Published online Feb 26, 2020. doi: 10.12998/wjcc.v8.i4.679
Peer-review started: September 29, 2019
First decision: November 21, 2019
Revised: December 21, 2019
Accepted: January 14, 2020
Article in press: January 14, 2020
Published online: February 26, 2020
Processing time: 150 Days and 6.8 Hours
Childhood obstructive sleep apnea hypopnea syndrome (OSAHS) is a common clinical disease that can cause serious complications if not treated in time. The preferred treatment for OSAHS in children is surgery.
To observe the effects of soft palate-pharyngoplasty on postoperative outcome, pharyngeal formation, and possible complications.
A total of 150 children with snoring, hernia, and mouth breathing were selected. A polysomnography test was performed to confirm the diagnosis of OSAHS. The children were randomly divided into experimental and control groups. The experimental group underwent adenoidectomy, tonsillectomy, and soft palate-pharyngoplasty. The control group underwent adenoidectomy and tonsillectomy. The t-test and chi-square test were used to compare conditions such as postoperative fever, postoperative hemorrhage, and pharyngeal reflux. Postoperative efficacy and complications were interrogated and observed in the form of outpatient follow-up and telephone follow-up at 6 mo and 1 year after surgery. The curative effects were divided into two groups: Cure (snoring, snoring symptoms disappeared) and non-cure.
The effective rate of the experimental group was significantly higher than that of the control group, but the difference was not statistically significant (P > 0.05). The incidence of postoperative bleeding was lower in the experimental group. There was no postoperative pharyngeal reflux in either group. In the experimental group, the incidence of hyperthermia (body temperature exceeded 38.5 °C) was lower than that in the control group. The difference in postoperative swallowing pain scores between the experimental and control groups was significant.
Soft palate-pharyngoplasty can more effectively enlarge the anteroposterior diameter and transverse diameter of the isthmus faucium. Compared with surgery alone, it can better treat OSAHS in children, improve the curative effect, reduce the risk of perioperative bleeding, close the surgical cavity, reduce the risk of postoperative infection, reduce the proportion of postoperative fever, and accelerate healing. Although this process takes more time, it is simple, safe, and effective.
Core tip: Adenoidectomy and tonsillectomy are currently the most important treatments for children with obstructive sleep apnea hypopnea syndrome. Soft palate-pharyngoplasty can more effectively enlarge the anteroposterior diameter and transverse diameter of the isthmus faucium. Compared with surgery alone, it can better treat obstructive sleep apnea hypopnea syndrome in children, improve the curative effect, reduce the risk of perioperative bleeding, close the surgical cavity, reduce the risk of postoperative infection, reduce the proportion of postoperative fever, and accelerate healing. Although this process takes more time, it is simple, safe, and effective.
- Citation: Ding XX, Zhao LQ, Cui XG, Yin Y, Yang HA. Clinical observation of soft palate-pharyngoplasty in the treatment of obstructive sleep apnea hypopnea syndrome in children. World J Clin Cases 2020; 8(4): 679-688
- URL: https://www.wjgnet.com/2307-8960/full/v8/i4/679.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i4.679
Obstructive sleep apnea hypopnea syndrome (OSAHS)[1,2] refers to a series of pathophysiological changes caused by the partial or total blockage of upper airway during sleep, disturbing the normal ventilation and sleep structure. OSAHS in children often manifests as snoring, mouth breathing, repeated wakefulness in sleep, enuresis, hyperhidrosis, which seriously affect their cardiovascular system, physical development, craniofacial development and neurological cognitive function development[3-5]. The main causes of OSAHS in children are adenoid hypertrophy, tonsil hypertrophy, and obesity[6-9]. Adenoidectomy and tonsillectomy are currently the most important treatments for children with OSAHS. Although generally considered safe and simple, the risk of tonsillectomy varies in frequency and severity. The incidence of postoperative bleeding[10,11], intraoperative infection, and delayed healing is still not low, and life-threatening postoperative bleeding events can also occur. It is necessary to pay attention to how to improve the curative effect, accurately diagnose the obstruction plane, and personalize the treatment plan, effectively expand the nasopharyngeal and oropharyngeal airway, reduce the incidence of adverse events such as postoperative bleeding, reduce the pain of children, and accelerate healing. The clinical data of 150 children with OSAHS who were hospitalized in our hospital from October 2016 to December 2017 are summarized.
In total, 150 children from our hospital from October 2016 to June 2018 were collected. These children were diagnosed with OSAHS by sleep respiration monitoring[12] (Alice 6), and were hospitalized for surgical treatment under general anesthesia. All children were 3-10 years old, with an average age of 5.9 years; there were 86 males and 64 females with a treatment course of 2 to 6 years (Table 1). The children had different degrees of the following symptoms: Snoring during sleep, suffocation, mouth breathing, double nasal congestion, night awakening, inattention, memory loss, and recurrent tonsillitis. Three dimensional computed tomography (CT) of the respiratory tract was done in the patients as a routine preoperative examination to obtain the ratio A/N (A is the thickness of the adenoid, i.e. the vertical distance from the most prominent point of the lower edge of the adenoid to the tangential line outside the occipital slope of the occipital slope; N is the thickness of the nasal cavity, i.e. the distance from the upper end of the hard palate to the connection point between the root of the wing plate and extracranial aspect of the occipital slope). The diagnostic criteria for adenoid pathological hypertrophy are A/N ≥ 0.71 with clinical symptoms. According to the horizontal position CT, the tonsil size and distances were evaluated. Those who met the inclusion criteria and obtained parental consent were treated with tonsillectomy.
| Informaton | Experiment | Control | S | χ2 | P value |
| Gender | |||||
| M | 41 (54.7) | 45 (60) | 0.44 | 0.51 | |
| F | 34 (45.3) | 30 (40) | |||
| Age | 6.1 | 5.6 | 0.76 | ||
| M | 5.2 | 5.8 | 2.14 | ||
| F | 7.1 | 5.5 | 2.05 | ||
| Diagnosis | |||||
| OSAHS | 75 (100) | 75 (100) | |||
| Chronic tonsillitis | 43 (57.3) | 39 (52) | 0.43 | 0.51 | |
| Others | 69 (92) | 71 (94.7) | 0.42 | 0.51 | |
| Complications | |||||
| Asthma | 4 (5.3) | 3 (4) | 0.15 | 0.70 | |
| Allergic rhinitis | 8 (10.7) | 6 (8) | 0.35 | 0.58 | |
| Obesity | 9 (12) | 7 (9.3) | 0.28 | 0.60 | |
| Hearing loss | 4 (5.3) | 2 (2.7) | 0.69 | 0.41 | |
Diagnostic criteria for OSAHS in children: OSAH index ≥ 1 time/h; and hypoxemia, defined as the lowest arterial oxygen saturation below 0.92[13-17].
Inclusion criteria: Children diagnosed with OSAHS; patients with chronic tonsillitis; and patients with double tonsils more than III degree.
Exclusion criteria: Patients who refused to undergo tonsillectomy; patients with partial tonsillectomy; and patients with OSAH index < 1 time/h.
Tonsil size degree: The tonsil size was divided into I-IV degrees[18]. Degree I: Confined to the tonsil socket; degree II: Protruding from the lingual arch and occupying 1/2 of the pharyngeal cavity; degree III: Protruding from the tonsil socket, occupying 3/4 of the pharyngeal cavity; and degree IV: The tonsils on both sides were almost opposite, blocking the pharyngeal cavity.
Diagnostic criteria for chronic tonsillitis: A diagnosis of chronic tonsillitis can be achieved by any of the following criteria: Occurs more than 7 times a year; occurs more than 5 times a year for more than 2 years; and occurs more than 3 times a year for more than 3 years[19].
The children were randomly divided into an experimental group and control group. The patients in the experimental group underwent soft palate-pharyngoplasty after resection of the tonsils. The control group was exposed after the tonsils were removed.
Stryker nasal endoscope (child 70°), Image Pickup System and Surgical Power System for Electric Cutting Drill.
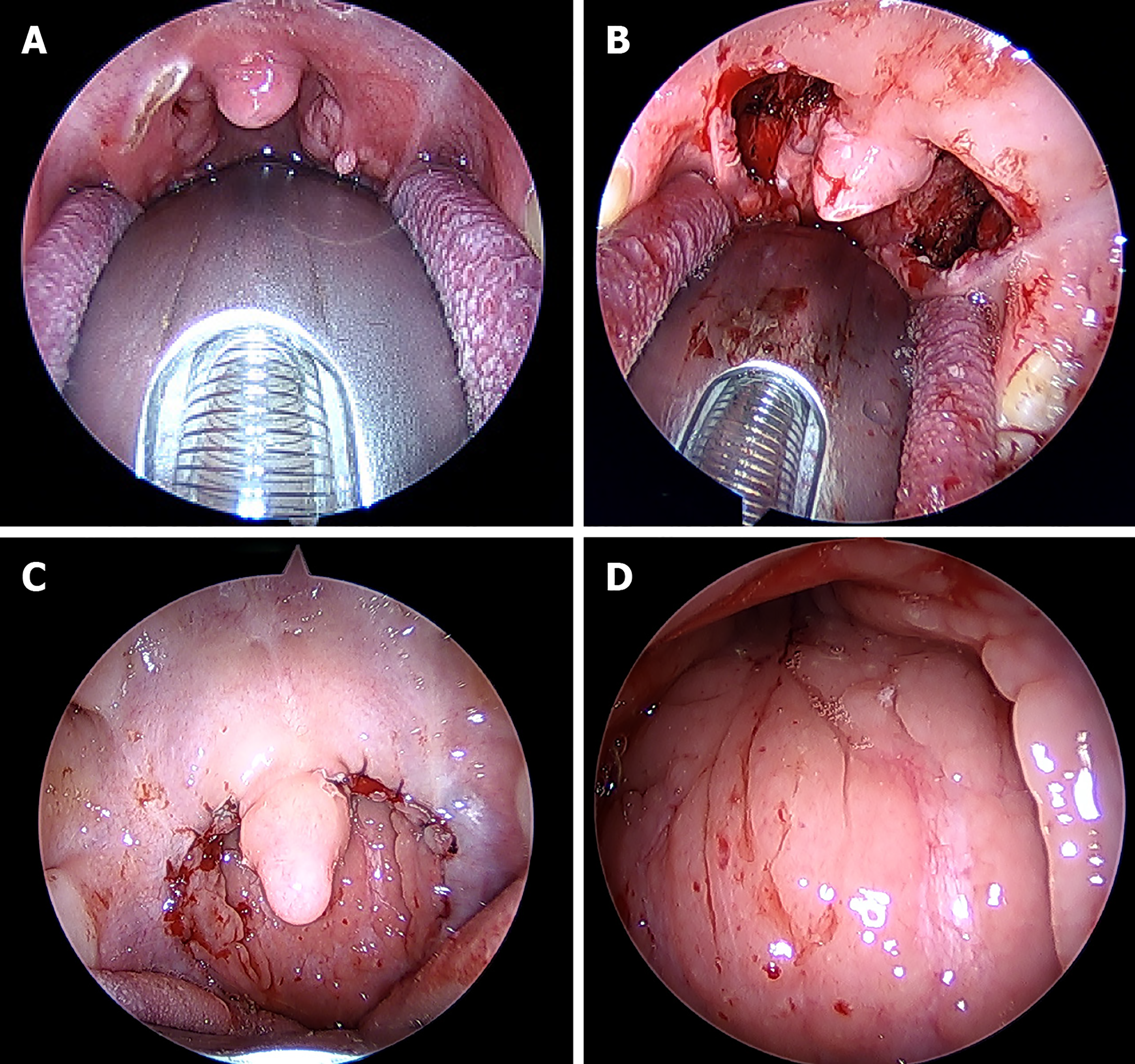
General anesthesia was performed in all cases, and the oropharynx was exposed with a Davis mouthpiece (Table 2). For soft palate-pharyngeal angioplasty, the anterior arch was cut with an electric knife to expose the upper pole of the tonsil. The tonsils were pulled inward with the Alice forceps, and then completely removed along the tonsil capsule. After complete resection, the tonsil socket was observed; electrocoagulation was performed with an electric knife until there was no bleeding in the operation area. The incision was sutured with absorbable thread, the posterior arch mucosa was sutured to the upper side, and the sutured soft palate was sutured to complete the soft palate-pharynx (Figure 1). For adenoid surgery, two thin catheters were sutured by soft palate through the nose, and the nasopharynx was directly viewed through a 70° nasal endoscope. Some of the posterior nostrils were obstructed by the hypertrophic adenoids, bilateral torus tubariusta and ostium pharyngeum tubaeauditivae were also squeezed. Adenoids were completely removed with the power system and then bleeding was stopped. Nasopharyngeal surgical wounds were observed until there was no bleeding or residual.
| Experiment | Control | χ2 | P value | |
| Anesthesia | ||||
| Inhalation | 73 (97.3) | 74 (98.7) | 0.34 | 0.56 |
| Intravenous injection | 2 (2.7) | 1 (1.3) | ||
| Surgical procedure | ||||
| Tonsillectomy and adenoidectomy | 71 (94.7) | 72 (96) | 0.34 | 0.56 |
| Tonsillectomy | 4 (5.3) | 3 (4) | ||
| Surgery duration (min) | ||||
| 20-30 | 48 (64) | 55 (73.3) | ||
| 30-40 | 24 (32) | 19 (25.3) | ||
| 40-50 | 2 (2.7) | 0 (0) | ||
| 50-60 | 1 (1.3) | 0 (0) | ||
Soft food was started 6 h after surgery, and antibiotics and mouthwashes were used as appropriate after surgery. Patients were discharged from the hospital 2 to 3 d after surgery and followed up in the clinic from 6 to 12 mo.
In the cure group, sleep snoring basically disappeared, there was no mouth breathing and sleep apnea, and the patient was able to sleep quietly. In the non-cure group, snoring and mouth breathing symptoms were reduced or there were no significant changes.
Postoperative fever, postoperative bleeding, and velopharyngeal incompetence.
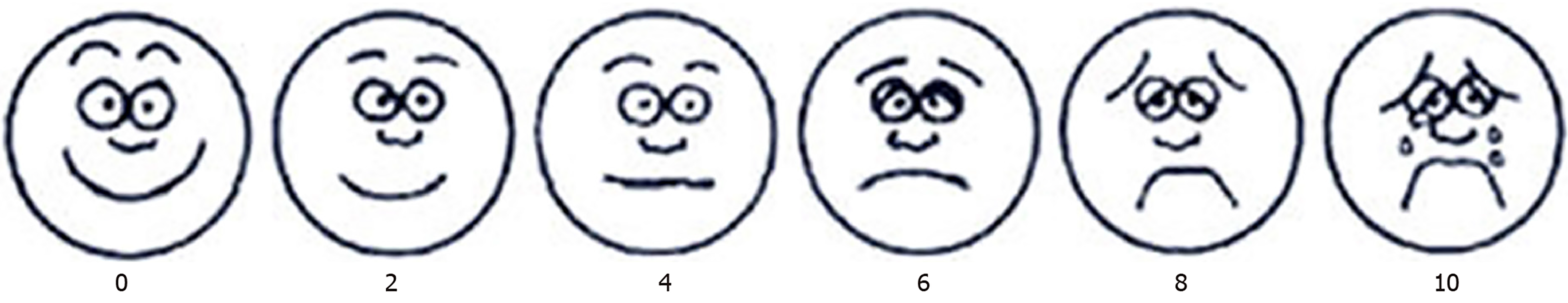
The Wong-Baker facial expression scale (Figure 2)[20] was used. The method uses six facial expressions, from smile, frowning to crying, to indicate different degrees of pain. The 10-point scale was used, and the degree of pain was proportional to the score. A score of 0 indicates no pain, 2 indicates a slight pain, 4 indicates mild pain, 6 indicates moderate pain, 8 indicates severe pain, and 10 indicates severe pain. The child indicated which expression best represented the degree of pain. The more the expression to the left, the lighter the pain; the more the expression to the right, the higher the pain. Postoperative pain was recorded continuously for 1 wk.
SPSS 17.0 statistical software was used, and the statistical methods were independent sample t-test and chi-square test, Statistical comparisons were made between the different groups of patients and controls by the χ2 test calculated on 2 × 2 contingency tables. The Fisher's exact test was used when expected cell values were less than 5. A P value less than 0.05 was considered statistically significant.
In the experimental group, 71 cases (94.7%) were cured and 4 cases were not cured (5.3%); in the control group, 68 cases (90.7%) were cured and 7 cases (9.3%) were not cured. The effective rate of the experimental group was significantly higher than that of the control group, but the difference was not statistically significant (P > 0.05, Table 3).
| Experiment | Control | χ2 | P value | |
| Cure | 71 (94.7) | 68 (90.7) | 0.883 | 0.347 |
| Non-cure | 4 (5.3) | 7 (9.3) |
Postoperative bleeding: There were four cases of postoperative bleeding in the control group, which appeared 5-10 d after surgery; and 0 cases in the experimental group (Table 4).
| Experiment (cases) | Congrol (cases) | χ2 | P value | |
| Hyperthermia | ||||
| > 38.5 | 1 | 8 | 5.79 | 0.02 |
| < 38.5 | 74 | 67 | ||
| Postoperative bleeding | ||||
| Male | 0 | 3 | 4.11 | 0.04 |
| Age | 6 yr | |||
| 7 yr | ||||
| 9 yr | ||||
| Female | 0 | 1 | ||
| 10 yr | ||||
| Velopharyngeal incompetence | 0 | 0 | ||
There were no symptoms of velopharyngeal incompetence in both groups.
In the experimental group, there was one case of hyperthermia (body temperature exceeded 38.5 °C), and eight cases of postoperative hyperthermia in the control group. The difference was statistically significant.
The postoperative swallowing pain scores of the experimental group and the control group were as follows: Postoperative day 1: 8 ± 1.6 and 6.9 ± 2.0; postoperative day 2: 7.1 ± 1.4 and 6.1 ± 1.9; postoperative day 3 : 5.5 ± 1.9 and 4.8 ± 1.6; postoperative day 4: 4.5 ± 2.1 and 3.2 ± 1.4; postoperative day 5: 3.6 ± 1.8 and 2.6 ± 1.4; postoperative day 6: 3.4 ± 1.4 and 2 ± 1.3, P < 0.05, the difference was statistically significant (Table 5).
| Days after surgery (d) | Control | Experiment | T | P value |
| 1 | 8 ± 1.6 | 6.9 ± 2.0 | 4.27 | < 0.05 |
| 2 | 7.1 ± 1.4 | 6.1 ± 1.9 | 4.90 | < 0.05 |
| 3 | 5.5 ± 1.9 | 4.8 ± 1.6 | 3.67 | < 0.05 |
| 4 | 4.5 ± 2.1 | 3.2 ± 1.4 | 5.64 | < 0.05 |
| 5 | 3.6 ± 1.8 | 2.6 ± 1.4 | 4.26 | < 0.05 |
| 6 | 3.4 ± 1.4 | 2 ± 1.3 | 7.29 | < 0.05 |
OSAHS refers to a series of pathophysiological changes caused by the partial or total blockage of upper airway during sleep, disturbing the normal ventilation and sleep structure. The incidence of OSAHS in children is 1%-5%, and the peak age of onset is 2-5 years old[21]. Children can be characterized by snoring, mouth breathing, hernia, bedwetting, restless sleep, and daytime sleepiness. Long-term mouth breathing can affect the development of the craniofacial region. In severe cases, it may even lead to growth retardation, decreased learning ability, and decreased memory[22,23]. Early diagnosis and early treatment are extremely important for children with OSAHS. The tonsils and adenoids are immune organs that are part of the pharyngeal lymphatic ring, and have protective effects on the body. However, hypertrophic tonsils and/or adenoids can cause upper airway stenosis, causing recurrent episodes of upper respiratory tract infection, leading to a series of pathological changes, which are important causes of OSHAS in children and play an important role in airway obstruction in children with OSAHS. Tonsillectomy and adenoidectomy are the preferred methods for relieving respiratory obstruction and treating OSAHS in children[24,25].
Surgery can quickly and effectively improve the symptoms of sleep snoring, improve the quality of sleep, and reduce the risk of OSAHS complications. The efficiency of adenoidectomy and tonsillectomy is different. How to improve the curative effect and maximize the benefits? In our study, the transverse diameter, anteroposterior diameter, and pharyngeal distance of the isthmus faucium in patients underwent soft palate-pharyngoplasty were significantly larger than those underwent tonsillectomy alone, which was more conducive to the relief of symptoms such as sleep snoring. After soft palate-pharyngoplasty, the reinforcement of the lateral pharyngeal wall and the closure of the wound surface are beneficial for children early eating. The swallowing action reduces the scar formation in the surgical cavity, and the scar formation is relatively small, which can improve the postoperative long-term discomfort. The cure rate of the experimental group was higher than that of the control group. Although there was no significant difference in the cure rate between the two groups, the patient did not receive sleep monitoring after the operation, and only relied on the family description of the child to interpret the recovery effect, which may affect the results. Besides, asthma attacks, allergic rhinitis and other attacks may affect the judgment of the cure effect, so the cure rate maybe deviated from the actual situation. Furthermore, soft palate-pharyngoplasty is beneficial to maintain the shape of soft palate. Although the difference in cure rate is not statistically significant in the near future, it is considered in the long-term that soft palate after suture has certain clinical significance for preventing soft palate relaxation and restenosis.
It has been reported that the bleeding rate after tonsillectomy is 2.1% to 12%[26-29], and the incidence of hemostasis requiring secondary surgery is 1.2% to 6%[30-32]. Chronic tonsillitis, age, and attention deficit/hyperactivity disorder are risk factors for postoperative bleeding in tonsillectomy[33]. Reliable hemostasis was performed after removal of the tonsils during surgery. However, after operation, due to pain, children may cry, shout, vomit, cough, or feel nausea, causing bleeding or even active bleeding on the wound surface. The soft palate-pharyngoplasty may seal off the wound so that it is no longer exposed to the mouth and strengthen the lateral pharyngeal wall, greatly reducing the risk of postoperative bleeding. Moreover, after the wound is sealed off, the area of tunica albuginea after surgery is reduced, and the probability of bleeding after premature detachment of the tunica albuginea due to food friction is also reduced. In the study, there was no significant difference in the incidence of chronic tonsillitis between the experimental group and the control group. In the control group, four patients developed postoperative bleeding, aged 7 years, 9 years, 6 years, and 10 years, two of them had chronic tonsillitis, and two had no history of tonsillitis; the 9-year-old patient returned to the operating room for hemostasis treatment, and the bleeding symptoms of the other three patients were controlled after compression and hemostasis. All bleeding occurred about 1 wk after surgery. In the experimental group, there was no bleeding after tonsillectomy. There was a statistically significant difference in the incidence of bleeding between the two groups, indicating that the closure of the surgery can greatly reduce the incidence of bleeding after tonsillectomy.
In the study, eight cases of postoperative hyperthermia occurred in the control group, and one case in the experimental group. The difference was statistically significant. After soft palate-pharyngoplasty, only a small amount of tunica albuginea was formed around the incision in the closed operation cavity, and the area of tunica albugine was significantly reduced compared with the control group, which reduced the postoperative inflammatory reaction and reduced the probability of fever. At the same time, the possibility of infectious fever due to poor formation of the tunica albuginea is also reduced.
Many studies have found that postoperative pain in children is often underestimated; compared with adult patients, postoperative analgesia is reported to be inadequate[34,35]. Dehydration and inadequate nutrient intake may be responsible for the increased pain after tonsillectomy[36-38]. After tonsillectomy, the mucous membrane was torn, the glossopharynx and the vagus nerve were stimulated, which caused pain. Tissues have local edema, inflammatory cell exudation and other symptoms after trauma, which leads to a large release of pain-causing substances, significantly increases the sensitivity of nerve endings receptors and causes local pain. The contraction of the pharyngeal muscles during swallowing causes compression of the tonsil fossa wounds, which in turn makes the pain significantly worse. The study found that the pain level and duration of pain in the experimental group were all less than the control group, which shows that the suture treatment of the surgery can relieve pain to some extent. The suture treatment of the surgical cavity can reduce the degree of wound exposure, and the nerve endings are wrapped in the wound surface, and the degree of swallowing stimulation generated by the child when eating is obviously reduced. And the wound after suturing is no longer exposed in the mouth, which is beneficial to the children to eat granular food, so as to avoid malnutrition caused by eating mainly liquid food.
In adult OSAHS patients, some people have velopharyngeal insufficiency after uvulopalatopharyngoplasty. However, there was almost no velopharyngeal insufficiency after adenoidectomy in children. In the study, no patients had a velopharyngeal insufficiency or an open nasal sound, either in the control group or the experimental group. At the same time, all patients had no complications of velopharyngeal insufficiency after suture, indicating that the application of soft palate-pharyngoplasty in children with OSAHS is safe[39].
For children with OSAHS, soft palate-pharyngoplasty and adenoidectomy can more effectively enlarge the airway, so snoring and mouth breathing symptoms are better after surgery. Although palate-pharyngoplasty will prolong the operation time, it can maximize the upper airway, improve the surgical cure rate, reduce the risk of bleeding, shorten the pain duration of children, and accelerate healing.
Childhood obstructive sleep apnea hypopnea syndrome (OSAHS) is a common clinical disease that can cause serious complications if not treated in time. The preferred treatment for OSAHS in children is surgery. In order to improve the postoperative outcome of children with OSAHS, and improve the safety of operation,this study made a clinical observation of the effect of soft palate-pharyngoplasty on postoperative outcome, pharyngeal formation and possible complications.
For children with OSAHS, soft palate-pharyngoplasty and adenoidectomy can more effectively enlarge the airway, so snoring and mouth breathing symptoms are better after surgery. Although generally considered safe and simple, the risk of tonsillectomy varies in frequency and severity. The incidence of postoperative bleeding, intraoperative infection, and delayed healing is still not low, and life-threatening postoperative bleeding events have also occurred. How to maximize the upper airway, improve the surgical cure rate, reduce the risk of bleeding, shorten the pain duration of children, and accelerate healing, is worth discussing.
These 150 children have different degrees of the following symptoms: Snoring during sleep, suffocation, mouth breathing, double nasal congestion, night awakening, inattention, memory loss, recurrent tonsillitis, etc. The child patients were given a 3D-computed tomography of the respiratory tract and were diagnosed as OSAHS by sleep respiration monitoring, and were hospitalized for surgical treatment under general anesthesia.
The children were randomly divided into experimental and control groups. The experimental group underwent adenoidectomy, tonsillectomy, and soft palate-pharyngoplasty. The control group underwent adenoidectomy and tonsillectomy. The t-test and χ2 test were used to compare conditions such as postoperative fever, postoperative hemorrhage, and pharyngeal reflux. Postoperative efficacy and complications were interrogated and observed in the form of outpatient follow-up and telephone follow-up at 6 mo and 1 year after surgery. The curative effect was divided into two groups, cure (snoring, snoring symptoms disappeared) and non-cure.
In the experimental group, 71 cases were cured (94.7%), 4 cases (5.3%) were not cured; in the control group 68 cases (90.7%) were cured, and 7 cases (9.3%) were not cured. The effective rate of the experimental group was significantly higher than that of the control group, but the difference was not statistically significant. Four cases of postoperative bleeding occurred in the control group, which occurred 5-10 d after surgery. There were 0 cases of postoperative bleeding in the experimental group. There was no postoperative pharyngeal reflux in either group. In the experiment group, 1 case of hyperthermia occured, and 8 cases occurred in the control group. The difference was statistically significant. The postoperative swallowing pain scores of the experiment group and the control group was statistically significant.
Soft palate-pharyngoplasty can more effectively enlarge the anteroposterior diameter and transverse diameter of the isthmus faucium. Compared with surgery alone, it can better treat OSAHS in children, improve the curative effect, reduce the risk of perioperative bleeding, close the surgical cavity, reduce the risk of postoperative infection, reduce the proportion of postoperative fever, and accelerate healing. Although this process takes more time, it is simple, safe and effective.And it is worthy of clinical promotion
We thank all the medical staff who agreed to participate in this study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Guerin A, Treepongkaruna S S-Editor: Wang JL L-Editor: Filipodia E-Editor: Liu JH
| 1. | Rosen CL, Larkin EK, Kirchner HL, Emancipator JL, Bivins SF, Surovec SA, Martin RJ, Redline S. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 479] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 2. | Allareddy V, Martinez-Schlurmann N, Rampa S, Nalliah RP, Lidsky KB, Allareddy V, Rotta AT. Predictors of Complications of Tonsillectomy With or Without Adenoidectomy in Hospitalized Children and Adolescents in the United States, 2001-2010: A Population-Based Study. Clin Pediatr (Phila). 2016;55:593-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Amin RS, Carroll JL, Jeffries JL, Grone C, Bean JA, Chini B, Bokulic R, Daniels SR. Twenty-four-hour ambulatory blood pressure in children with sleep-disordered breathing. Am J Respir Crit Care Med. 2004;169:950-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 245] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 4. | Amin RS, Kimball TR, Bean JA, Jeffries JL, Willging JP, Cotton RT, Witt SA, Glascock BJ, Daniels SR. Left ventricular hypertrophy and abnormal ventricular geometry in children and adolescents with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1395-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 291] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 5. | Nieminen P, Löppönen T, Tolonen U, Lanning P, Knip M, Löppönen H. Growth and biochemical markers of growth in children with snoring and obstructive sleep apnea. Pediatrics. 2002;109:e55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 144] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Rosen CL, Larkin ME, Clark AK, O’Malia B, Graham G, Redline S. Persistence of sleep disordered breathing in children post tonsillectomy. Respir Crit Care Med. 2001;163:A184. |
| 7. | Mitchell RB, Kelly J. Adenotonsillectomy for obstructive sleep apnea in obese children. Otolaryngol Head Neck Surg. 2004;131:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Gorman D, Ogston S, Hussain SS. Improvement in symptoms of obstructive sleep apnoea in children following tonsillectomy versus tonsillotomy: a systematic review and meta-analysis. Clin Otolaryngol. 2017;42:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Zhang F, Tian Z, Peng S, Li J, Yang X, Mo H, Tan J, Yao H, Li B. Exposure to intermittent hypoxia impairs learning and memory ability in rats. Sleep Biol Rhythms. 2018;16:331-336. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Bennger M, Walner D. Coblatiaon: improving outcomes for children following adenotcoms for adenotonsillectom. Clin Cornerstone. 2007;9 Suppl 1:S13-23. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Liu JH, Anderson KE, Willging JP, Myer CM, Shott SR, Bratcher GO, Cotton RT. Posttonsillectomy hemorrhage: what is it and what should be recorded? Arch Otolaryngol Head Neck Surg. 2001;127:1271-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Riva T, Tanya E, Jyoti KR. Persistence of Obstructive Sleep Apnea Syndrome in Children After Adenotonsillectomy. J Pediatrics. 2006;12:804 808. |
| 13. | Marcus CL, Omlin KJ, Basinki DJ, Bailey SL, Rachal AB, Von Pechmann WS, Keens TG, Ward SL. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis. 1992;146:1235-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 524] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 14. | Montgomery-Downs HE, O'Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117:741-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 348] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 15. | Standards and indications for cardiopulmonary sleep studies in children. American Thoracic Society. Am J Respir Crit Care Med. 1996;153:866-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 817] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 16. | Berry RB, Budhiraja R. Rules for scoring respiratory events in sleep :update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med. 2012;8:597-619. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2718] [Cited by in RCA: 3724] [Article Influence: 286.5] [Reference Citation Analysis (0)] |
| 17. | Aurora RN, Zak RS, Karippot A, Lamm CI, Morgenthaler TI, Auerbach SH, Bista SR, Casey KR, Chowdhuri S, Kristo DA, Ramar K; American Academy of Sleep Medicine. Practice parameters for the respiratory indications for polysomnography in children. Sleep. 2011;34:379-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 267] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 18. | Liner LH, Marcus CL. Ventilatory management of sleep-disordered breathing in children. Curr Opin Pediatr. 2006;18:272-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Mitchell RB, Archer SM, Ishman SL, Rosenfeld RM, Coles S, Finestone SA, Friedman NR, Giordano T, Hildrew DM, Kim TW, Lloyd RM, Parikh SR, Shulman ST, Walner DL, Walsh SA, Nnacheta LC. Clinical Practice Guideline: Tonsillectomy in Children (Update). Otolaryngol Head Neck Surg. 2019;160:S1-S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 308] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 20. | Wong DL, Baker CM. Pain in children:conlparison of assessment scales. Pediatr Nurs. 1988;4:9-17. |
| 21. | Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 969] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 22. | Davies CR, Harrington JJ. Impact of Obstructive Sleep Apnea on Neurocognitive Function and Impact of Continuous Positive Air Pressure. Sleep Med Clin. 2016;11:287-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Liu JF, Tsai CM, Su MC. Application of desaturation index in post-surgery follow-up in children with obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2016;17:156–157. |
| 24. | Baugh RF, Archer SM, Rosenfeld RM. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144:S1eS30. |
| 25. | Hill CA, Litvak A, Canapari C, Cummings B, Collins C, Keamy DG, Ferris TG, Hartnick CJ. A pilot study to identify pre- and peri-operative risk factors for airway complications following adenotonsillectomy for treatment of severe pediatric OSA. Int J Pediatr Otorhinolaryngol. 2011;75:1385-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 26. | Francis DO, Fonnesbeck C, Sathe N, McPheeters M, Krishnaswami S, Chinnadurai S. Postoperative Bleeding and Associated Utilization following Tonsillectomy in Children. Otolaryngol Head Neck Surg. 2017;156:442-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Marcus CL, Moore RH, Rosen CL. A randomized trial of adenotonsillectomy for childhood sleep apnea. Otolaryngol Head Neck Surg. 2013;368:2366–2376. |
| 28. | Weinstock TG, Rosen CL, Marcus CL, Garetz S, Mitchell RB, Amin R, Paruthi S, Katz E, Arens R, Weng J, Ross K, Chervin RD, Ellenberg S, Wang R, Redline S. Predictors of obstructive sleep apnea severity in adenotonsillectomy candidates. Sleep. 2014;37:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 29. | Willging JP, Wiatrak BJ. Harmonic scalpel tonsillectomy in children: a randomized prospective study. Otolaryngol Head Neck Surg. 2003;128:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Bhattacharyya N, Shapiro NL. Associations between socioeconomic status and race with complications after tonsillectomy in children. Otolaryngol Head Neck Surg. 2014;151:1055-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 31. | Duval M, Wilkes J, Korgenski K, Srivastava R, Meier J. Causes, costs, and risk factors for unplanned return visits after adenotonsillectomy in children. Int J Pediatr Otorhinolaryngol. 2015;79:1640-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Mueller J, Boeger D, Buentzel J, Esser D, Hoffmann K, Jecker P, Mueller A, Radtke G, Geißler K, Bitter T, Guntinas-Lichius O. Population-based analysis of tonsil surgery and postoperative hemorrhage. Eur Arch Otorhinolaryngol. 2015;272:3769-3777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Spektor Z, Saint-Victor S, Kay DJ, Mandell DL. Risk factors for pediatric post-tonsillectomy hemorrhage. Int J Pediatr Otorhinolaryngol. 2016;84:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Ali S, Chambers A, Johnson DW, Newton AS, Vandermeer B, Williamson J, Curtis SJ. Reported practice variation in pediatric pain management: a survey of Canadian pediatric emergency physicians. CJEM. 2014;16:352-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Ferrante P, Cuttini M, Zangardi T, Tomasello C, Messi G, Pirozzi N, Losacco V, Piga S, Benini F; the PIPER Study Group. Pain management policies and practices in pediatric emergency care: A nationwide survey of Italian hospitals. BMC Pediatr. 2013;13:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Ericsson E. Health and Well-being of Children and Young Adults in Relation to Surgery of the Tonsils. Doctorial Thesis, Linko¨ping University, 2007. Available from: www.diva-portal.org/smash/get/diva2:23621/FULLTEXT01.pdf. |
| 37. | Schmidt R, Herzog A, Cook S, O'Reilly R, Deutsch E, Reilly J. Complications of tonsillectomy: a comparison of techniques. Arch Otolaryngol Head Neck Surg. 2007;133:925-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Acevedo JL, Shah RK, Brietzke SE. Systematic review of complications of tonsillotomy versus tonsillectomy. Otolaryngol Head Neck Surg. 2012;146:871-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Chiu PH, Ramar K, Chen KC, Tsai YJ, Lin CM, Chiang YC, Lu CY, Chiang RP. Can pillar suturing promote efficacy of adenotonsillectomy for pediatric OSAS? A prospective randomized controlled trial. Laryngoscope. 2013;123:2573-2577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |