Published online Feb 6, 2020. doi: 10.12998/wjcc.v8.i3.614
Peer-review started: November 21, 2019
First decision: December 23, 2019
Revised: December 25, 2019
Accepted: January 2, 2020
Article in press: January 2, 2020
Published online: February 6, 2020
Processing time: 76 Days and 20 Hours
Erdheim-Chester disease (ECD) is a rare multi-system or multi-organ histiocytic proliferative disease with diverse clinical manifestations, and the development of the disease is complex, which makes clinical diagnosis and treatment difficult. The characteristic clinical manifestations include multi-organ involvement, especially in the symmetrical diaphysis and metaphysis of the bilateral extremities. ECD with a unilateral talus lesion is extremely rare. Here, we report an unusual case of ECD invading the asymmetric talus and tibia without involving other organs. The patient had good outcome after surgery.
We report a case of a 67-year-old man who was referred to our outpatient department because of left ankle chronic pain for 5 years, which exacerbated after a foot sprain 6 mo previously. We discovered multiple sclerotic lesions of the tibia and talus on his previous X-ray films, which were initially missed in a local hospital. Therefore, enhanced computer computed tomography (CT) and magnetic resonance imaging were performed. These examinations showed multiple lesions in the bone marrow cavity of the left tibia, and cortical sclerosis and osteonecrosis of the left talus. Specimens were collected via bone puncture from the two lesions, and a final diagnosis of ECD was confirmed by pathological and immunohistochemical examinations. In addition, other auxiliary examinations including head CT, pulmonary CT, spinal CT, abdominal CT, cardiac ultrasound and thyroid ultrasound showed no obvious abnormalities. The patient underwent surgery for the tibia lesion scraping and talus lesion scraping combined with cement casting. The patient started on a progressive rehabilitation at 4 wk, and felt no pain after surgery. During a 2-year follow-up period, the patient exercised normally without pain, and there were no signs of recurrence.
This study shows that surgery treatment may also achieve good results for ECD patients with only bone involvement.
Core tip: Erdheim-Chester disease (ECD) is a subtype of non-Langerhans’s cell histiocytosis, for which diagnosis is difficult and no treatment guidelines have been available. In addition, ECD is a systemic disease with multi-organ involvement, and especially affects the symmetrical diaphysis and metaphysis of the bilateral extremities. We report the first case of ECD invading the unilateral tibia and talus without involvement of any other organs. The patient was treated with surgery, and had a good prognosis, which added evidence to the diagnosis and treatment of this disease.
- Citation: Xia Q, Tao C, Zhu KW, Zhong WY, Li PL, Jiang Y, Mao MZ. Erdheim-Chester disease with asymmetric talus involvement: A case report. World J Clin Cases 2020; 8(3): 614-623
- URL: https://www.wjgnet.com/2307-8960/full/v8/i3/614.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i3.614
Erdheim-Chester disease (ECD) is a subtype of non-Langerhans’s cell histiocytosis with multi-system invasion and poor prognosis, which is now thought to be an inflammatory histiocytic disease caused by oncogene mutations[1-5]. Studies have shown that almost half of the patients with ECD have the BRAFV600E mutation, and this oncogene mutation activates the mitogen-activated protein kinase pathway, which is involved in the histiocytosis pathogenesis of inflammation and fibrosis[3,4,6,7]. In addition, typical histopathology revealed activated fibro-inflammatory histiocytes and Touton giant cells. This disease mainly occurs in adults between the fourth and seventh decades of life, with a slight male predominance[6,8].
Due to its multiple clinical manifestations and lack of specificity, diagnosis of ECD is difficult. ECD can potentially occur in all organs of the body with multi-system involvement, most commonly affecting bones, the central nervous system, cardiovascular system, lungs and retroperitoneal cavities[9]. Bone pain is the most common clinical presentation, and other frequent extra-skeletal presentations include periaortic infiltration as “coated aorta” in the cardiovascular system, and perinephric fat infiltration as “hairy kidney” in the urinary system. Therefore, it may become a fatal illness when extensive central nervous, pulmonary and other systems are affected[10].
The characteristic X-ray presentation of ECD is the symmetrical osteosclerosis of the extremities, and generally on the diaphysis of appendicular long bones. In addition to typical osteosclerosis, it may also be associated with partial epiphysis sclerosis, periostitis, and even bone infarction[11]. The disease rarely involves only bone or unilateral limbs, and most patients with bone involvement are accompanied by extra-bone lesions. In addition, asymmetric talus and tibia involvement without other organs affected has not been reported in the literature.
There are few prospective studies or randomized controlled clinical studies on ECD, so the treatments are mainly based on case reports or empirical summaries, and there is still a lack of high-level evidence. In general, except for a small number of asymptomatic patients, all patients should start treatment immediately after the disease is found rather than observe, and many therapies have been used for this disease. Currently, through in-depth studies of the pathogenesis of ECD, treatments such as interferon-α recommended as the first-line therapy, anakinra (IL-1 receptor antagonist), vemurafenib (specific BARF inhibitor) and cobimetinib (specific MEK inhibitor) have been proven to have various efficacies[4,12-18]. Besides, surgical treatment can also achieve good results[19-21]. However, there is still a lack of standard guidelines for the surgical treatment of focal bone lesions with ECD involvement. We aim to share our experience in managing a case of ECD with asymmetric bone involvement, and suggest the feasibility of surgery.
A 67-year-old man complained of left ankle pain with slight limitation of motion.
The patient had a 5-year history of left ankle chronic pain, which exacerbated after a foot sprain 6 mo previously. The patient suffered from pain in his left ankle during walking. He noted some swelling in the left medial malleolus region but no fever, chills or loss of weight.
His past history was unremarkable.
His family history was unremarkable.
Physical examination showed swelling in his left medial malleolus region with tenderness. He presented with pain and weakness in eversion.
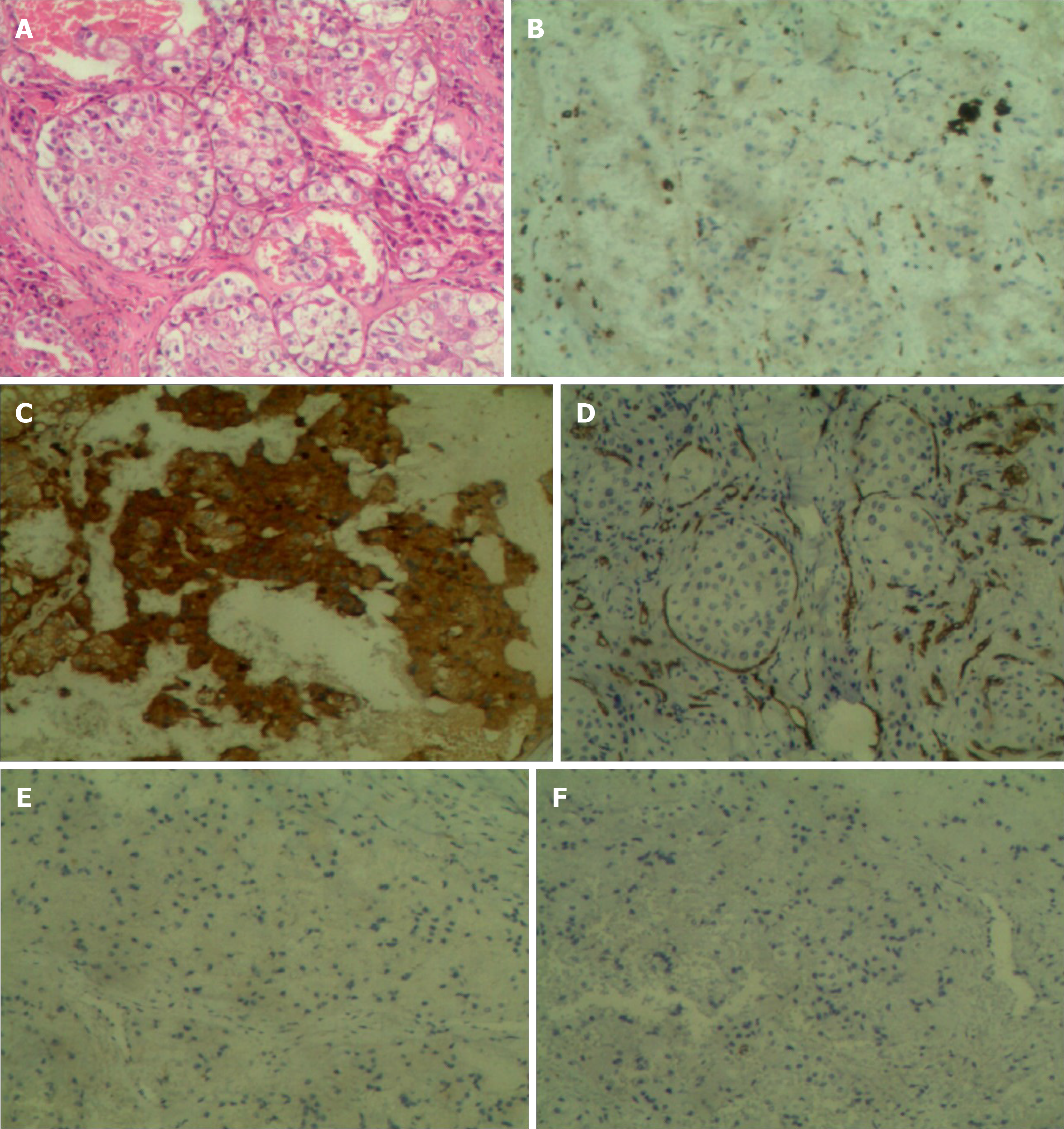
The laboratory tests showed a white blood cell count of 6.09 × 109/L, C-reactive protein of 1.64 mg/L and erythrocyte sedimentation rate of 2.00 mm/h. Biopsies were obtained from the affected tibia and talus for pathological and immunohistochemical examinations. Microscopy revealed foamy histiocytes filled with lipids and granulomatous inflammation containing fibrous tissue, lymphoid tissue, plasma cells and Touton giant cells. Immunohistochemical examination showed positive expression of CD68 and AACT, but negative expression of CD34, S100 and CD1a (Figure 1). In addition, molecular pathologic examination was negative for the BRAFV600E mutation.
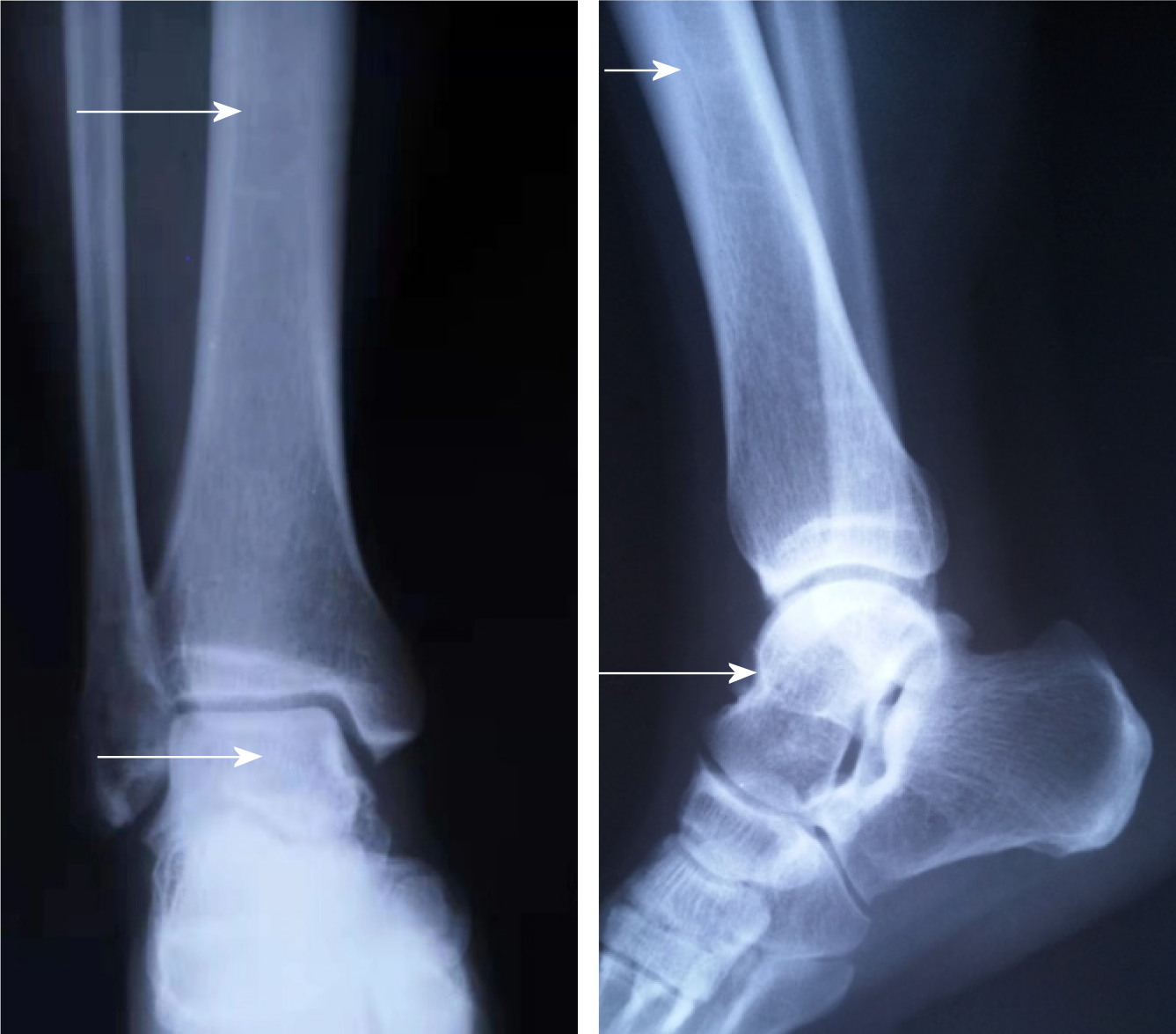
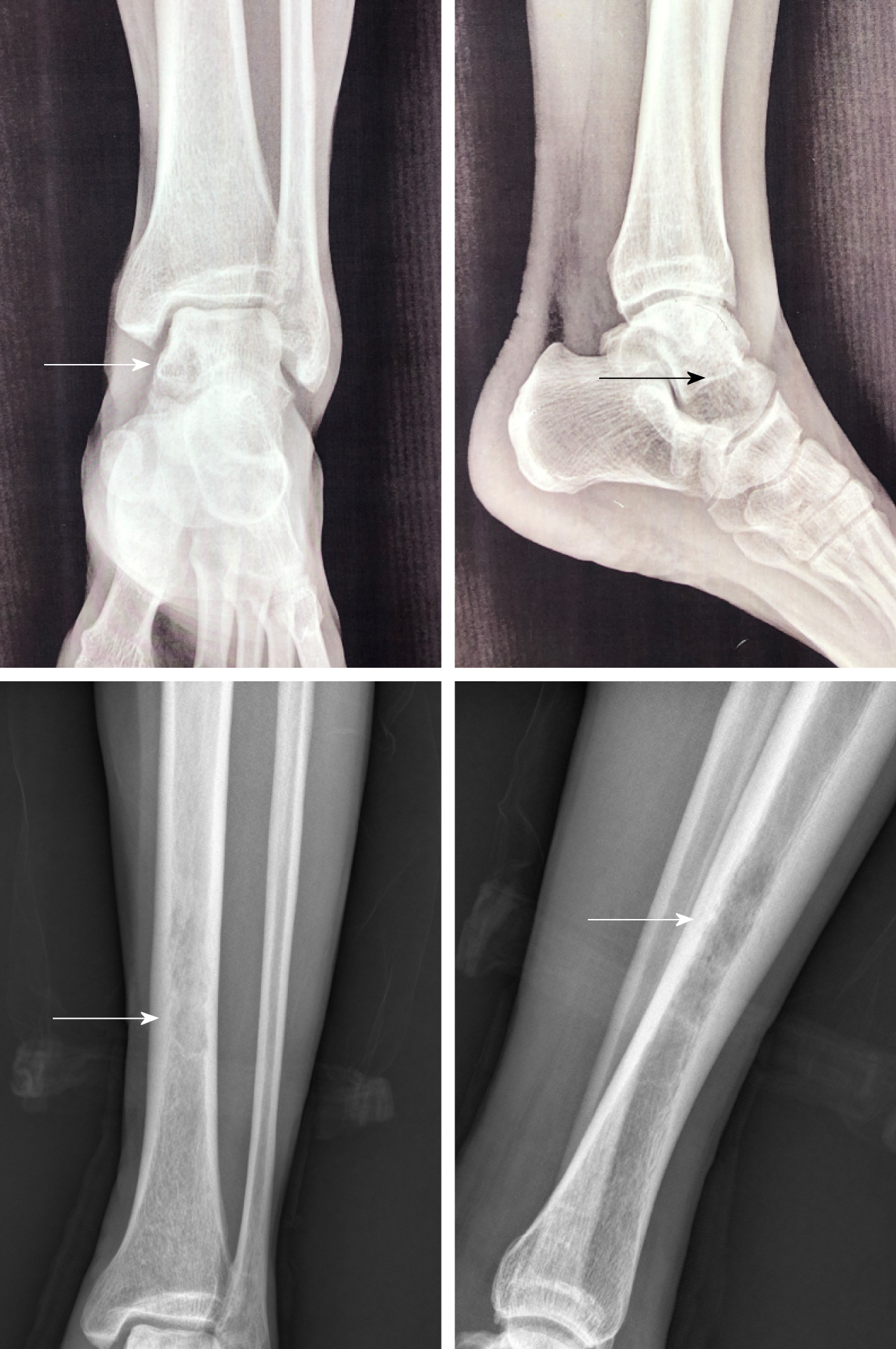
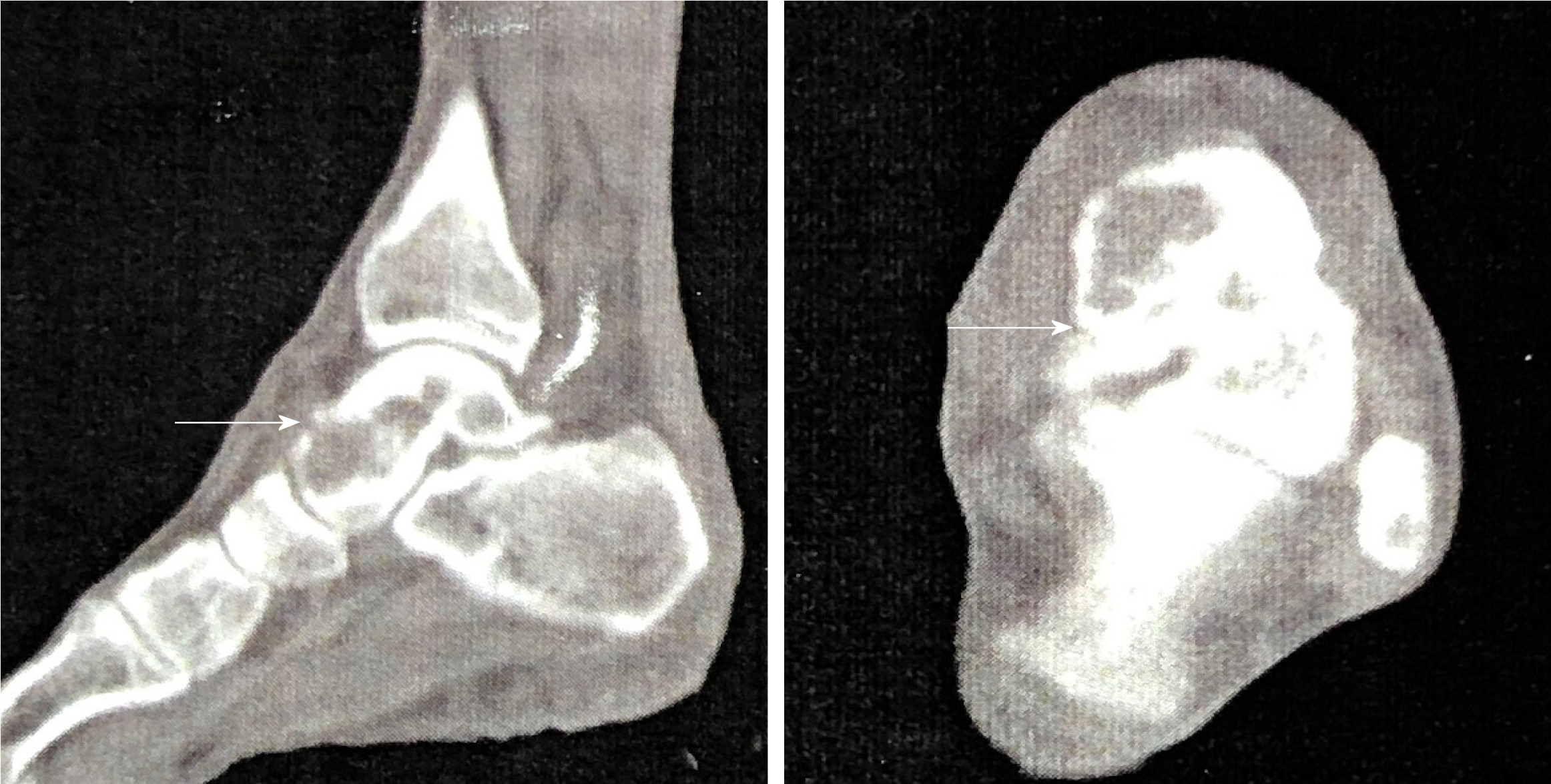
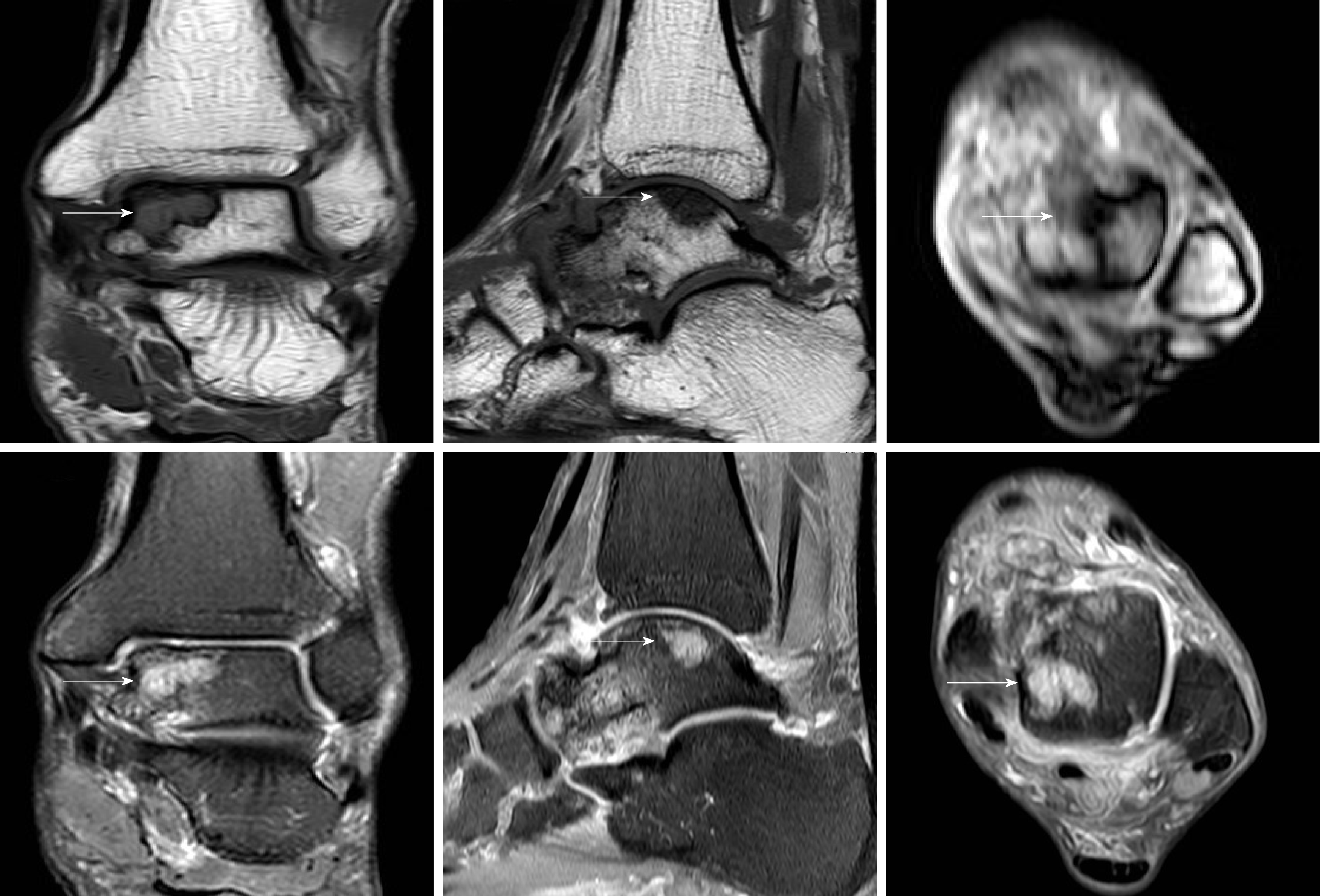
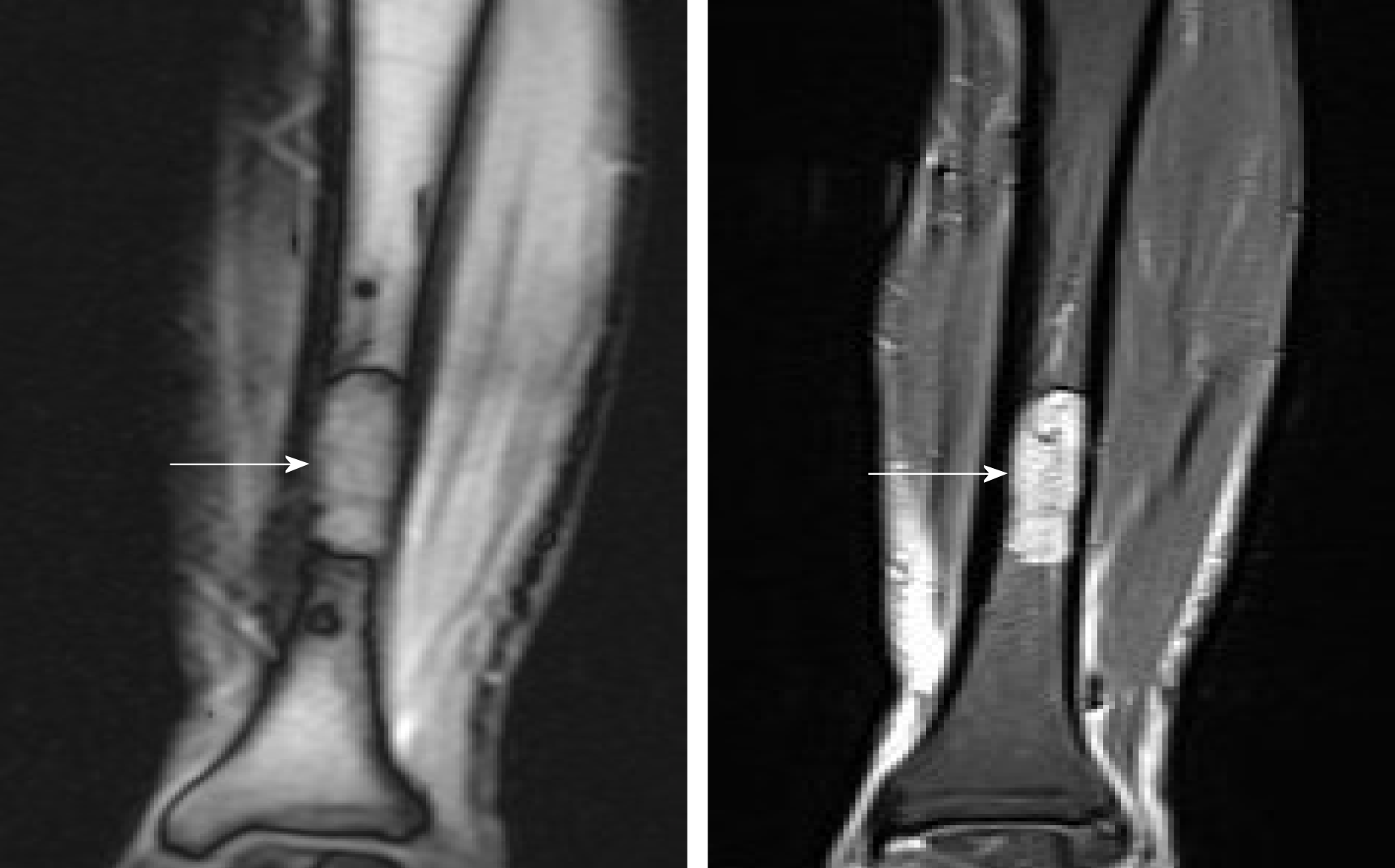
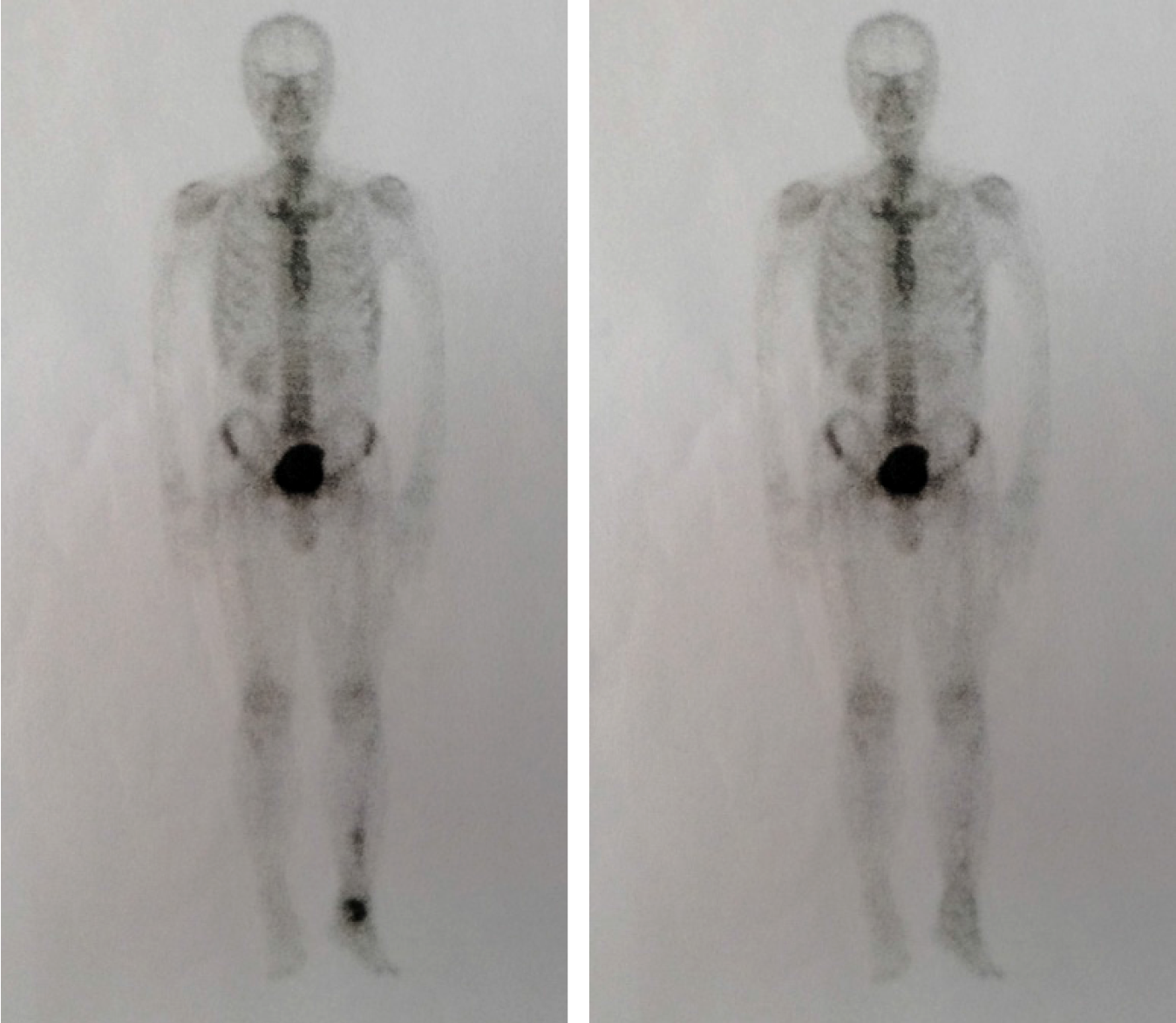
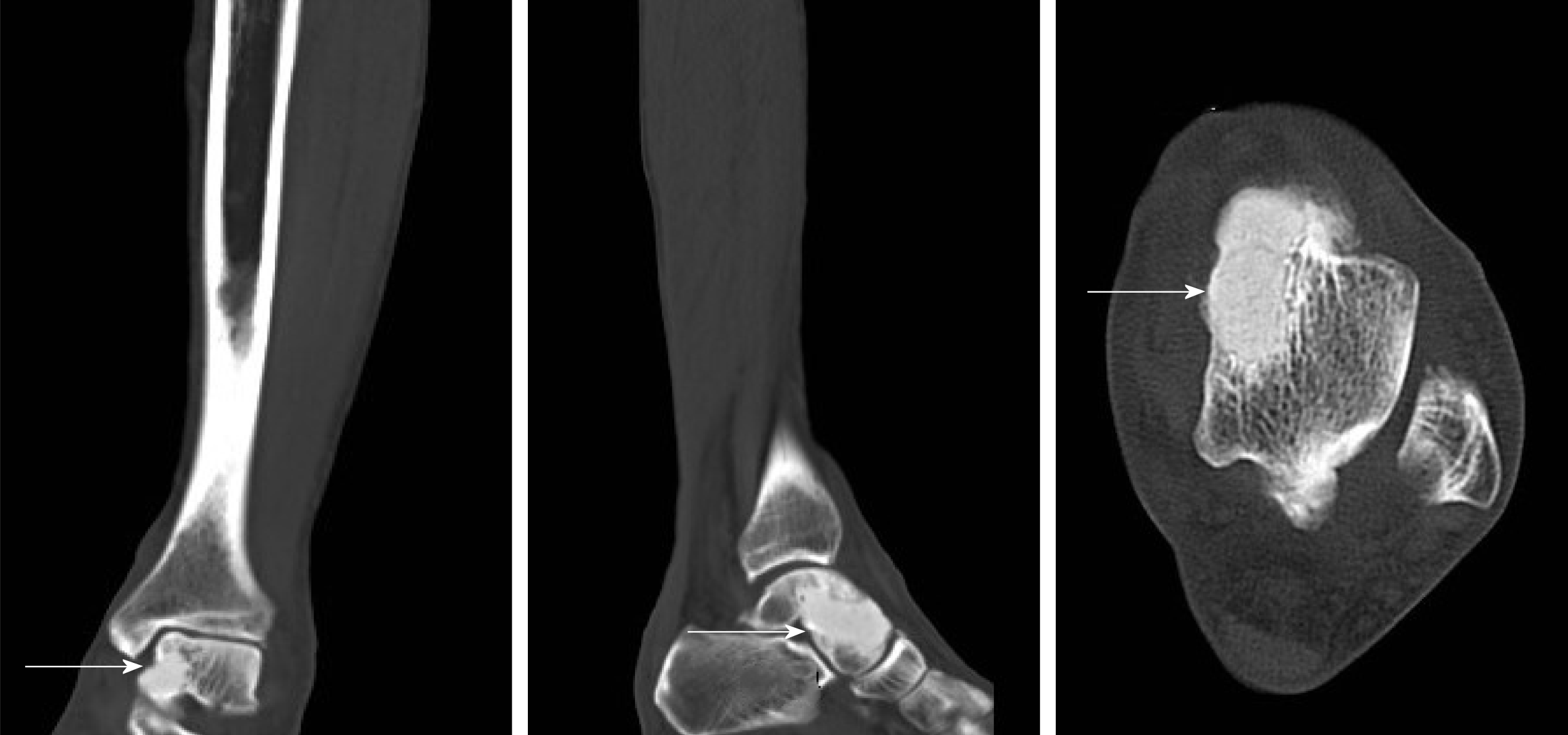
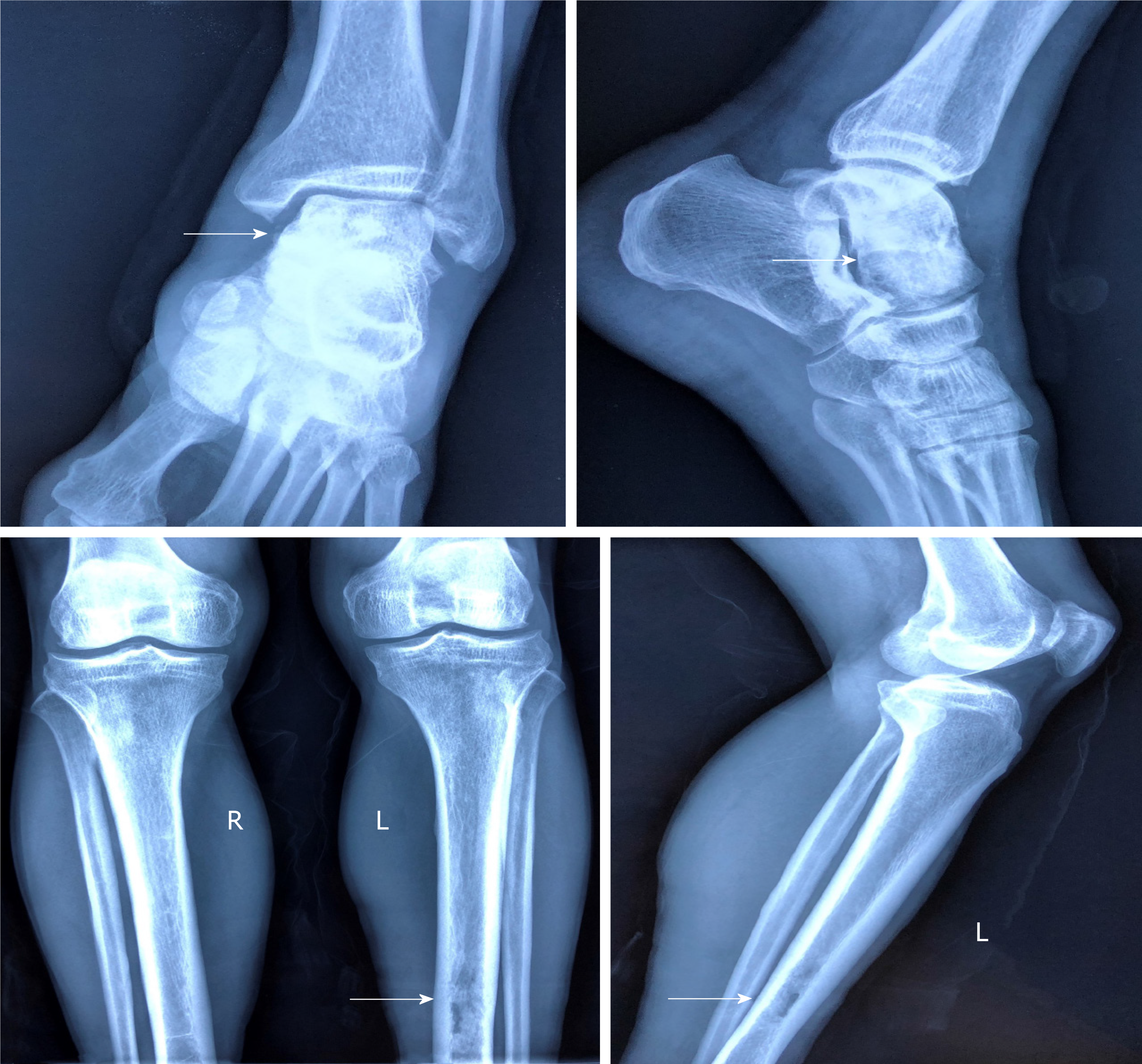
We discovered osteosclerosis of the talus and tibia on the previous plain X-ray film taken by the patient 5 years ago, which was initially missed at a local hospital (Figure 2). His recent left tibiofibular X-ray showed irregular osteolytic lesions in the talus, and focal osteosclerosis in the distal tibia (Figure 3). The computer tomography (CT) revealed left talus osteolytic destruction and cortical discontinuity of the anterior articular surface (Figure 4). Magnetic resonance imaging (MRI) revealed lesions of the talus and tibia hypointense on T1-weighted images (T1WI) and hyperintense on T2-weighted images (T2WI) (Figures 5 and 6). The whole-body technetium bone scan showed increased asymmetric uptake at the left talus and tibia (Figure 7).
Erdheim-Chester disease with a wild-type mutation in the BRAFV600E gene.
Based on the X-ray findings over a 5-year period, the patient was found to have relatively slow progress of the disease, and the other organs were not involved. Moreover, side effects of IFN and other drugs were frequently reported, so surgery was performed after consent was obtained from the patient. The patient was placed in a supine position with a thigh tourniquet. An anterior medial incision was made to avoid osteotomy of the medial malleolus. Intraoperatively, synovial hyperplasia was found in the ankle joint. A tawny and soft osteochondral lesion without obvious envelope was found in the neck and body of the talus. Complete resection of the astragalar lesion was performed, and specimens were sent for pathological examination. As there was a large area of defective bones in the internal side of the talus, combined with a small area of defective cartilage, bone cement was cast into this defective area without extending beyond the borders and damaging the surrounding tissues. A small incision was made in the distal tibia, and the diseased tissue in the medullary cavity of the tibia was completely scraped away. After sufficient hemostasis, the incision was covered with a dressing.
Close follow-up was conducted after operation. At 1 mo after surgery, the patient was examined in our outpatient department. No postoperative incision infections or complications were found. At 6 mo post-operatively, the patient could walk normally without pain or limited mobility, and CT showed that the lesion in the talus was completely removed and the bone was filled with cement (Figure 8). During a 2-year follow-up period after the surgery, X-ray and whole-body bone scans showed no abnormalities, and no evidence of recurrence or metastasis was recorded (Figures 7 and 9).
The diagnosis of ECD is based on characteristic pathological findings, clinical manifestations and unique imaging features[5,9]. The most common pathological manifestation is diffuse sclerosis characterized by foam-like adipose tissue infiltration and granulomatous inflammation containing fibrous tissue, lymphoid tissue, plasma cells and Touton giant cells. Histologically, CD68(+), CD34(-), CD1a(-) and S100(-) were observed in immunostaining[22]. The clinical manifestations range from mild asymptomatic focal lesions to severe life-threatening multiple organ dysfunction syndrome, which are especially common in the diaphysis and metaphysis of long bones[23]. Affected extra-osseous organs include cardiovascular, respiratory, central and neuroendocrine, orbital, retroperitoneal and other rare sites[22].
In recent years, with the deepening of imaging research, diagnosis, evaluation, monitoring and follow-up of ECD become increasingly important. X-ray is the primary method of examining changes in bone structure; CT, MRI and radionuclide bone scans are more sensitive to early and hidden lesions. The unique imaginary feature is the symmetrical osteosclerosis of the long bone, and it may also be associated with partial epiphysis sclerosis, periostitis, and even bone infarction[11]. MRI is highly sensitive to bone marrow infiltration, which shows that normal intramedullary fat is replaced by abnormal hyperplasia tissue. In addition to assessing the extent of bone marrow infiltration, MRI can clearly show changes in the periosteum and cartilage.
ECD affecting the unilateral talus is rare, and may be easily missed in diagnosis. Up to date, there are only about 1000 cases reported in the literature. The disease may be ignored by inexperienced clinicians, leading to delayed diagnosis. In general, ECD can occur in any site of the body, but almost all patients have symmetrical osteosclerosis of the limb long bone, such as the femur, tibia and fibula. Our patient only had unilateral talus and tibia involvement, while no lesions were found in the rest of the body. According to the literature review, all the affected bones are above the ankle plate. Epiphysis, articular cartilage and talus involvement has been scarcely reported previously[9-11,22]. Anatomically, there is no muscular attachment to the talus, all the bones are surrounded by the articular surface of cartilage, and there is also cartilage continuation at the edge of the upper articular surface; in addition, the main blood supply comes from the dorsal foot artery, which passes into the body from the talus anterolateral neck[24]. In the case of trauma, destruction of the talus is likely to occur due to cartilage damage or ischemic necrosis caused by blood supply interruption. Extensive synovial hyperplasia, articular cartilage damage and severe bone destruction of the affected talus were discovered during surgery. Upon reaching this point, we are faced with two possibilities: One would be the disease itself, which exacerbates bone and cartilage destruction. Considering that the patient had a history of trauma, the other possibility is that trauma as an inducement would lead to bone destruction and articular cartilage damage of the affected talus, which further exacerbates the deterioration of ECD.
To date, many different drugs for ECD have been explored, but few prospective studies or randomized controlled clinical studies are being conducted. Currently, the best ECD treatment is IFN-α and PEG-IFN-α therapy. A prospective, non-randomized, observational cohort analysis of 53 ECD patients revealed that 46 patients with ECD who received IFN-α- or PEG-IFN-α-based treatment had increased overall survival[12]. Numerous adverse events such as fever, fatigue, gastrointestinal symptoms and depression were observed. Anakinra has also been available as an IL-1 receptor antagonist[15,16]. In addition, based on the pathogenesis that almost half ECD patients have the BRAFV600E mutation and almost all ECD tissue samples are active in extracellular signal–regulated kinase phosphorylation, the use of vemurafenib as a specific BRAF inhibitor and cobimetinib as a specific MEK inhibitor resulted in good therapeutic effects as well[13,14,25]. However, the most frequent side effects with vemurafenib or cobimetinib were skin complications, gastrointestinal symptoms and rhabdomyolysis. There are very few studies on the surgical treatment of ECD. Alfieri et al[19] have confirmed that surgical resection and radiation therapy can be a good treatment choice for cerebral lesions; Wimpissinger et al[20] have reported that renal complications in ECD should be considered as part of the surgical strategy; and Mahoozi et al[21] have concluded that surgical treatment is an option in the management of isolated cardiac ECD. Here, we describe a unique case of ECD that only affected the focal talus and tibia, but no other organs. The lesions were completely removed by surgery, and there was no recurrence or metastasis during the 2-year follow-up period.
Although different drug treatments for ECD have been reported, surgery for bone involvement alone should also be considered to avoid side effects caused by drugs. Given its rarity, further research on ECD, as well as a comparison of the clinical efficacy of surgery and medication, will undoubtedly be vital and difficult.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alimehmeti RH, Ghoch ME, Lee SH S-Editor: Zhang L L-Editor: Filipodia E-Editor: Liu MY
| 1. | Badalian-Very G, Vergilio JA, Degar BA, MacConaill LE, Brandner B, Calicchio ML, Kuo FC, Ligon AH, Stevenson KE, Kehoe SM, Garraway LA, Hahn WC, Meyerson M, Fleming MD, Rollins BJ. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 857] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 2. | Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O'Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA; BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5824] [Cited by in RCA: 5943] [Article Influence: 424.5] [Reference Citation Analysis (0)] |
| 3. | Haroche J, Charlotte F, Arnaud L, von Deimling A, Hélias-Rodzewicz Z, Hervier B, Cohen-Aubart F, Launay D, Lesot A, Mokhtari K, Canioni D, Galmiche L, Rose C, Schmalzing M, Croockewit S, Kambouchner M, Copin MC, Fraitag S, Sahm F, Brousse N, Amoura Z, Donadieu J, Emile JF. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120:2700-2703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 506] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 4. | Cohen-Aubart F, Emile JF, Carrat F, Helias-Rodzewicz Z, Taly V, Charlotte F, Cluzel P, Donadieu J, Idbaih A, Barete S, Amoura Z, Haroche J. Phenotypes and survival in Erdheim-Chester disease: Results from a 165-patient cohort. Am J Hematol. 2018;93:E114-E117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 5. | Papo M, Cohen-Aubart F, Trefond L, Bauvois A, Amoura Z, Emile JF, Haroche J. Systemic Histiocytosis (Langerhans Cell Histiocytosis, Erdheim-Chester Disease, Destombes-Rosai-Dorfman Disease): from Oncogenic Mutations to Inflammatory Disorders. Curr Oncol Rep. 2019;21:62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7459] [Cited by in RCA: 7634] [Article Influence: 331.9] [Reference Citation Analysis (0)] |
| 7. | Hervier B, Haroche J, Arnaud L, Charlotte F, Donadieu J, Néel A, Lifermann F, Villabona C, Graffin B, Hermine O, Rigolet A, Roubille C, Hachulla E, Carmoi T, Bézier M, Meignin V, Conrad M, Marie L, Kostrzewa E, Michot JM, Barete S, Taly V, Cury K, Emile JF, Amoura Z; French Histiocytoses Study Group. Association of both Langerhans cell histiocytosis and Erdheim-Chester disease linked to the BRAFV600E mutation. Blood. 2014;124:1119-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 8. | Nadjiri J, Woertler K, Specht K, Harrasser N, Toepfer A. Erdheim-Chester disease with bilateral Achilles tendon involvement. Skeletal Radiol. 2016;45:1437-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Munoz J, Janku F, Cohen PR, Kurzrock R. Erdheim-Chester disease: characteristics and management. Mayo Clin Proc. 2014;89:985-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Haroche J, Amoura Z, Dion E, Wechsler B, Costedoat-Chalumeau N, Cacoub P, Isnard R, Généreau T, Wechsler J, Weber N, Graef C, Cluzel P, Grenier P, Piette JC. Cardiovascular involvement, an overlooked feature of Erdheim-Chester disease: report of 6 new cases and a literature review. Medicine (Baltimore). 2004;83:371-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 194] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Perez A, Crahes M, Laquerrière A, Proust F, Derrey S. Neurological form of Erdheim-Chester disease: Case report and review of the literature. Neurochirurgie. 2014;60:316-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 12. | Arnaud L, Hervier B, Néel A, Hamidou MA, Kahn JE, Wechsler B, Pérez-Pastor G, Blomberg B, Fuzibet JG, Dubourguet F, Marinho A, Magnette C, Noel V, Pavic M, Casper J, Beucher AB, Costedoat-Chalumeau N, Aaron L, Salvatierra J, Graux C, Cacoub P, Delcey V, Dechant C, Bindi P, Herbaut C, Graziani G, Amoura Z, Haroche J. CNS involvement and treatment with interferon-α are independent prognostic factors in Erdheim-Chester disease: a multicenter survival analysis of 53 patients. Blood. 2011;117:2778-2782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 288] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 13. | Haroche J, Cohen-Aubart F, Emile JF, Maksud P, Drier A, Tolédano D, Barete S, Charlotte F, Cluzel P, Donadieu J, Benameur N, Grenier PA, Besnard S, Ory JP, Lifermann F, Idbaih A, Granel B, Graffin B, Hervier B, Arnaud L, Amoura Z. Reproducible and sustained efficacy of targeted therapy with vemurafenib in patients with BRAF(V600E)-mutated Erdheim-Chester disease. J Clin Oncol. 2015;33:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 223] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 14. | Diamond EL, Subbiah V, Lockhart AC, Blay JY, Puzanov I, Chau I, Raje NS, Wolf J, Erinjeri JP, Torrisi J, Lacouture M, Elez E, Martínez-Valle F, Durham B, Arcila ME, Ulaner G, Abdel-Wahab O, Pitcher B, Makrutzki M, Riehl T, Baselga J, Hyman DM. Vemurafenib for BRAF V600-Mutant Erdheim-Chester Disease and Langerhans Cell Histiocytosis: Analysis of Data From the Histology-Independent, Phase 2, Open-label VE-BASKET Study. JAMA Oncol. 2018;4:384-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 286] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 15. | Aouba A, Georgin-Lavialle S, Pagnoux C, Martin Silva N, Renand A, Galateau-Salle F, Le Toquin S, Bensadoun H, Larousserie F, Silvera S, Provost N, Candon S, Seror R, de Menthon M, Hermine O, Guillevin L, Bienvenu B. Rationale and efficacy of interleukin-1 targeting in Erdheim-Chester disease. Blood. 2010;116:4070-4076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Tran TA, Pariente D, Lecron JC, Delwail A, Taoufik Y, Meinzer U. Treatment of pediatric Erdheim-Chester disease with interleukin-1-targeting drugs. Arthritis Rheum. 2011;63:4031-4032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Cohen Aubart F, Emile JF, Maksud P, Galanaud D, Cluzel P, Benameur N, Aumaitre O, Amoura Z, Haroche J. Efficacy of the MEK inhibitor cobimetinib for wild-type BRAF Erdheim-Chester disease. Br J Haematol. 2018;180:150-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Al Bayati A, Plate T, Al Bayati M, Yan Y, Lavi ES, Rosenblatt JD. Dabrafenib and Trametinib Treatment for Erdheim-Chester Disease with Brain Stem Involvement. Mayo Clin Proc Innov Qual Outcomes. 2018;2:303-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Alfieri A, Gazzeri R, Galarza M, Neroni M. Surgical treatment of intracranial Erdheim-Chester disease. J Clin Neurosci. 2010;17:1489-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Wimpissinger TF, Schernthaner G, Feichtinger H, Stackl W. Compression of kidneys in Erdheim-Chester disease of retroperitoneum: Open surgical approach. Urology. 2005;65:798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Mahoozi HR, Zittermann A, Hakim Meibodi K, Burchert W, Gummert JF, Mirow N. Erdheim-Chester disease in a female cardiac surgery patient. Thorac Cardiovasc Surg. 2012;60:428-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Campochiaro C, Tomelleri A, Cavalli G, Berti A, Dagna L. Erdheim-Chester disease. Eur J Intern Med. 2015;26:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 23. | Carpinteri R, Patelli I, Casanueva FF, Giustina A. Pituitary tumours: inflammatory and granulomatous expansive lesions of the pituitary. Best Pract Res Clin Endocrinol Metab. 2009;23:639-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Trovato A, El-Rich M, Adeeb S, Dhillon S, Jomha N. Geometric analysis of the talus and development of a generic talar prosthetic. Foot Ankle Surg. 2017;23:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Nelson DS, Quispel W, Badalian-Very G, van Halteren AG, van den Bos C, Bovée JV, Tian SY, Van Hummelen P, Ducar M, MacConaill LE, Egeler RM, Rollins BJ. Somatic activating ARAF mutations in Langerhans cell histiocytosis. Blood. 2014;123:3152-3155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |