder Carcinoma: A Diagnostic and Therapeutic Challenge. Contribution Using a New Case and Review of the Literature. Clin Genitourin Cancer. 2017;15:e507-e515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Elzevier HW, Venema PL, Kropman RF, Kazzaz BA. Lymphoepithelioma-like carcinoma of the kidney. J Urol. 2002;167:2127-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Fukunaga M, Ushigome S. Lymphoepithelioma-like carcinoma of the renal pelvis: a case report with immunohistochemical analysis and in situ hybridization for the Epstein-Barr viral genome. Mod Pathol. 1998;11:1252-1256. [PubMed] |
| 10. | Cohen RJ, Stanley JC, Dawkins HJ. Lymphoepithelioma-like carcinoma of the renal pelvis. Pathology. 1999;31:434-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Yamada Y, Fujimura T, Yamaguchi T, Nishimatsu H, Hirano Y, Kawamura T, Teshima S, Takeuchi T, Kitamura T. Lymphoepithelioma-like carcinoma of the renal pelvis. Int J Urol. 2007;14:1093-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Tamas EF, Nielsen ME, Schoenberg MP, Epstein JI. Lymphoepithelioma-like carcinoma of the urinary tract: a clinicopathological study of 30 pure and mixed cases. Mod Pathol. 2007;20:828-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Perez-Montiel D, Wakely PE, Hes O, Michal M, Suster S. High-grade urothelial carcinoma of the renal pelvis: clinicopathologic study of 108 cases with emphasis on unusual morphologic variants. Mod Pathol. 2006;19:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Modi H, Beckley I, Bhattarai S, Spencer J, Cartledge J. Lymphoepithelioma-like carcinoma of the renal pelvis: Pathological and therapeutic implications. Can Urol Assoc J. 2013;7:E590-E593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Lopez-Beltran A, Paner G, Blanca A, Montironi R, Tsuzuki T, Nagashima Y, Chuang SS, Win KT, Madruga L, Raspollini MR, Cheng L. Lymphoepithelioma-like carcinoma of the upper urinary tract. Virchows Arch. 2017;470:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Chen YC, Chen HW, Chueh KS, Wei YC. A rare pure lymphoepithelioma-like carcinoma of the renal pelvis mimicking upper tract urothelial carcinoma: A potential diagnostic pitfall. Kaohsiung J Med Sci. 2018;34:657-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Ahn H, Sim J, Kim H, Yi K, Han H, Chung Y, Rehman A, Paik SS. Lymphoepithelioma-like Carcinoma of the Renal Pelvis: A Case Report and Review of the Literature. Korean J Pathol. 2014;48:458-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Allende DS, Desai M, Hansel DE. Primary lymphoepithelioma-like carcinoma of the ureter. Ann Diagn Pathol. 2010;14:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Chalik YN, Wieczorek R, Grasso M. Lymphoepithelioma-like carcinoma of the ureter. J Urol. 1998;159:503-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Liu A, Cui W, Sun M, Wang H, Liu J. Lymphoepithelioma-like Carcinoma of the Renal Pelvis–A Case Report and Literature Review. OMICS J Radiol. 2014;3:167. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Ma P, Leonard T, Trussell JC. Lymphoepithelioma-like carcinoma of the ureter discovered intraoperatively during a hysterectomy. Can J Urol. 2008;15:4421-4424. [PubMed] |
| 22. | Ng KF, Chen TC, Chang PL. Lymphoepithelioma-like carcinoma of the ureter. J Urol. 1999;161:1277-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Roig JM, Amérigo J, Velasco FJ, Giménez A, Guerrero E, Soler JL, González-Cámpora R. Lymphoepithelioma-like carcinoma of ureter. Histopathology. 2001;39:106-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Terai A, Terada N, Ichioka K, Matsui Y, Yoshimura K, Wani Y. Lymphoepithelioma-like carcinoma of the ureter. Urology. 2005;66:1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Wen SC, Shen JT, Jang MY, Tsai KB, Chang SF, Tsai LJ, Wu WJ. Lymphoepithelioma-like carcinoma of ureter-a rare case report and review of the literature. Kaohsiung J Med Sci. 2012;28:509-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Haga K, Aoyagi T, Kashiwagi A, Yamashiro K, Nagamori S. Lymphoepithelioma-like carcinoma of the renal pelvis. Int J Urol. 2007;14:851-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Val-Bernal JF, González-Márquez P, Ballestero R, Zubillaga S. Primary lymphoepithelioma-like carcinoma of the ureter. Ann Diagn Pathol. 2011;15:218-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Ouzzane A, Ghoneim TP, Udo K, Verhasselt-Crinquette M, Puech P, Betrouni N, Rouprêt M, Villers A, Leroy X, Colin P. Small cell carcinoma of the upper urinary tract (UUT-SCC): report of a rare entity and systematic review of the literature. Cancer Treat Rev. 2011;37:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | David KA, Mallin K, Milowsky MI, Ritchey J, Carroll PR, Nanus DM. Surveillance of urothelial carcinoma: stage and grade migration, 1993-2005 and survival trends, 1993-2000. Cancer. 2009;115:1435-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol. 2016;70:106-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 1204] [Article Influence: 133.8] [Reference Citation Analysis (0)] |