Published online Feb 6, 2020. doi: 10.12998/wjcc.v8.i3.527
Peer-review started: December 9, 2019
First decision: December 30, 2019
Revised: January 12, 2020
Accepted: January 19, 2020
Article in press: January 19, 2020
Published online: February 6, 2020
Processing time: 58 Days and 13.7 Hours
Distant metastasis occasionally occurs in patients who have been diagnosed with colorectal cancer (CRC), but it occurs in a few patients with stage I CRC. The vagina as a metastasis site has also been reported, albeit rarely. Most reported cases of vaginal metastasis (VM) report their origin from advanced CRC. We encountered a patient who was diagnosed with isolated VM originating from stage I colon cancer (T2N0) and herein present the case of this patient.
A 63-year-old woman visited the outpatient clinic because of a positive result from a stool occult blood test. She underwent laparoscopic anterior resection and was pathologically diagnosed with stage I (T2N0) sigmoid colon cancer. Neither lymphovascular invasion nor perineural invasion was observed. Ten months following the surgery, isolated vaginal metastases were detected on gynecologic examination. The examination was performed due to vaginal spotting. A transvaginal wide excision was performed, and no other adjuvant treatment was provided after discussion with a multidisciplinary team and the patient. Subsequently, a new VM was discovered after 33 mo. An additional transvaginal excision was performed. To date, there has been no evidence of further disease progression. From the time of diagnosis of VM, the patient’s overall survival has been 54 mo.
VM can occur as a result of early-stage colorectal cancer. Surgeons should consider the possibility of VM following complaints of gynecologic symptoms following surgery.
Core tip: Vaginal metastasis (VM) from colorectal cancer is rare. To our knowledge, this is the first case of VM from stage I colorectal cancer to be reported in English. Although the prognosis of VM is considered dismal, it seems to be favorable when it occurs isolated, without other metastases. The standard treatment has not yet been established because of rarity. The treatment needs to be established individually, and multidisciplinary teams can be helpful. Most patients with VM complain about gynecologic symptoms such as vaginal bleeding, so it should not be overlooked even in patient with stage I colorectal cancer.
- Citation: Kwon SK, Yu CS, Lee SW, Kim J, Song I, Lee JL, Kim CW, Yoon YS, Park IJ, Lim SB, Kim JC. Isolated vaginal metastasis from stage I colon cancer: A case report. World J Clin Cases 2020; 8(3): 527-534
- URL: https://www.wjgnet.com/2307-8960/full/v8/i3/527.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i3.527
Distant metastasis occurs in 20%–30% of patients who have been diagnosed with colorectal cancer (CRC). Their median survival time is 9 mo and their 5-year survival rate is only 9%–14%[1,2]. Distant metastasis occurs in only 6.5% of patients with stage I CRC[2]. The most common sites of distant metastases are the liver and lung[1,2], but the vagina as a metastasis site has also been reported, albeit rarely[3]. Since Whitelaw et al[4] reported the first case of distant vaginal metastasis (VM) in 1956, less than one hundred cases have been reported[3]. Because of the rarity, the mechanism of VM remains to be elucidated[3,5,6], and a treatment strategy has not yet been established[3]. Distant VM has been known to have a dismal prognosis in general, primarily because it often appears with other metastases as a result of the dissemination of the tumor[5]. The prognosis, however, seems to be favorable when it is revealed as an isolated metastasis[3,6].
As we experienced one patient with a favorable prognosis after treatment with local excision who was diagnosed with an isolated VM originating from stage I colon cancer (T2N0), we herein present the case of this patient.
A 63-year-old woman presented with a complaint about her vaginal spotting.
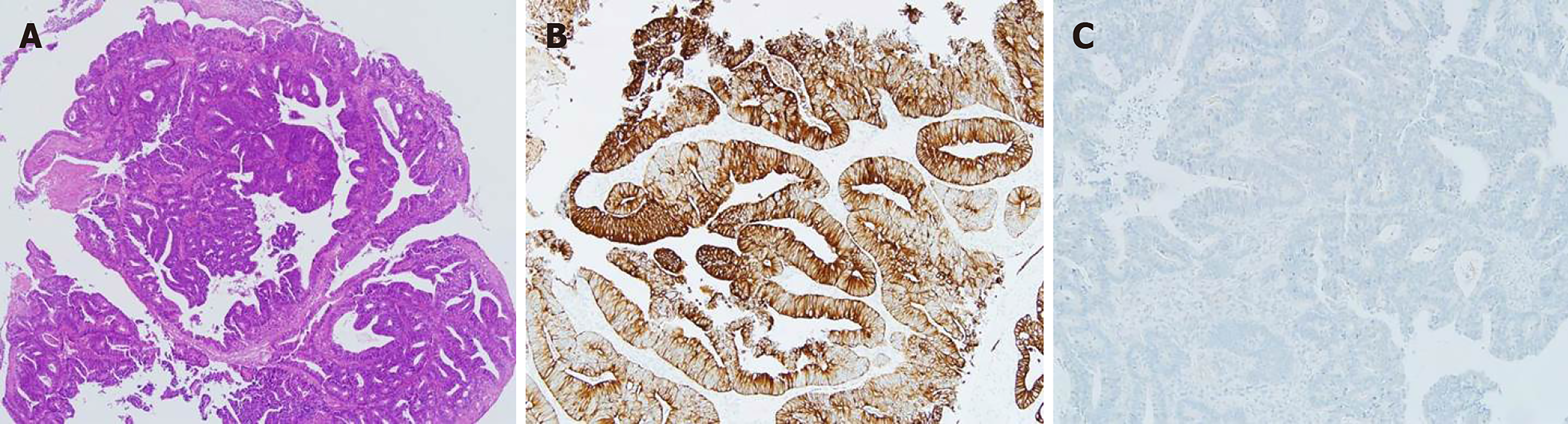
The patient visited the gynecologic clinic at our institution because of vaginal spotting. Submucosal leiomyoma was diagnosed and hysteroscopic myomectomy was performed. However, the symptoms persisted and the patient, therefore, visited a gynecologic clinic outside our institution. Two masses were detected on the right wall of her vagina. This occurred 10 mo after the previous surgery for sigmoid colon cancer. One mass was 1 cm (3 cm from the introitus) and the other was 1.2 cm (5 cm from the introitus) in size. Excisional biopsies were performed, which revealed a moderately differentiated adenocarcinoma originating from the colon cancer (Figure 1). The patient was then referred to our institution.
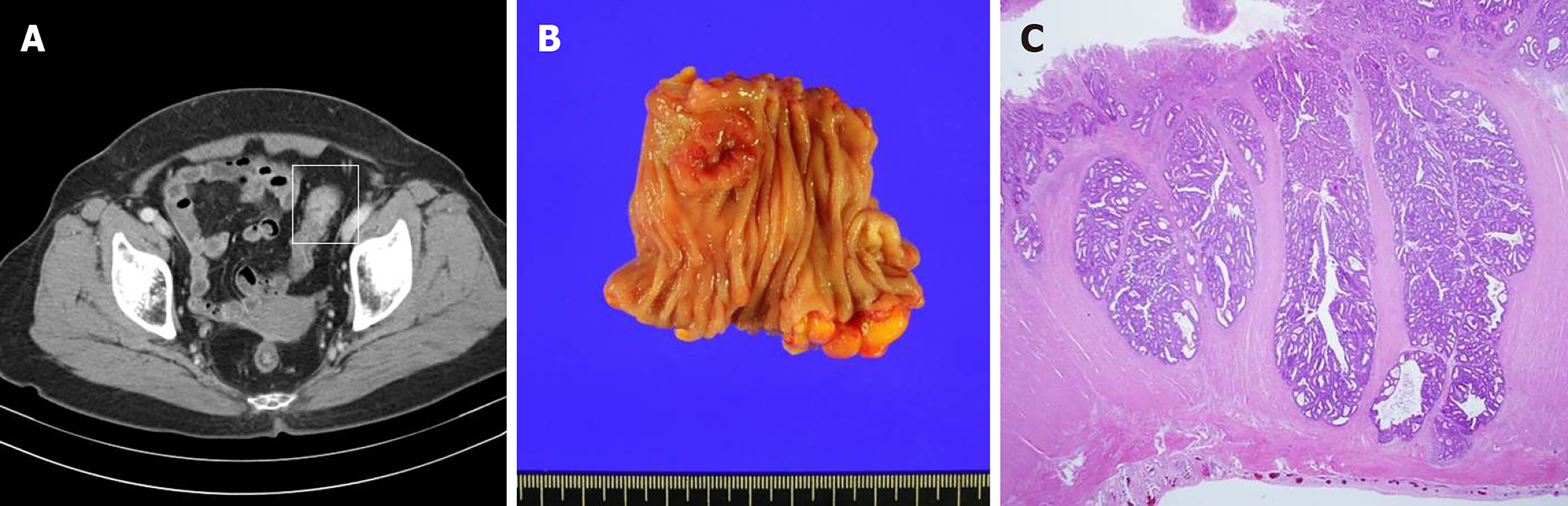
The patient first visited the outpatient clinic because of a positive result from a stool occult blood test that was performed as part of the National Cancer Screening Program. Four months before visiting the gynecologic clinic, she was diagnosed with sigmoid colon cancer after colonoscopic evaluation, and a laparoscopic anterior resection was performed (Figure 2). Following surgery, the patient was discharged and had no complications.
The preoperative serum-carcinoembryonic antigen level was 1.2 ng/mL. The size of the tumor was 2.5 cm × 2.3 cm. Pathologic examination showed a moderately differentiated adenocarcinoma of the sigmoid colon that invaded the proper muscle. There was no lymph node metastasis within the 17 resected lymph nodes (T2N0M0, Stage I). Neither lymphovascular invasion nor perineural invasion was observed. MLH1 and MSH2 were positive as per the immunohistochemial examination and the microsatellites were stable.
None.
The gynecologist at our institution found only blood tinged mucosa in her vagina.
All laboratory results were normal. The carcinoembryonic antigen level at this time was 1.8 ng/mL.
A positron emission tomography was performed, and no significant hypermetabolic lesion was detected. Abdominopelvic computed tomography scan was also performed and no abnormal finding was detected.
The final diagnosis of the presented case is isolated vaginal metastasis from stage I colorectal cancer.
After discussion with a multidisciplinary team (MDT) and the patient, we decided not to perform any adjuvant treatment considering the primary colon cancer was stage I and there was no evidence of recurrence or other metastasis. In addition, the patient was reluctant to undergo chemotherapy. Transvaginal wide excision of the vagina was performed for local control. The right posterior vaginal mucosal flap was dissected and resected in a triangular shape. There was no residual tumor in the specimen on pathologic examination.
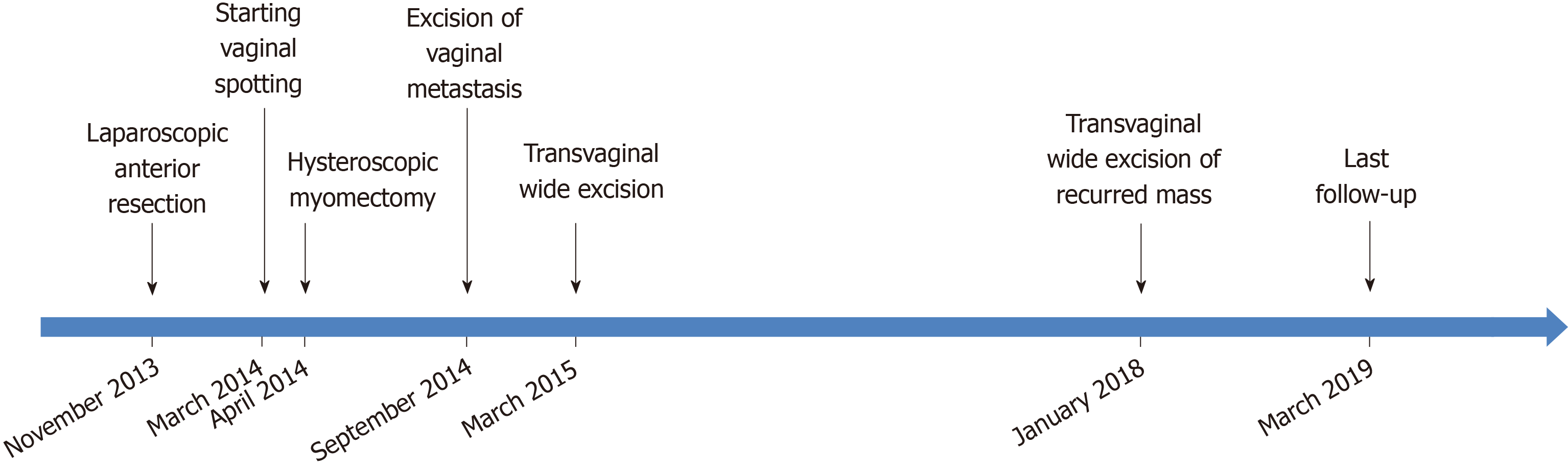
Thirty-three months following the wide excision, a 1-cm sized polypoid mass was identified on the right vaginal wall during gynecologic follow-up. An excisional biopsy was performed and it was confirmed that a moderately differentiated adenocarcinoma was present that had originated from the colon. Computed tomography scan was performed and the results showed no evidence of a recurrence or any other metastasis. Transvaginal wide excision of the mass was performed and no adjuvant treatment was subsequently conducted. After 14 mo, to date, there is no evidence of disease. From the time of diagnosis of VM to the patient’s last follow-up, her overall survival was 54 mo. The timeline of the patient's treatment (Figure 3).
The vagina is occasionally stricken by colorectal cancer through direct invasion, but distant metastasis to the vagina is a rare occurrence. To the best of our knowledge, only 57 cases have been reported worldwide[3,6-15]. Among the reported cases, 24 reports included descriptions of the case details and were written in English. Except for the two cases that suggested that VM developed through direct invasion rather than distant metastasis, we reviewed the 22 eligible cases (Table 1)[6,8,9,16-27]. The primary CRCs in these cases were all relatively advanced (above T3N0) CRCs except for this single case. Among the cases that had the exact CRC stage indicated, 33% (6/18) were stage II, 50% (12/18) were stage III, and 16% (3/18) were stage IV. Mazur et al[7] found 11 vaginal metastatic tumors that derived from CRC among 129 metastatic vaginal tumors and they reported that all the primary lesions were Dukes’ stage B or C. However, it seems the VM could occur even in stage I CRC because the primary colon cancer in this case was identified as stage I CRC (T2N0).
| No | Age (yr) | Location | Stage | Synchro-nous meta-stasis1 | Time interval | Treatment for VM2 | Isolated VM3 | Metachro-nous metastasis | Outcome | Ref. |
| 1 | 52 | SC | NM | No | Sync | TVE, cervicectomy, IRT | Yes | - | 39 mo, Dead | Whitelaw et al[4] |
| 2 | 46 | DC | Dukes B | No | 13 mo | IRT | No | Peritoneum, omentum, brain | 39 mo, Dead | Raider et al[5] |
| 3 | 48 | SC | Dukes C | Ovaries, uterus | 4 mo | IRT | No | Pleura, liver | 21 mo, Dead | Raider et al[5] |
| 4 | 63 | RS | Dukes C | No | 21 mo | TVE, nitrogen mustard→ EBRT, IRT | Yes | - | 48 mo, NED | Raider et al[5] |
| 5 | 46 | RS | Dukes C | No | 41 mo | EBRT, IRT | No | Ovary, ileum, peritoneum, media-stinum | 10 mo, Dead | Raider et al[5] |
| 6 | 81 | RS | T3N+ | No | Sync | TVE | Yes | - | 12 mo, NED | Lee et al[16] |
| 7 | 57 | SC | T?N+ | No | 18 mo | IRT → observation | Yes | - | 12 mo, NED | Lee et al[16] |
| 8 | 56 | DC | T4N1 | Peritoneum | Sync | Partial resection | No | Peritineum | 8 mo, AWD | Singh et al[17] |
| 9 | 83 | SC | T4N1 | No | 3 wk | NM | No | Liver, skin | NM | Chagpar et al[18] |
| 10 | 44 | Rec | T3N1 | Liver | 13 mo | Incisional Bx., IRT, CTx | No | - | NM | Yagci et al[19] |
| 11 | 81 | SC | T4N0 | No | Sync | TVE, TAH/BSO, EBRT, IRT | Yes | - | 39 mo, NED | Marchal et al[20] |
| 12 | 67 | AC | T4N0 | No | 3 mo | TVE, EBRT | Yes | - | 48 mo, NED | Costa et al[21] |
| 13 | 63 | Rec | NM | No | Sync | Partial resection, EBRT | Yes | - | 12 mo, NED | Funada et al[22] |
| 14 | 72 | Rec | T4N2 | No | Sync | TVE, BO, CTx | Yes | - | 13 mo, NED | Ceccaroni et al[23] |
| 15 | 64 | SC | T?N1 | No | 34 mo | En-bloc resection (Vagina partial resection, TAH/BSO, radical cystectomy) | No | Brain | 10 mo, Dead | Lorente et al[24] |
| 16 | 62 | Rec | NM | No | Sync | TVE | Yes | - | 12 mo, NED | Sabbagh et al[25] |
| 17 | 78 | Rec | uT3N0 | No | Sync | TVE, CTx, EBRT | Yes | - | 10 mo, NED | Sabbagh et al[25] |
| 18 | 53 | RS | T3N2 | No | 24 mo | CTx, EBRT | No | Peritoneum, systemic LNs | 24 mo, AWD | Macedo et al[26] |
| 19 | 64 | SC | T4N0 | No | 36 mo | EBRT | No | 8 mo, NED | Tanaka et al[27] | |
| 20 | 67 | SC | T3N0 | No | Sync | TVE | Yes | NM | D'Arco et al[8] | |
| 21 | 87 | Rec | T4N? | No | 24 mo | EBRT | Yes | NM, AWD | Ng et al[9] | |
| 22 | 78 | Rec | T4N1 | No | Sync | TVE, TAH/BSO | No | 22 mo, AWD | Sadatomo et al[6] | |
| 23 | 63 | SC | T2N0 | No | 10 mo | TVE→ TVE | Yes | 47 mo, NED | This case |
Theoretically, cancer cells can spread from the colon and rectum to the vagina through three pathways, which are the transcoelomic, lymphatic, and hematogenous spreading pathways[3,5,6]. In our case, transcoelomic implantation and lymphatic spreading are difficult to consider because the stage of the primary tumor was T2N0 without lymphovascular invasion. In 87% (20/23) of the summarized cases, including this case, the tumors originated from the rectum or sigmoid colon. This may be because the rectum and sigmoid colon are near the systemic venous system, and cancer cells can travel through this system. The venous system, which is associated with the vertebral vein, appears to be the route of choice from the rectum and colon to the vagina[5]. Therefore, the VM seems to have traveled through the hematogenous pathway in this case.
The standard treatment for VM has not yet been established[3]. As in primary vaginal carcinomas[10], radiotherapy is the primary treatment followed by surgical resection. Approximately half of the patients among the summarized cases (14 out of 23) had been treated with radiotherapy. Earlier cases were managed with internal radiotherapy, and recent cases have been managed with external beam radiotherapy. One patient received both internal radiotherapy and external beam radiotherapy. Seven patients (30%) were treated with surgical resection. The other patients were treated with a combination of surgical resection, chemotherapy, and radiotherapy. After reviewing the reported cases, Ng et al[3] suggested that adjuvant therapy after resection of VM could be beneficial. Sadatomo et al[6] suggested that chemotherapy is a more appropriate adjuvant treatment. However, chemotherapy was provided to only four patients among the summarized cases. Like as treatment for primary vaginal carcinomas, the maintaining functional status of vagina also needs to be considered in determining the treatment[10]. Nonetheless, the patient in this case did not receive any adjuvant treatment because the MDT decided not to offer it because the metastasis had occurred only in the vagina, the primary lesion was stage I and the patient was hesitant about receiving adjuvant treatment. As the appropriate treatment for this rare metastatic disease has not been elucidated yet, the treatment needs to be tailored and established individually and the MDT seemed to be helpful.
Although the patient in this case did not present with any other distant metastases, local recurrence at the vagina occurred at 33 mo after excision and further excision was performed. Among the summarized cases, two patients experienced local recurrence at the vagina and a total of 13% (3/23) experienced vaginal recurrence. One patient experienced vaginal recurrence at 4 mo after the initial treatment with excision and a course of nitrogen mustard[3]. The other patient experienced vaginal recurrence at 1 year after the initial treatment with internal radiotherapy (192Ir implantation). Including our case, vaginal recurrence developed without other distant metastases in the three patients. It appears that careful follow-up of the vaginal condition is necessary after local treatment for VM.
As the patients with female genital tract metastasis have a poor prognosis[7], the prognosis of patients with VM has been known to be dismal. This is primarily because VM often appears as a part of the dissemination[5,18,19,26,27] or is a herald of dissemination[5,6,24]. Raider[5] reported three patients who presented with VM as part of a widespread dissemination, and they died within 10–39 mo after the detection of vaginal lesions among the four reported patients. Consequently, intensive evaluation for searching other metastases appears to be necessary if VM is found. Careful follow-up is required even when the vagina is the only metastatic site because it could be the beginning of dissemination. In addition, when it occurs as isolated metastasis without any other metastases, the prognosis seems favorable[4,5,16,20-23,25]. In the summarized cases, considering the outcomes, 10 of the 11 patients with isolated VM were still alive and 4 of the 8 patients with disseminated disease were still alive at the time of this report. When the patients with isolated VM were compared with those with disseminated disease, the mean time interval between diagnosis of the CRC and diagnosis of the VM was shorter (5.7 ± 9.2 mo and 16.6 ± 16.0 mo, respectively). The mean age of the patients with isolated VM was higher for more than 10 years (58.2 ± 13.8 years vs 68.7 ± 10.4 years, respectively). This may be due to older patients having a lower risk of metastasis[2].
Our patient complained of vaginal spotting for several months. Most of the patients with VM complain about gynecologic symptoms, and asymptomatic VM is uncommon[3,6]. Although some reports in the literature have shown the usefulness of magnetic resonance imaging (MRI) in detecting VM[6,8,9], two cases of VM were not detected on MRI[25]. Therefore, MRI seems insufficient to detect VM in some patients. Meanwhile, a careful history assessment seems more important than imaging studies. Although Sabbagh et al[25] recommended offering a keen gynecologic examination to all patients with CRC, it seems impracticable considering its rarity. Instead, colorectal surgeons should concentrate on their patient’s gynecologic complaints and be aware of the possibility of VM even if the primary cancer was at an early stage. Of the summarized patients, 87% (20/23) were diagnosed with VM synchronously or within two years. Therefore, gynecologic symptoms such as vaginal bleeding should not be overlooked, especially during early postoperative periods.
Herein, we report a patient with VM derived from stage I (T2N0) sigmoid colon cancer. VM could occur even in patients with stage I CRC and most VM occurs within 2 years after the diagnosis of colorectal cancer. If the VM occurs solely, the prognosis seems favorable. As was with this patient, most patients with VM present with gynecologic symptoms, such as vaginal bleeding, and imaging studies could miss the VM. Colorectal surgeons should not overlook the patient’s gynecologic symptoms, especially during early postoperative periods.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lin JM, Mihai C, Osawa S, Sun XT S-Editor: Zhang L L-Editor: A E-Editor: Liu JH
| 1. | Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2526] [Cited by in RCA: 2912] [Article Influence: 364.0] [Reference Citation Analysis (3)] |
| 2. | Riihimäki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016;6:29765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 420] [Cited by in RCA: 681] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 3. | Ng HJ, Aly EH. Vaginal metastases from colorectal cancer. Int J Surg. 2013;11:1048-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Whitelaw GP, Leard SE, Parsons L, Sherwin RP. Carcinoma of the large bowel with metastasis to the genitalia; report of two cases. AMA Arch Surg. 1956;73:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Raider L. Remote vaginal metastases from carcinoma of the colon. Am J Roentgenol Radium Ther Nucl Med. 1966;97:944-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 12] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Sadatomo A, Koinuma K, Horie H, Lefor AK, Sata N. An isolated vaginal metastasis from rectal cancer. Ann Med Surg (Lond). 2016;5:19-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Mazur MT, Hsueh S, Gersell DJ. Metastases to the female genital tract. Analysis of 325 cases. Cancer. 1984;53:1978-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | D'Arco F, Pizzuti LM, Romano F, Natella V, Laccetti E, Storto G, Maurea S, Mainenti PP. MRI findings of a remote and isolated vaginal metastasis revealing an adenocarcinoma of the mid-sigmoid colon. Pol J Radiol. 2014;79:33-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Ng QJ, Namuduri RP, Yam KL, Lim-Tan SK. Vaginal metastasis presenting as postmenopausal bleeding. Singapore Med J. 2015;56:e134-e136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Hacker NF, Eifel PJ, van der Velden J. Cancer of the vagina. Int J Gynaecol Obstet. 2015;131 Suppl 2:S84-S87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Baba H, Kuwabara H, Wakabayashi M, Nakamura H, Sanada T, Baba H, Nakajima K, Goseki N. [A case of vaginal metastasis of rectal cancer post -operation]. Gan To Kagaku Ryoho. 2012;39:2255-2257. [PubMed] |
| 12. | Quaranta D, Delotte J, Bongain A, François E, Bereder JM, Bernard JL. [Vaginal metastasis revealing an adenocarcinoma of the transverse colon]. Gynecol Obstet Fertil. 2014;42:622-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Katsumoto Y, Maruyama K, Furukawa J, Nagai K, Maruyama N, Tanaka J, Yokouchi H, Nakaguchi K, Sue F. [A case of metastatic vaginal tumor of rectal cancer]. Gan To Kagaku Ryoho. 2002;29:2406-2409. [PubMed] |
| 14. | Okayama S, Yoshimatsu K, Yokomizo H, Yano Y, Yamada Y, Satake M, Sakuma A, Matsumoto A, Fujimoto T, Usui T, Yamaguchi K, Shiozawa S, Shimakawa T, Katsube T, Naritaka Y. [A Case of Recurrence in the Posterior Wall of the Virginal after Radical Resection for Rectal Cancer Well Responded in a Long Period by Chemo-Radiotherapy]. Gan To Kagaku Ryoho. 2017;44:1197-1199. [PubMed] |
| 15. | Yasuyama A, Noura S, Matsumura T, Hirota M, Takada A, Koga C, Kameda C, Murakami M, Kawabata R, Shimizu J, Hasegawa J. [A Resected Case of the Vaginal Metastasis from Rectal Cancer]. Gan To Kagaku Ryoho. 2017;44:1434-1436. [PubMed] |
| 16. | Lee SM, Whiteley HW. Unusual metastatic sites of colonic and rectal carcinoma: Report of four cases. Dis Colon Rectum. 1974;17:560-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Singh P, O'Reilly AP, Prabhakaran K, Ratnam SS. Colonic adenocarcinoma presenting with vaginal bleeding. Aust N Z J Obstet Gynaecol. 1987;27:264-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Yagci G, Cetiner S, Dede M, Gunhan O. True vaginal metastasis of rectal cancer. Indian J Surg. 2005;67:270-272 Available from: URL: http://www.bioline.org.br/request?is05084. |
| 20. | Marchal F, Leroux A, Hoffstetter S, Granger P. Vaginal metastasis revealing colon adenocarcinoma. Int J Colorectal Dis. 2006;21:861-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Costa SRP, Antunes RCP, Abraão AT. Single vaginal metastasis from cancer of the right colon: case report. Einstein. 2009;7:219-221. |
| 22. | Funada T, Fujita S. A case of vaginal metastasis from a rectal cancer. Jpn J Clin Oncol. 2010;40:482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Ceccaroni M, Paglia A, Ruffo G, Scioscia M, Bruni F, Pesci A, Minelli L. Symptomatic vaginal bleeding in a postmenopausal woman revealing colon adenocarcinoma metastasizing exclusively to the vagina. J Minim Invasive Gynecol. 2010;17:779-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Lorente L, Alonso S, Pascual M, Pera M. Vaginal metastasis of colon cancer. Rev Esp Enferm Dig. 2011;103:435-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Sabbagh C, Fuks D, Regimbeau JM, Degremont R, Jarry-Tossou V, Mauvais F. Isolated vaginal metastasis from rectal adenocarcinoma: a rare presentation. Colorectal Dis. 2011;13:e355-e356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Macedo MP, Andrade Lde B, Coudry R, Crespo R, Gomes M, Lisboa BC, Aguiar S, Soares FA, Carraro DM, Cunha IW. Multiple mutations in the Kras gene in colorectal cancer: review of the literature with two case reports. Int J Colorectal Dis. 2011;26:1241-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Tanaka T, Kanda T, Sakaguchi S, Munakata S, Ohmichi M. Vaginal stump metastasis from sigmoid colon cancer. Acta Cytol. 2012;56:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |