Published online Feb 6, 2020. doi: 10.12998/wjcc.v8.i3.487
Peer-review started: September 24, 2019
First decision: November 4, 2019
Revised: December 13, 2019
Accepted: January 1, 2020
Article in press: January 1, 2020
Published online: February 6, 2020
Processing time: 134 Days and 14 Hours
Although gastrointestinal stromal tumors (GISTs) are rare, with an incidence of 1/100000 per year, they are the most common sarcomas in the peritoneal cavity. Despite considerable progress in the diagnosis and treatment of GIST, about half of all patients are estimated to experience recurrence. With only two drugs, sunitinib and regorafenib, approved by the Food and Drug Administration, selecting treatment options after imatinib failure and coordinating multidisciplinary care remain challenging. In addition, physicians across the Middle East face some additional and unique challenges such as lack of published local data from clinical trials, national disease registries and regional scientific research, limited access to treatment, lack of standardization of care, and limited access to mutational analysis. Although global guidelines set a framework for the management of GIST, there are no standard local guidelines to guide clinical practice in a resource-limited environment. Therefore, a group of 11 experienced medical oncologists from across the Gulf and Levant region, part of the Rare Tumors Gastrointestinal Group, met over a period of one year to conduct a narrative review of the management of GIST and to describe regional challenges and gaps in patient management as an essential step to proposing local clinical practice recommendations.
Core tip: Challenges faced by Middle Eastern clinicians in the management of gastrointestinal stromal tumor patients are numerous. Firstly, a lack of experience and equipment at non-cancer centres, lack of histopathologists with sarcoma expertise and limited access to radiological assessments present a hurdle to diagnosis. Secondly, management of patients by surgeons and gastroenterologists with limited oncology expertise, lack of access by healthcare authorities to guidelines, inadequate training of onco-surgeons and lack of radiological assessment to inform treatment can result in poor patient management. Furthermore, patient access to novel tyrosine kinase inhibitors or trials and a lack of patient understanding of treatment compliance also present challenges.
- Citation: Rare Tumors GI Group, Farhat F, Farsi AA, Mohieldin A, Bahrani BA, Sbaity E, Jaffar H, Kattan J, Rasul K, Saad K, Assi T, Morsi WE, Abood RA. Comprehensive review into the challenges of gastrointestinal tumors in the Gulf and Levant countries. World J Clin Cases 2020; 8(3): 487-503
- URL: https://www.wjgnet.com/2307-8960/full/v8/i3/487.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i3.487
Gastrointestinal stromal tumors (GISTs) are rare, with an incidence of 1/100000 per year[1]; nonetheless, they are the most common mesenchymal tumors of the GI tract[2]. Epidemiological data concerning GIST in the Gulf and Levant countries is scarce, with several studies describing cases within the region, including Kuwait[3], Qatar[4], Saudi Arabia[5] and Lebanon[6]. GIST is the most common sarcoma in the peritoneal cavity, and metastatic spread can be found in extravisceral locations such as the omentum, mesentery, and retroperitoneum[7,8]. Clinical signs and symptoms depend on the tumor’s location and size, with GI bleeding the most common symptom, followed by abdominal discomfort, pain, abdominal distention, and weight loss[7]. Small, asymptomatic, indolent GISTs are discovered incidentally, whereas highly malignant GISTs are typically large and symptomatic at the time of diagnosis[7].
In the past, malignant GISTs were misdiagnosed mainly as leiomyosarcomas, and were considered one of the tumor types most refractory to conventional chemotherapy and/or radiation therapy[2]. However, the development of imatinib led to a paradigm shift in the management of metastatic GIST, and imatinib became the treatment of choice in the metastatic setting, later being used in earlier stages[2]. Given the success with surgery and targeted therapy, it is estimated that the prevalence of GIST is likely to be 10 times that of the reported incidence, with the number of GIST survivors approaching 135-155 per million per year[2].
Despite the progress in treatment strategies, about half of all GIST patients will experience disease recurrence[9]. With only two drugs - sunitinib and regorafenib – approved by the Food and Drug Administration (FDA) for GIST after imatinib failure, managing patients with primary and secondary resistance or those with refractory disease poses a huge challenge[9]. Moreover, appropriate GIST management requires a multidisciplinary approach, as the correct characterization of the tumor at diagnosis requires a specialized endoscopist, radiologist, and nuclear medicine physician, with treatment potentially involving a surgeon and a clinical oncologist[10].
Patients and physicians across the Middle East face additional unique challenges, including the lack of published epidemiological data resulting in limited knowledge about unique disease features and molecular patterns. Challenges related to drug availability, lack of standardization of care, and limited access to mutational analysis further impede appropriate GIST management across the region.
Although global guidelines set a framework for management, there are no local practice guidelines that meet the practical needs of regional physicians in a resource-limited environment. A consensus on the diagnosis and management of GIST tumors for the Gulf and Levant countries is necessary to improve health education, diagnostic capabilities, patient identification and screening, treatment access, and disease monitoring. Such guidelines would reiterate the need for local clinical trials to generate data to help benchmark local disease biology and genetics relative to published global data.
Therefore, a group of 11 experienced medical oncologists practicing across the Gulf and Levant region created the Rare Tumors GI Group. Through a series of short meetings conducted over a period of 1 year (2016-2017), the Rare Tumors GI Group drafted a narrative review and placing this in the context of the local challenges could highlight the need to standardize care across the region. This paper aims to review current practices in the region and describe regional GIST management challenges; in preparation for proposing local clinical practice recommendations.
GIST includes tumors with a wide biological spectrum at all sites of occurrence, with diverse patterns including nodular, cystic, and diverticular tumors[11]. GISTs are commonly seen in patients > 50 years of age[12]. GISTs are most commonly located in the stomach (60%-70%), followed by the duodenum (20%-25%), the anus and rectum (5%), and the esophagus and colon (< 5%)[7]. They are predominantly seen in women[1]. Signs and symptoms of GIST depend on the anatomic location and size of the tumor, with GI bleeding being the most common clinical manifestation. Pain due to tumor rupture, GI obstruction, or appendicitis can occur[11]. GIST commonly metastasizes to the abdominal cavity and the liver; uncommonly to the lymph nodes and lungs; and rarely to the bones, soft tissue, and skin[11].
Microscopic features of GIST tumors depend on the site. They may be cellular or hypocellular, with most being spindle-cell tumors (70%-80%) and a minority being epithelioid or mixed spindle, epithelioid (20%-30%); or, rarely, pleomorphic[7,11,12]. GIST tumors may have prominent vascularity[7].
GIST tumors are immunohistochemically positive for KIT [cluster of differentiation (CD)117] (94.7%) and Discovered on GIST-1 (DOG1) (94.7%), and about 70%-80% co-express CD34[7,12]. GISTs may be positive for smooth-muscle actin (30%-40%), and rarely for S100 protein (5%), desmin and keratin (1%-2%)[12,13].
The clinicopathologic heterogeneity of GIST is associated with its molecular diversity, with the majority being spontaneous activating mutations in KIT (approximately 78.5%), and sometimes PDGFRA (5%-10%)[14]. About 10%-15% of GISTs do not harbor KIT/PDGFRA mutations and are known as wild type[13,15]. Given that the treatment of GIST depends on the mutations present, genotyping is integral to GIST management[16].
For tumors suspected to be GIST, biopsy is necessary to confirm diagnosis for surgical planning and initiating tyrosine kinase inhibitor (TKI) therapy[7,12]. For tumors < 2 cm detected within the esophagus, stomach, or duodenum, excision is necessary to make a histological diagnosis, as endoscopic biopsy is difficult[1]. As the majority of GIST tumors < 2 cm are likely to be low risk, the standard approach includes endoscopic ultrasound assessment and follow-up, with further excision only for patients with growing or symptomatic tumors[1]. Endoscopic ultrasound is preferred over percutaneous biopsies due to potential intraperitoneal tumor spillage with the latter[7].
Tumors ≥ 2 cm in size are at a high risk of progression and biopsy excision is standard practice[1,13]. Multivisceral resection using multiple-core needle biopsies and endoscopic ultrasound guidance or an ultrasound-/computed tomography (CT)-guided percutaneous method is a common approach[1]. For patients presenting with metastatic disease, laparotomy for diagnostic purposes may not be necessary and a biopsy of the metastatic focus is sufficient[1].
Plain abdominal imaging: Plain abdominal imaging is not specific for GIST diagnosis. Barium studies can suggest GIST by detecting a filling defect that is sharply demarcated and elevated compared with the surrounding mucosa[17].
Ultrasonography: Abdominal ultrasonography, although not optimal for GIST diagnosis, can evaluate liver involvement and the presence of tumor necrosis. Endoscopic ultrasonography (EUS) is useful for characterizing and assessing localization of lesions, especially < 2 cm[18].
Computed tomography scanning of the abdomen and pelvis: CT is the method of choice for diagnosing and staging GISTs[19]. It provides comprehensive information regarding tumor size and multiplicity, presence of calcifications, irregular margins, ulcerations, heterogeneity, regional lymphadenopathy, evidence of extraluminal and mesenteric fat infiltration, location, and relationship to adjacent structures[20].
Magnetic resonance imaging (MRI): MRI provides similar information to CT but is more accurate in identifying rectal GISTs and liver metastasis, hemorrhage, and necrosis[18].
Positron emission tomography (PET) scanning with 2-(F-18)-fluoro-2-deoxy-D-glucose: PET scanning with 2-(F-18)-fluoro-2-deoxy-D-glucose can be used as an adjunct to CT scanning for preoperative staging work-up, to distinguish viable lesions from necrotic tissue, benign from malignant tissue, and scar tissue from recurrent tumor. PET scanning facilitates monitoring of early clinical responses to neoadjuvant therapies and identification of early recurrence[21].
In addition to tumor location, morphology, and immunohistochemistry, mutational analyses of KIT and PDGFRA genes are important for diagnosis[13]. About 80% of GIST tumors have an oncogenic mutation in the KIT tyrosine kinase domain, mostly encoded by KIT exon 11, although some occur in exons 9, 13, and 17[13]. A subset of GIST tumors typically demonstrating an epithelioid morphology and expressing little or no KIT may also have an activating mutation in the KIT-homologous tyrosine kinase PDGFRA but this can only be determined through molecular analysis[13]. An estimated 5%-7.5% of GIST tumors, predominantly in the stomach, harbor the PDGFRA mutation, with two-thirds of these having the PDGFRA D842V mutation[22].
The National Comprehensive Cancer Network (NCCN) strongly recommends undertaking mutational analysis, especially if imatinib therapy is required for unresectable or metastatic disease or in patients with primary disease, particularly for high-risk tumors[13]. The European Society for Medical Oncology (ESMO) recommends mutational analysis as standard practice in diagnostic work-up of all GISTs due to its prognostic value and ability to predict sensitivity to therapy[1].
Risk classification systems have been developed and validated to predict the probability of postoperative relapse, including the National Institutes of Health (NIH) consensus classification (Fletcher’s criteria), Armed Forces Institute of Pathology criteria (Miettinen’s criteria), the “modified NIH” classification (Joensuu’s criteria), and the modified Fletcher risk classification[23-26].
Stratifying GISTs into low-, intermediate-, and high-risk categories is preferred to classification into benign or malignant, as a small number of GISTs with a histologically benign appearance may recur or metastasize[12]. Such categorization helps select patients for adjuvant imatinib therapy[26]. Unlike other classification systems, the “modified NIH” classification includes “tumor rupture”, a prognostic indicator for predicting the benefit of further treatment with adjuvant imatinib therapy[27].
Tumor size and mitotic index are important prognostic features in risk stratification[13]. Assuming that all GISTs have malignant potential, Miettinen and colleagues demonstrated that the anatomic location of the tumor affects the risk of recurrence and progression[13].
Surgery: Complete surgical resection with negative margins, without causing tumor rupture and with economic resection of the underlying organ, is the mainstay curative treatment for localized GIST[1,28]. This is feasible due to the exophytic growth pattern of these tumors. Negative margins can be easily achieved with organ-sparing segmental or wedge resections of the organ[23,28,29]. Furthermore, as lymphatic spread of the tumor is rare and lymph node dissection is generally not necessary, complete surgical resection can be achieved without sacrificing organ function[23,27,29].
Potential complications of surgery, especially for large tumors, include intraoperative bleeding and tumor rupture, resulting in spillage of tumoral contents into the peritoneal cavity[24,28].
Role of laparoscopic surgery: Surgeons have increasingly adopted a minimally invasive surgical approach. Evidence suggests that, in select patients, endoscopic or laparoscopic removal of GISTs yields recurrence rates comparable to open resection, improves long-term survival and enables better short-term postoperative outcomes[24,29,30].
The technical feasibility of performing an oncologically safe and effective laparoscopic or endoscopic procedure, without risk of rupture or incomplete removal, should be predetermined based on preoperative tumor evaluation[24,29,30]. The stomach is the only organ where either laparoscopic or endoscopic procedures can be performed safely and reliably in well-selected patients[29].
Although complete surgical resection of localized GISTs is successful in approximately 95% of cases, relapse affects approximately 40% of patients, particularly within the first 5 years after surgery[25,30-32]. The liver and peritoneum are the most common sites of recurrence[32]. Estimating tumor prognosis and the risk of postoperative recurrence is essential to tailoring patient management[23,25,26].
Imatinib adjuvant therapy: Depending on the risk of recurrence following complete surgical resection, adjuvant therapy should be initiated[33]. Traditional chemotherapy and radiotherapy are ineffective against GISTs[34,35]. Molecular targeted therapies, such as TKIs imatinib, sunitinib, and regorafenib have gained approval by the FDA for treatment of GIST[27,36]. Imatinib is an oral, selective TKI that inhibits KIT and PDGFRA, preventing tumor proliferation. It is regarded as the primary adjuvant treatment for postoperative GIST patients with a high risk of recurrence and is generally well tolerated[28,31,35].
Several clinical trials have confirmed the clinical benefits and acceptable safety profile of imatinib adjuvant treatment in surgically resected GIST patients with substantial risk of relapse. A randomized placebo-controlled study evaluated the impact of 1-year adjuvant imatinib therapy (400 mg daily) in patients with primary, localized, KIT-positive GIST (> 3 cm) who had undergone gross surgical excision and had low, intermediate, or high risk of recurrence. A significant difference was observed in the 1-year recurrence-free survival (RFS) rates (imatinib 98% vs placebo 83%) but not for overall survival (OS)[37].
The efficacy of 2-year imatinib adjuvant therapy (400 mg daily) was investigated in surgically resected, KIT-positive GIST patients showing high or intermediate risk of recurrence. The 3-year RFS rates were higher in the adjuvant imatinib group (84%) vs placebo (66%), with no impact on survival outcomes[38].
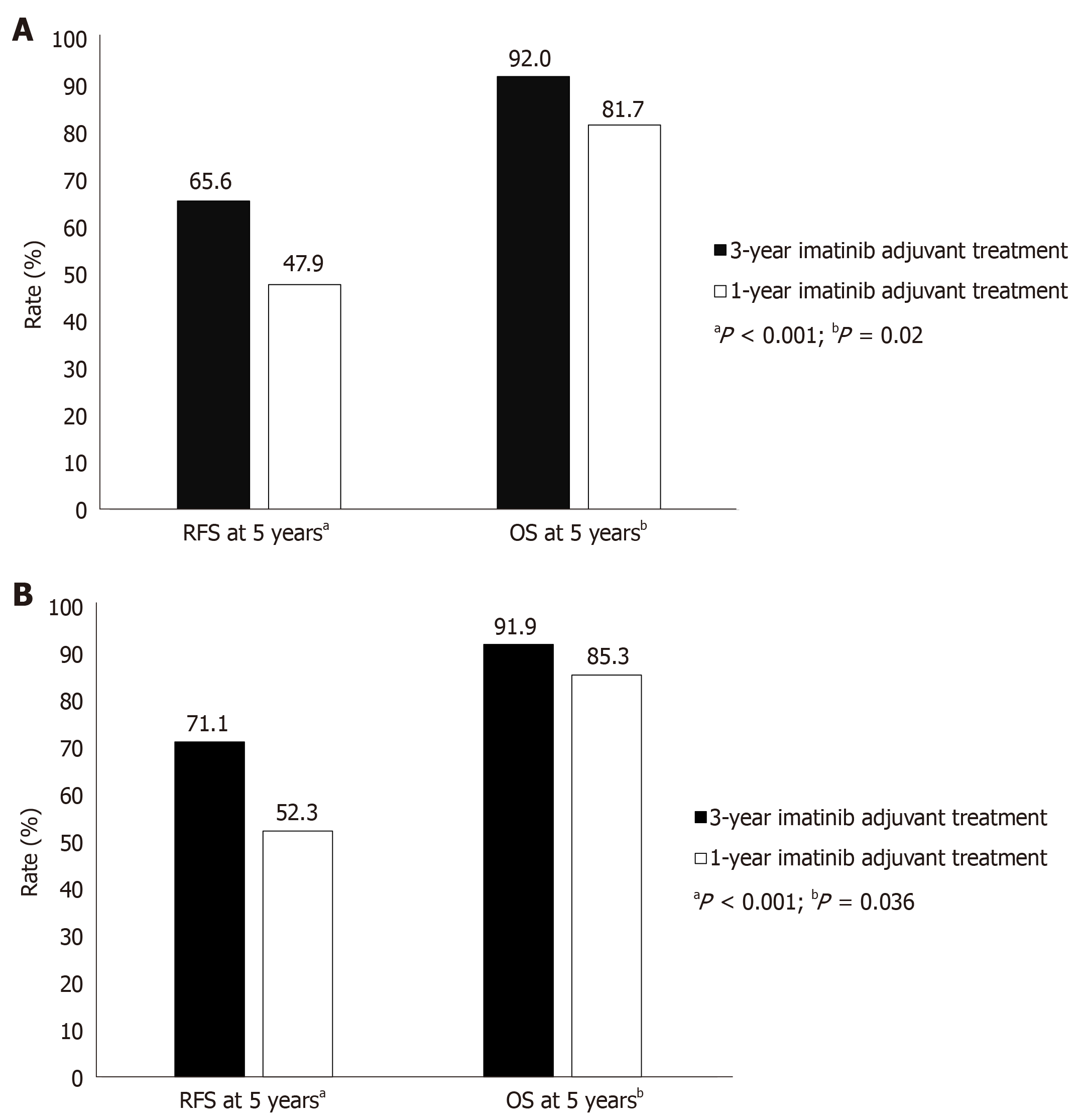
A further trial demonstrated the efficacy of 3-year imatinib adjuvant treatment in GIST patients who had undergone tumor resection and had high risk of recurrence. At a median follow-up of 54 mo, 5-year RFS and OS were significantly greater in imatinib patients treated for 3 years vs 1 year (Figure 1A), with acceptable tolerability[39]. In a subset of patients with centrally confirmed GIST and without macroscopic metastases at study entry, with a median follow-up of 90 mo, 3-year treatment resulted in significantly higher RFS and OS than did 1-year treatment (Figure 1B)[40].
Therefore, adjuvant imatinib therapy in postoperative high-risk GIST patients improves RFS, with an acceptable tolerability profile. Length of imatinib treatment influences treatment response, with greater survival with 3 years of treatment vs 1 year. Therefore, 3-year adjuvant imatinib treatment is recommended to improve RFS and OS in high-risk GIST patients who have undergone complete surgical resection of the primary localized tumor[1,23,27].
Controversies surrounding the optimal treatment duration and its role in patients with intermediate risk continue[1,25,27,35]. As there are insufficient data available and a 10% risk of relapse, a standard recommendation cannot be made. A shared decision with the patients regarding adjuvant therapy is necessary for intermediate-risk patients[39].
Whilst the current standard of practice is 3-year adjuvant imatinib therapy in high-risk patients, further investigation of longer treatment duration and outcomes is ongoing. The PERSIST-5 trial, in high-risk GIST patients, demonstrated that 5-year adjuvant imatinib treatment achieves 5-year RFS and OS in 90% and 95% of patients, respectively, with an acceptable tolerability profile[41]. Further ongoing clinical trials aim to compare the efficacy and safety of 5- and 6-year adjuvant imatinib treatment with additional 3-year treatment in high-risk GIST patients, and the results will likely affect treatment recommendations[25].
The efficacy of imatinib varies with KIT/PDGFRA mutation type[25]. Clinical data suggest that adjuvant imatinib treatment improves RFS in GIST patients with deletions in KIT exon 11, but not in patients with some mutations in KIT exon 11 or 9[42,43]. Despite the absence of data in adjuvant studies, dose escalation up to 800 mg, instead of the standard 400 mg dose, could be beneficial in patients with KIT exon 9 mutations[1,34]. Furthermore, adjuvant imatinib treatment is not recommended in patients with the PDGFRA D842V mutation or those with GIST WT, as treatment is ineffective[1,25,27]. Therefore, in addition to the assessment of postoperative recurrence risk, mutation analysis should part of the decision-making process prior to imatinib adjuvant therapy initiation, as recommended by the ESMO and EUROCAN[1,27,33].
If tumor rupture, an unfavorable prognostic factor, occurs before or during surgery, adjuvant imatinib therapy should be initiated due to the high risk of peritoneal relapse, which has significant impact on progression-free survival (PFS)[44]. Patients with gastric GIST with KIT exon 11 mutation (codon 557 and 558) are at increased risk of tumor rupture[44]. The optimal duration of treatment in these cases remains undetermined[1].
Imatinib neoadjuvant therapy for localized GIST: Neoadjuvant imatinib should be considered in patients with initially unresectable or borderline resectable bulky tumors. A prospective phase 2 trial evaluated an 8-12 wk short course of 600 mg neoadjuvant imatinib in 63 GIST KIT+ or recurrent resectable tumors[45]. The estimated 5-year PFS and OS rates for localized disease were 55% and 77%, respectively, with only 7% achieving a partial remission (PR)[45]. A multinational phase 2 study involving patients with gastric GISTs ≥ 10 cm demonstrated that neoadjuvant imatinib therapy (400 mg once daily) for 6-9 mo allowed a substantial proportion of patients to undergo R0 surgery (90%)[46]. Furthermore, 2-year PFS and OS rates of 89% and 98% were achieved, respectively, over a median follow-up of 32 mo[46].
Based on the encouraging findings from clinical studies, neoadjuvant imatinib treatment is indicated if reducing the tumor bulk prior to surgery would permit less-mutilating organ-preserving surgery with R0 margins and reduce the risk of intraoperative tumor rupture and bleeding or if achievement of microscopically negative margins is not feasible. Administering imatinib for approximately 3-12 mo, depending on the strictness of radiological follow-up and the burden of tumor, is advised to limit surgical morbidity. Functional imaging is advised to assess response to treatment, and unresponsive patients should undergo surgery without delay[1]. Furthermore, mutational analysis can identify patients with imatinib-resistant forms, such as PDGFRA D842V, or those who require higher doses of imatinib in order to prevent delays in surgery.
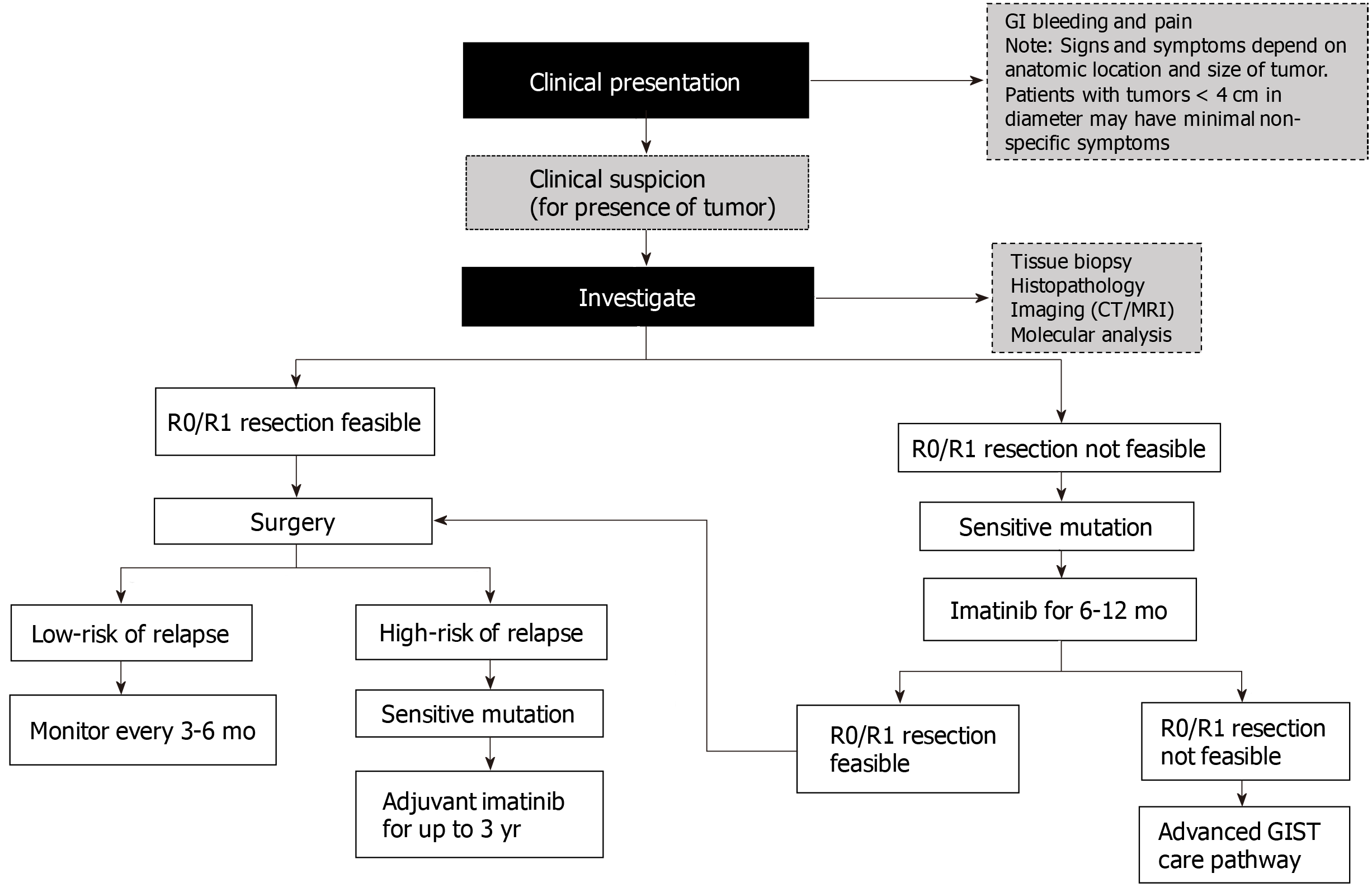
Postoperative follow-up: Postoperative follow-up is important for early detection and treatment of relapses[27]. Optimal follow-up timings remain undefined due to insufficient data on the frequency and intervals of routine postoperative follow-up visits[1,27]. Recurrences most commonly occur in the liver and/or the peritoneum; therefore, abdominal and pelvic contrast CT is adequate for detecting relapses. In young patients, MRI should be used to avoid the ionizing radiation risk associated with CT. The risk of relapse is particularly high during the first few years after surgery and following discontinuation of adjuvant imatinib therapy[27]. Therefore, based on a patient’s risk stratification (based on mitotic count, tumor size, and tumor site), CT or MRI can be used for monitoring, according to the schedules shown in Table 1[1]. Figure 2 shows an algorithm for managing localized primary GIST.
| Risk | Monitoring frequency |
| High-risk tumors | Every 3 to 6 mo for the first 3 yr of adjuvant therapy |
| Every 3 mo for 2 yr after stopping adjuvant imatinib, then every 6 mo for 5 yr, then once a year for 5 yr | |
| Low-risk tumors | Every 6 to 12 mo for 5 yr |
| Very low-risk tumors | Routine follow-up may not be necessary; however, risk of recurrence is not nil |
Role of surgery: The role of complete surgical resection of localized GIST is well established; however, for patients with locally advanced, metastatic GIST, responding to imatinib therapy, the role of surgery is unclear[47]. A small Chinese trial of 41 GIST patients demonstrated higher 2-year PFS and OS rates in the surgery with imatinib group (88%; not reached) vs the imatinib group (58%; 49 mo). Despite this uncertainty, surgery in patients with advanced GIST has the potential to be used as an adjunct to imatinib in responding patients with metastases or recurrent disease in an effort to improve disease-free survival and OS[48]. Ideally, surgery should be avoided in those with imatinib-resistant disease unless for emergency palliative intervention[48].
Although patients with advanced unresectable or metastatic GIST may achieve PR or stable disease while on imatinib, about half are highly likely to develop secondary resistance within 2 years[47]. Cytoreductive surgery may be considered in patients with metastatic GIST who respond to imatinib, especially if R0/R1 resection can be achieved. In patients with multifocal progression, surgery leads to poor outcomes[47]. In patients with metastatic GIST treated with sunitinib, surgery may be feasible; however, resections are commonly incomplete, associated with complications and have unclear survival benefit[47]. Imatinib should be continued even if the surgical resection is complete.
First line: Imatinib, at the standard dose of 400 mg per day, has demonstrated efficacy in advanced, metastatic GIST with an average prolongation of median PFS time of 24 mo[49,50]. Higher doses have largely shown no clinical benefit. In two phase 3 trials, clinical benefit rates for imatinib 400 mg and 800 mg per day in patients with metastatic GIST were approximately 90% and 88%, respectively[49,51]. However, this benefit can vary according to GIST mutation. Pooled analysis of 768 patients across four clinical trials revealed that patients with mutations in KIT exon 11 and 9 and those with GIST WT, had objective response rates (complete or partial response) of 72%, 38%, and 28%, respectively[52,53]. A dose-dependent improvement in response was, however, seen in patients with KIT exon 9 mutations[53]. GIST mutational status can also contribute to differences in overall OS and time to progression (TTP) events. Patients testing positive for KIT exon 11 and 9 mutations and WT GIST genotypes had TTPs of 25, 17, and 12.8 mo, respectively. The corresponding OS improvement in these patients was 60, 38, and 49 mo, respectively[53]. Patients with GISTs harboring the PDGFRA D842V mutation appear to be resistant to imatinib[53]. Furthermore, GIST patients with SDH deficiency or NF1 mutation rarely respond to imatinib[54,55]. Imatinib treatment interruption poses a threat to control of metastatic disease, as discontinuation has resulted in disease progression that may not be fully reversed by rechallenge[56,57].
Cytoreductive surgery, following a response to imatinib, has improved survival[58,59]. In fact, no evidence of disease was found after the procedure in 78% of patients who had achieved stable disease before surgery. The 12-mo OS and PFS rates in these patients were 95% and 80%, respectively.
These findings suggest testing patient genotype before starting treatment for metastatic GIST. In patients intolerant to or who progress on imatinib therapy, second-line therapy with sunitinib may be considered. Before progressing to second-line options, however, physicians should ensure patient compliance with imatinib therapy for at least 2 additional months with modification of the timing of tablet intake[60]. Furthermore, imatinib plasma levels can be checked; if low (< 1000 ng/mL) increasing the dose to 800 mg daily may be beneficial; if high, switching to second-line therapy is recommended[61]. Physicians should carefully consider potential drug interactions with imatinib: proton pump inhibitors are known to decrease imatinib plasma levels to subtherapeutic levels[53,62].
Second line: Sunitinib, an FDA-approved multitargeted TKI, is indicated in imatinib-refractory or imatinib-intolerant GIST patients[63,64]. This indication was approved following an international phase 3 trial of sunitinib vs. placebo in 312 GIST patients after imatinib failure. Patients receiving sunitinib had longer TTP (27 mo) vs placebo (6 mo), despite low objective response rates (7%) and an absence of OS benefits over time. Sunitinib is recommended at a daily dose of 50 mg for 4 wk followed by a 2-wk rest interval; lower (37.5 mg), continuous daily dosing can also be used[63,65,66].
Response to sunitinib may also be driven by mutation type. Clinical benefit was observed to be higher in patients with KIT exon 9 mutation and GIST WT (58% and 56%) than with KIT exon 11 mutation (34%). After initial progression to imatinib, TTP was 19 mo in patients with KIT exon 9 or PDGFRA mutation vs 5 mo in patients with KIT exon 11 mutation. In patients with secondary KIT exon 13 and 14 mutations, both OS and PFS were significantly longer than in those with KIT exon 17 or 18 mutations. Common side effects relating to sunitinib include renal toxicity (proteinuria), hematological effects (myelosuppression, thrombotic microangiopathy), thyroid dysfunction (hypothyroidism), hypertension, and GI bleeding or perforation.
Third line: Regorafenib, at a daily dose of 160 mg taken for 21 d in 28-d cycles, has been approved for the treatment of patients with unresectable or metastatic GIST after failure of or intolerance to imatinib and sunitinib therapy. A phase 2 trial of 34 GIST patients who experienced failure of imatinib and sunitinib demonstrated 26 patients with a clinical benefit with regorafenib (four had partial response), with a median PFS of 10 mo. In a confirmatory phase 3 trial of 129 GIST patients, PFS was higher for regorafenib (4.8) than placebo (0.9), without OS benefit[67]. In contrast to sunitinib, regorafenib may be beneficial for patients with metastatic and/or unresectable GIST harboring KIT exon 11 mutations, and SDH-deficient GIST, but not GIST with secondary KIT exon 17 mutations[68]. The most common side effects associated with regorafenib include hypertension, hand-foot syndrome, and diarrhea.
According to the NCCN guidelines, later lines of therapy after failure of the three FDA-approved drugs (imatinib, sunitinib, and regorafenib) include sorafenib and third generation TKIs such as pazopanib, nilotinib, ponatinib, or dasatinib. Sorafenib, at a dose of 400 mg twice daily, has also demonstrated efficacy in patients with metastatic GIST who have progressed on imatinib and sunitinib therapy[69]. In contrast, in the multicenter, phase 2 trial of sorafenib including 38 KIT-expressing GIST patients, the disease control rate was 68%, median PFS 5.2 mo, and median OS 11.6 mo[70].
Pazopanib achieved favorable 4-mo PFS rates in a phase 2 trial compared with placebo (45% vs 18%, respectively; P = 0.03) [71]. Ponatinib has been shown to suppress all KIT secondary mutations with the exception of V654A[72]. The clinical benefit rate achieved with ponatinib therapy was 55% in heavily pretreated (including regorafenib) patients with GIST harboring primary KIT exon 11 mutation[73]. Dasatinib has proven to be active against KIT WT tumors, particularly PDGFRA D842V, which is normally resistant to imatinib[74]. Further details on these emerging therapies are provided in Table 2.
| Target | Class of agent (specific activity) | Drug(s) | Trial number/ study phase | Results | Ref. |
| KIT/PDGFRA | Multitargeted TKI (KIT exon 17 D816-mutant kinases) | Ponatinib | NCT01874665 Phase 2 | 37% CBR at 16 weeks | [78] |
| KIT exon 13 resistance mutations | Ponatinib | NCT03171389 Phase 2 | Awaited | [79] | |
| Multitargeted TKI (PDGFRA D842V) | Dasatinib | Phase 2 | 32% PR; 21% PFS at 6 months | [74] | |
| NCT01643278 Phase 1 (+ ipilimumab) | Awaited | [80] | |||
| Multitargeted TKI | Crenolanib | NCT01243346 Phase 1/2 study | 31% CBR | [81] | |
| KIT D816V/PDGFRA D842V inhibitor | BLU285 | NCT02508532 Phase 1 | ORR 84% in PDGFRA D842V GIST and ORR 20% for fourth-line or later; tumor reduction 98% in PDGFRA D842V and 60% in fourth-line or later | [82,83] | |
| KIT exon 9, 11, 13, 14, 17, and 18 inhibitor | DCC-2618 | NCT02571036 Phase 1 | ORR 14%, 3-month DCR 22%, mPFS at 24 weeks 56% | [84] | |
| PI3K | PI3K inhibitor | BYL719 | NCT01735968 Phase 1 | Awaited | [85] |
| Selective PI3K catalytic p110α subunit inhibitor | Buparlisib | NCT01468688 Phase 1 | Awaited | [86] | |
| BRAF V600E | BRAF inhibitor | Vemurafenib | NCT02304809 Phase 2 | Awaited | [87] |
| MEK | MEK inhibitor | Binimetinib | NCT01991379 Phase 1b/2 (+ imatinib) | 33% PR | [88] |
| MET | Dual MET and KIT small-molecule inhibitor | Cabozantinib | Phase 1 | Long-lasting SD as best response | [89] |
| NCT02216578 Phase 2 | Awaited | [90] | |||
| FGFR | Pan-FGFR inhibitor | BGJ398 | NCT02257541 Phase 1b/2 (+ imatinib) | Awaited | [91] |
| IGF1R | IGF1R inhibitor | Linsitinib | NCT01560260 Phase 2 | 45% CBR; 52% PFS, 80% OS at 9 months | [92] |
| HSP90 | Non-ansamycin HSP90 inhibitor | Onalespib | NCT01560260 Phase 1 | 36% CBR | [93] |
| NCT01294202 Phase 2 (± imatinib) | Awaited | [94] | |||
| CTLA4 | Anti-CTLA4 antibody | Ipilimumab | NCT01738139 Phase 1 (+ imatinib) | Single PR | [95] |
| NCT01643278 Phase 1 (+ dasatinib) | Single durable SD for 59+ weeks | [80] | |||
| CDK | CDK4/6 inhibitor | Palbociclib | NCT01907607 Phase 2 | Awaited | [96] |
Clinical trials for GIST are based on recent advances and understanding of molecular differences. Several new pathways have been targeted, alone or in combination, in clinical trials to overcome primary and/or secondary acquired resistance to existing GIST treatment. Table 2 outlines potential key therapeutic targets for GIST.
Correct characterization of GIST at the time of initial diagnosis is crucial to the proper management of these tumors. Clinical decision making is based on histopathology, immunohistochemistry, and molecular diagnosis of the cancer subtype. Therefore, a multidisciplinary team at a comprehensive cancer care center is necessary for formulating patient care plans based on the best-available published evidence. Before a diagnostic and therapeutic strategy is initiated, suspected GIST patients require a discussion with a multidisciplinary tumor board, including sarcoma experts in medical oncology, surgical oncology, radiation oncology, radiology, and pathology. The development of local and national multidisciplinary meetings in the Middle Eastern region is mandatory but faces several obstacles, mainly the private medical system.
Pretreatment biopsy of large tumors is mandatory in order to prevent unnecessary measures. Specialized endoscopists, diagnostic/intervention radiologists, and sarcoma surgeons are integral to the process of tumor sampling and staging. A tumor tissue sample helps ascertain subtype for a GIST diagnosis. Lack of experience and proper tools at a non-cancer facility contribute to poor tumor sampling and poor fixation and preservation of tumor structure. Therefore, training programs and awareness campaigns for medical doctors and surgeons on the proper management of GIST patients are essential to decrease the removal of uncharacterized tumors that might benefit from medical therapy only, such as lymphomas.
Another challenge is the lack of experts in pathological analysis of different types of sarcomas including GIST. First, without a wide immunostaining panel and molecular analyses, it is difficult to differentiate GIST from other pathologies. Proper pathological assessment with wide molecular profiling should be implemented through medical societies and regional groups. There is a lack of histopathologists with proper expertise in sarcoma in the Middle Eastern region, which highlights the need to implement targeted formations for specialists, with special focus on sarcomas and on different diagnostic methods (molecular analysis in GIST). Recent data have demonstrated the importance of next-generation DNA sequencing in identifying all possible mutations within a tumor sample and determining the correct treatment. However, next-generation sequencing is expensive, has a long runtime, and requires technical and interpretational expertise.
The final diagnostic challenge is the limited access to radiological assessments such as PET scans due to their high cost and limited availability, presenting a significant hurdle to proper diagnosis and subsequent management.
Surgery is critical to GIST management and remains the only potentially curative treatment for resectable GIST; however, oncologic surgery is still in its nascent stage and onco-surgeons are often inappropriately trained. The lack of harmony between the onco-/general surgeon and the medical oncologist is another challenge in defining the steps before and after diagnosis and staging. For those with locally advanced GISTs, preoperative imatinib mesylate for 6–9 mo to shrink the tumor, followed by complete cytoreductive surgery, is the optimal plan; early surgery by a general surgeon carries an increased risk of surgery-related morbidity and worse oncological outcomes. Most patients are managed by surgeons and gastroenterologists with limited expertise in oncology.
There is a lack of radiological availability in some regions (CT scans, MRIs, or PET scans), which may limit the initiation of neoadjuvant therapy or the optimal follow-up of GIST during therapy. In addition, health authorities across the Gulf region do not have access to any guidelines that regulate management of cancer patients at general hospitals or in the private sector. This has the potential to lead to poor management of patients outside a specialized cancer center by a non-specialized team. Comprehensive cancer care centers can guarantee the availability of specialized manpower and access to latest technology.
Medication access and local formulary approvals are a big challenge and need to be optimized to enable optimal treatment of GIST patients. The medical systems in the region do not allow all patients full access to recently developed TKIs or even clinical trials. For example, the Arabian Gulf, which has a population of 20 million, has a healthcare system that is publicly funded. The treatment of GIST with TKIs represents a new era of molecular targeted therapy. Expensive drugs such as TKIs are reimbursed by the national health insurance system for its citizens; however, non-citizen residents have to find alternative methods to pay for treatment, which varies based on the Gulf country they reside in. For instance, in the United Arab Emirates, third-party insurance can cover treatment-related expenses within an annual budget; in Kuwait, surgery for cancer patients is allowed in the private sector but anti-cancer treatment is not allowed to be prescribed outside the Ministry of Health Cancer Center. Charities, such as the Patients Helping Fund Society in Kuwait takes the lead to reimburse treatment for non-citizen residents after a long process of financial assessment. Across the Gulf region, imatinib is reimbursed up to a dose of 400-800 mg orally per day for metastatic disease and for up to 3 years for adjuvant treatment of high-risk GISTs. Sunitinib can be prescribed and is reimbursed after imatinib failure, and regorafenib has recently become available for routine use, except in Iraq where it is not a formulary drug. In Lebanon, where the drug is reimbursed by all insurers including the Ministry of Public Health, the challenge is the sub-standard generics that might be included in the therapeutic arsenal. In some other countries, due to economic restrictions or war situations, such drugs might not be reimbursed.
The importance of setting guidelines in this region is to offer physicians an insight into proper management and drug usage with the available amenities. Another important drawback in the management of GIST patients is the limited access to international clinical trials in which patients might benefit from the latest treatment novelties without added costs.
On the patient level, a better understanding of the risks associated with poor treatment compliance is needed. Early discontinuation of imatinib has severe consequences with an increase in relapse rates (up to 49% of early discontinuation rates in the PERSIST trial). Frequent physician visits and closer follow-up are recommended to ensure optimal compliance of TKI intake.
The Middle Eastern society is traditionally conservative with strong religious and cultural beliefs[75]. Cancer diagnosis is still considered, in some regions, as a death certificate and family bonds have an impact on limiting patients’ access to information about their health status[76]. Up to 40% of patients are unaware of their diagnosis, which could also impact their compliance with treatment[77]. In general, patients are apt to discontinue oral medications because of a lack of information concerning their initial diagnosis and prognosis. These limitations can be overcome by empowering the physician-patient relationship.
Overall, the lack of sufficient clinical trials, national disease registries, and regional scientific research into GIST epidemiology, tumor characteristics, prognostic features, tolerance to treatment, and quality of life of patients highlights the long road ahead in establishing standards of care that are consistent across treatment centers irrespective of geographical reach[6].
Counseling of patients and their family members concerning the value of preoperative treatment remains a challenge faced by some oncologists due to the risk of primary resistance to the treatment and the possibility of disease progression.
Multiple challenges remain for recurrent/metastatic disease management, including limited affordability of care, lack of proper testing of resistance to imatinib mesylate, and limited availability of subsequent lines of therapy after imatinib mesylate failure.
Medical writing support in the development of this manuscript was provided by Leris D’Costa of OPEN Health Dubai.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Lebanon
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chiu KW, Cerwenka H S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ
| 1. | Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Brodowicz T. Broto JM, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dileo P, Eriksson M, Fedenko A, Ferraresi V, Ferrari A, Ferrari S, Frezza AM, Gasperoni S, Gelderblom H, Gil T, Grignani G, Gronchi A, Haas RL, Hassan B, Hohenberger P, Issels R, Joensuu H, Jones RL, Judson I, Jutte P, Kaal S, Kasper B, Kopeckova K, Krákorová DA, Le Cesne A, Lugowska I, Merimsky O, Montemurro M, Pantaleo MA, Piana R, Picci P, Piperno-Neumann S, Pousa AL, Reichardt P, Robinson MH, Rutkowski P, Safwat AA, Schöffski P, Sleijfer S, Stacchiotti S, Sundby Hall K, Unk M, Van Coevorden F, van der Graaf WTA, Whelan J, Wardelmann E, Zaikova O, Blay JY, ESMO Guidelines Committee and EURACAN. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv68-iv78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 289] [Article Influence: 41.3] [Reference Citation Analysis (1)] |
| 2. | Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 520] [Article Influence: 57.8] [Reference Citation Analysis (1)] |
| 3. | Makar RR, al-Waheeb S, John B, Junaid TA. Gastrointestinal stromal tumors: clinicopathological and immunohistochemical features. Med Princ Pract. 2002;11:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Al-Thani H, El-Menyar A, Rasul KI, Al-Sulaiti M, El-Mabrok J, Hajaji K, Elgohary H, Tabeb A. Clinical presentation, management and outcomes of gastrointestinal stromal tumors. Int J Surg. 2014;12:1127-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Bokhary RY, Al-Maghrabi JA. Gastrointestinal stromal tumors in western Saudi Arabia. Saudi Med J. 2010;31:437-441. [PubMed] |
| 6. | El Rassy E, Nasr F, Assi T, Ibrahim T, Rassy N, Bou Jaoude J, Massoud M, Chahine G. Epidemiology and Survival Analysis of Gastrointestinal Stromal Tumors in Lebanon: Real-life study from a Hospital tumor registry 2000-2015. Gulf J Oncolog. 2017;1:38-42. [PubMed] |
| 7. | Levy AD, Manning MA, Al-Refaie WB, Miettinen MM. Soft-Tissue Sarcomas of the Abdomen and Pelvis: Radiologic-Pathologic Features, Part 1-Common Sarcomas: From the Radiologic Pathology Archives. Radiographics. 2017;37:462-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Li H, Ren G, Cai R, Chen J, Wu X, Zhao J. A correlation research of Ki67 index, CT features, and risk stratification in gastrointestinal stromal tumor. Cancer Med. 2018;7:4467-4474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Mei L, Du W, Idowu M, von Mehren M, Boikos SA. Advances and Challenges on Management of Gastrointestinal Stromal Tumors. Front Oncol. 2018;8:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Sanchez-Hidalgo JM, Duran-Martinez M, Molero-Payan R, Rufian-Peña S, Arjona-Sanchez A, Casado-Adam A, Cosano-Alvarez A, Briceño-Delgado J. Gastrointestinal stromal tumors: A multidisciplinary challenge. World J Gastroenterol. 2018;24:1925-1941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466-1478. [PubMed] |
| 12. | Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 2003;23:283-304, 456; quiz 532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 324] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 13. | Demetri GD, Benjamin RS, Blanke CD, Blay JY, Casali P, Choi H, Corless CL, Debiec-Rychter M, DeMatteo RP, Ettinger DS, Fisher GA, Fletcher CD, Gronchi A, Hohenberger P, Hughes M, Joensuu H, Judson I, Le Cesne A, Maki RG, Morse M, Pappo AS, Pisters PW, Raut CP, Reichardt P, Tyler DS, Van den Abbeele AD, von Mehren M, Wayne JD, Zalcberg J; NCCN Task Force. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw. 2007;5 Suppl 2:S1-29; quiz S30. [PubMed] |
| 14. | Charville GW, Longacre TA. Surgical Pathology of Gastrointestinal Stromal Tumors: Practical Implications of Morphologic and Molecular Heterogeneity for Precision Medicine. Adv Anat Pathol. 2017;24:336-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Wada R, Arai H, Kure S, Peng WX, Naito Z. "Wild type" GIST: Clinicopathological features and clinical practice. Pathol Int. 2016;66:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Szucs Z, Thway K, Fisher C, Bulusu R, Constantinidou A, Benson C, van der Graaf WT, Jones RL. Molecular subtypes of gastrointestinal stromal tumors and their prognostic and therapeutic implications. Future Oncol. 2017;13:93-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Yamashita F, Sasatomi E, Kiyama M, Fukumori K, Yano Y, Kato O, Sakai T, Kiyomatsu K, Hirose N, Yamamoto H, Tokunaga O, Tanaka M, Toyonaga A, Sata M. Radiographic observation of a case of gastrointestinal stromal tumor in stomach. Kurume Med J. 2001;48:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Vernuccio F, Taibbi A, Picone D, LA Grutta L, Midiri M, Lagalla R, Lo Re G, Bartolotta TV. Imaging of Gastrointestinal Stromal Tumors: From Diagnosis to Evaluation of Therapeutic Response. Anticancer Res. 2016;36:2639-2648. [PubMed] |
| 19. | Lanke G, Lee JH. How best to manage gastrointestinal stromal tumor. World J Clin Oncol. 2017;8:135-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Belloni M, De Fiori E, Mazzarol G, Curti A, Crosta C. Endoscopic ultrasound and computed tomography in gastric stromal tumours. Radiol Med. 2002;103:65-73. |
| 21. | Stroobants S, Goeminne J, Seegers M, Dimitrijevic S, Dupont P, Nuyts J, Martens M, Van den Borne B, Cole P, Sciot R. 18 FDG-positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec®). Eur J Cancer. 2003;39:2012-2020. [RCA] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 352] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 22. | Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, Kiese B, Eisenberg B, Roberts PJ, Singer S, Fletcher CD, Silberman S, Dimitrijevic S, Fletcher JA. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342-4349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1817] [Cited by in RCA: 1646] [Article Influence: 74.8] [Reference Citation Analysis (1)] |
| 23. | Akahoshi K, Oya M, Koga T, Shiratsuchi Y. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol. 2018;24:2806-2817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 180] [Cited by in RCA: 237] [Article Influence: 33.9] [Reference Citation Analysis (9)] |
| 24. | Lim KT. Surgical treatment of gastrointestinal stromal tumors of the stomach: current status and future perspective. Transl Gastroenterol Hepatol. 2017;2:104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 25. | Duffaud F, Le Cesne A. Recent advances in managing gastrointestinal stromal tumor. F1000Res. 2017;6:1689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Poveda A, del Muro XG, López-Guerrero JA, Martínez V, Romero I, Valverde C, Cubedo R, Martín-Broto J. GEIS 2013 guidelines for gastrointestinal sarcomas (GIST). Cancer Chemother Pharmacol. 2014;74:883-898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 323] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 28. | Thacoor A. Gastrointestinal stromal tumours: advances in surgical and pharmacological management options. J Gastrointest Oncol. 2018;9:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Gluzman MI, Kashchenko VA, Karachun AM, Orlova RV, Nakatis IA, Pelipas IV, Vasiukova EL, Rykov IV, Petrova VV, Nepomniashchaia SL, Klimov AS. Technical success and short-term results of surgical treatment of gastrointestinal stromal tumors: an experience of three centers. Transl Gastroenterol Hepatol. 2017;2:56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Poškus E, Petrik P, Petrik E, Lipnickas V, Stanaitis J, Strupas K. Surgical management of gastrointestinal stromal tumors: a single center experience. Wideochir Inne Tech Maloinwazyjne. 2014;9:71-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Koumarianou A, Economopoulou P, Katsaounis P, Laschos K, Arapantoni-Dadioti P, Martikos G, Rogdakis A, Tzanakis N, Boukovinas I. Gastrointestinal Stromal Tumors (GIST): A Prospective Analysis and an Update on Biomarkers and Current Treatment Concepts. Biomark Cancer. 2015;7:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Bamboat ZM, DeMatteo RP. Metastasectomy for gastrointestinal stromal tumors. J Surg Oncol. 2014;109:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Reichardt P, Blay JY, Boukovinas I, Brodowicz T, Broto JM, Casali PG, Decatris M, Eriksson M, Gelderblom H, Kosmidis P, Le Cesne A, Pousa AL, Schlemmer M, Verweij J, Joensuu H. Adjuvant therapy in primary GIST: state-of-the-art. Ann Oncol. 2012;23:2776-2781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Nakano K, Takahashi S. Current Molecular Targeted Therapies for Bone and Soft Tissue Sarcomas. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Zhao R, Wang Y, Huang Y, Cui Y, Xia L, Chen Y, Zhuang W, Zhou Y, Wu X. Adjuvant imatinib for patients with high-risk gastrointestinal stromal tumors: a retrospective cohort study. Sci Rep. 2017;7:16834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, Kane JM, Keedy V, Kim E, Koon H, Mayerson J, McCarter M, McGarry SV, Meyer C, Morris ZS, O'Donnell RJ, Pappo AS, Paz IB, Petersen IA, Pfeifer JD, Riedel RF, Ruo B, Schuetze S, Tap WD, Wayne JD, Bergman MA, Scavone JL. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:536-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 460] [Article Influence: 76.7] [Reference Citation Analysis (1)] |
| 37. | Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, Blackstein ME, Blanke CD, von Mehren M, Brennan MF, Patel S, McCarter MD, Polikoff JA, Tan BR, Owzar K; American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097-1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1075] [Cited by in RCA: 971] [Article Influence: 60.7] [Reference Citation Analysis (1)] |
| 38. | Casali PG, Le Cesne A, Poveda Velasco A, Kotasek D, Rutkowski P, Hohenberger P, Fumagalli E, Judson IR, Italiano A, Gelderblom H, Adenis A, Hartmann JT, Duffaud F, Goldstein D, Broto JM, Gronchi A, Dei Tos AP, Marréaud S, van der Graaf WT, Zalcberg JR, Litière S, Blay JY. Time to Definitive Failure to the First Tyrosine Kinase Inhibitor in Localized GI Stromal Tumors Treated With Imatinib As an Adjuvant: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in Collaboration With the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J Clin Oncol. 2015;33:4276-4283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 39. | Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schütte J, Ramadori G, Hohenberger P, Duyster J, Al-Batran SE, Schlemmer M, Bauer S, Wardelmann E, Sarlomo-Rikala M, Nilsson B, Sihto H, Monge OR, Bono P, Kallio R, Vehtari A, Leinonen M, Alvegård T, Reichardt P. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 682] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 40. | Joensuu H, Eriksson M, Sundby Hall K, Reichardt A, Hartmann JT, Pink D, Ramadori G, Hohenberger P, Al-Batran SE, Schlemmer M, Bauer S, Wardelmann E, Nilsson B, Sihto H, Bono P, Kallio R, Junnila J, Alvegård T, Reichardt P. Adjuvant Imatinib for High-Risk GI Stromal Tumor: Analysis of a Randomized Trial. J Clin Oncol. 2016;34:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 41. | Raut CP, Espat NJ, Maki RG, Araujo DM, Williams TF, Wolff JE, DeMatteo RP. Extended treatment with adjuvant imatinib (IM) for patients (pts) with high-risk primary gastrointestinal stromal tumor (GIST): The PERSIST-5 study. J Clin Oncol. 2017;35:11009-11009. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Corless CL, Ballman KV, Antonescu CR, Kolesnikova V, Maki RG, Pisters PW, Blackstein ME, Blanke CD, Demetri GD, Heinrich MC, von Mehren M, Patel S, McCarter MD, Owzar K, DeMatteo RP. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32:1563-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 221] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 43. | Joensuu H, Wardelmann E, Sihto H, Eriksson M, Sundby Hall K, Reichardt A, Hartmann JT, Pink D, Cameron S, Hohenberger P, Al-Batran SE, Schlemmer M, Bauer S, Nilsson B, Kallio R, Junnila J, Vehtari A, Reichardt P. Effect of KIT and PDGFRA Mutations on Survival in Patients With Gastrointestinal Stromal Tumors Treated With Adjuvant Imatinib: An Exploratory Analysis of a Randomized Clinical Trial. JAMA Oncol. 2017;3:602-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 44. | Boye K, Berner JM, Hompland I, Bruland ØS, Stoldt S, Sundby Hall K, Bjerkehagen B, Hølmebakk T. Genotype and risk of tumour rupture in gastrointestinal stromal tumour. Br J Surg. 2018;105:e169-e175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Wang D, Zhang Q, Blanke CD, Demetri GD, Heinrich MC, Watson JC, Hoffman JP, Okuno S, Kane JM, von Mehren M, Eisenberg BL. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: long-term follow-up results of Radiation Therapy Oncology Group 0132. Ann Surg Oncol. 2012;19:1074-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 46. | Eisenberg BL, Harris J, Blanke CD, Demetri GD, Heinrich MC, Watson JC, Hoffman JP, Okuno S, Kane JM, von Mehren M. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009;99:42-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 329] [Cited by in RCA: 283] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 47. | Keung EZ, Fairweather M, Raut CP. The Role of Surgery in Metastatic Gastrointestinal Stromal Tumors. Curr Treat Options Oncol. 2016;17:8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 48. | Ford SJ, Gronchi A. Indications for surgery in advanced/metastatic GIST. Eur J Cancer. 2016;63:154-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Heinrich MC, Owzar K, Corless CL, Hollis D, Borden EC, Fletcher CD, Ryan CW, von Mehren M, Blanke CD, Rankin C, Benjamin RS, Bramwell VH, Demetri GD, Bertagnolli MM, Fletcher JA. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008;26:5360-5367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 459] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 50. | Zalcberg JR, Verweij J, Casali PG, Le Cesne A, Reichardt P, Blay JY, Schlemmer M, Van Glabbeke M, Brown M, Judson IR; EORTC Soft Tissue and Bone Sarcoma Group, the Italian Sarcoma Group; Australasian Gastrointestinal Trials Group. Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer. 2005;41:1751-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 51. | Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M, Bertulli R, Judson I. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1270] [Cited by in RCA: 1205] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 52. | Debiec-Rychter M, Dumez H, Judson I, Wasag B, Verweij J, Brown M, Dimitrijevic S, Sciot R, Stul M, Vranck H, Scurr M, Hagemeijer A, van Glabbeke M, van Oosterom AT; EORTC Soft Tissue and Bone Sarcoma Group. Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2004;40:689-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 345] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 53. | Debiec-Rychter M, Sciot R, Le Cesne A, Schlemmer M, Hohenberger P, van Oosterom AT, Blay JY, Leyvraz S, Stul M, Casali PG, Zalcberg J, Verweij J, Van Glabbeke M, Hagemeijer A, Judson I; EORTC Soft Tissue and Bone Sarcoma Group; Italian Sarcoma Group; Australasian GastroIntestinal Trials Group. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;42:1093-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 644] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 54. | Mussi C, Schildhaus HU, Gronchi A, Wardelmann E, Hohenberger P. Therapeutic consequences from molecular biology for gastrointestinal stromal tumor patients affected by neurofibromatosis type 1. Clin Cancer Res. 2008;14:4550-4555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 55. | Boikos SA, Pappo AS, Killian JK, LaQuaglia MP, Weldon CB, George S, Trent JC, von Mehren M, Wright JA, Schiffman JD, Raygada M, Pacak K, Meltzer PS, Miettinen MM, Stratakis C, Janeway KA, Helman LJ. Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors: A Report From the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol. 2016;2:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 271] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 56. | Le Cesne A, Ray-Coquard I, Bui BN, Adenis A, Rios M, Bertucci F, Duffaud F, Chevreau C, Cupissol D, Cioffi A, Emile JF, Chabaud S, Pérol D, Blay JY; French Sarcoma Group. Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomised phase 3 trial. Lancet Oncol. 2010;11:942-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 57. | Patrikidou A, Chabaud S, Ray-Coquard I, Bui BN, Adenis A, Rios M, Bertucci F, Duffaud F, Chevreau C, Cupissol D, Domont J, Pérol D, Blay JY, Le Cesne A; French Sarcoma Group. Influence of imatinib interruption and rechallenge on the residual disease in patients with advanced GIST: results of the BFR14 prospective French Sarcoma Group randomised, phase III trial. Ann Oncol. 2013;24:1087-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 58. | Raut CP, Posner M, Desai J, Morgan JA, George S, Zahrieh D, Fletcher CD, Demetri GD, Bertagnolli MM. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol. 2006;24:2325-2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 296] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 59. | Rutkowski P, Nowecki Z, Nyckowski P, Dziewirski W, Grzesiakowska U, Nasierowska-Guttmejer A, Krawczyk M, Ruka W. Surgical treatment of patients with initially inoperable and/or metastatic gastrointestinal stromal tumors (GIST) during therapy with imatinib mesylate. J Surg Oncol. 2006;93:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 60. | Patrikidou A, Domont J, Chabaud S, Ray-Coquard I, Coindre JM, Bui-Nguyen B, Adenis A, Rios M, Bertucci F, Duffaud F, Chevreau C, Cupissol D, Pérol D, Emile JF, Blay JY, Le Cesne A; French Sarcoma Group. Long-term outcome of molecular subgroups of GIST patients treated with standard-dose imatinib in the BFR14 trial of the French Sarcoma Group. Eur J Cancer. 2016;52:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 61. | Demetri GD, Wang Y, Wehrle E, Racine A, Nikolova Z, Blanke CD, Joensuu H, von Mehren M. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009;27:3141-3147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 302] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 62. | van Leeuwen RWF, Jansman FGA, Hunfeld NG, Peric R, Reyners AKL, Imholz ALT, Brouwers JRBJ, Aerts JG, van Gelder T, Mathijssen RHJ. Tyrosine Kinase Inhibitors and Proton Pump Inhibitors: An Evaluation of Treatment Options. Clin Pharmacokinet. 2017;56:683-688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 63. | Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, Desai J, Fletcher CD, George S, Bello CL, Huang X, Baum CM, Casali PG. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1942] [Cited by in RCA: 1918] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 64. | Demetri GD, Garrett CR, Schöffski P, Shah MH, Verweij J, Leyvraz S, Hurwitz HI, Pousa AL, Le Cesne A, Goldstein D, Paz-Ares L, Blay JY, McArthur GA, Xu QC, Huang X, Harmon CS, Tassell V, Cohen DP, Casali PG. Complete longitudinal analyses of the randomized, placebo-controlled, phase III trial of sunitinib in patients with gastrointestinal stromal tumor following imatinib failure. Clin Cancer Res. 2012;18:3170-3179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 65. | George S, Blay JY, Casali PG, Le Cesne A, Stephenson P, Deprimo SE, Harmon CS, Law CN, Morgan JA, Ray-Coquard I, Tassell V, Cohen DP, Demetri GD. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45:1959-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 274] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 66. | Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith D, Town A, McKinley A, Ou WB, Fletcher JA, Fletcher CD, Huang X, Cohen DP, Baum CM, Demetri GD. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008;26:5352-5359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 579] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 67. | Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, Hohenberger P, Leahy M, von Mehren M, Joensuu H, Badalamenti G, Blackstein M, Le Cesne A, Schöffski P, Maki RG, Bauer S, Nguyen BB, Xu J, Nishida T, Chung J, Kappeler C, Kuss I, Laurent D, Casali PG; GRID study investigators. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 942] [Cited by in RCA: 1032] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 68. | George S, Wang Q, Heinrich MC, Corless CL, Zhu M, Butrynski JE, Morgan JA, Wagner AJ, Choy E, Tap WD, Yap JT, Van den Abbeele AD, Manola JB, Solomon SM, Fletcher JA, von Mehren M, Demetri GD. Efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: a multicenter phase II trial. J Clin Oncol. 2012;30:2401-2407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 69. | Park SH, Ryu MH, Ryoo BY, Im SA, Kwon HC, Lee SS, Park SR, Kang BY, Kang YK. Sorafenib in patients with metastatic gastrointestinal stromal tumors who failed two or more prior tyrosine kinase inhibitors: a phase II study of Korean gastrointestinal stromal tumors study group. Invest New Drugs. 2012;30:2377-2383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 70. | Kindler HL, Campbell NP, Wroblewski K, Maki RG, D'Adamo DR, Chow WA, Gandara DR, Antonescu C, Stadler WM, Vokes EE. Sorafenib (SOR) in patients (pts) with imatinib (IM) and sunitinib (SU)-resistant (RES) gastrointestinal stromal tumors (GIST): Final results of a University of Chicago Phase II Consortium trial. J Clin Oncol. 2011;29:10009-10009. [RCA] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 71. | Mir O, Cropet C, Toulmonde M, Cesne AL, Molimard M, Bompas E, Cassier P, Ray-Coquard I, Rios M, Adenis A, Italiano A, Bouché O, Chauzit E, Duffaud F, Bertucci F, Isambert N, Gautier J, Blay JY, Pérol D; PAZOGIST study group of the French Sarcoma Groupe-Groupe d'Etude des Tumeurs Osseuses (GSF-GETO). Pazopanib plus best supportive care versus best supportive care alone in advanced gastrointestinal stromal tumours resistant to imatinib and sunitinib (PAZOGIST): a randomised, multicentre, open-label phase 2 trial. Lancet Oncol. 2016;17:632-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 72. | Garner AP, Gozgit JM, Anjum R, Vodala S, Schrock A, Zhou T, Serrano C, Eilers G, Zhu M, Ketzer J, Wardwell S, Ning Y, Song Y, Kohlmann A, Wang F, Clackson T, Heinrich MC, Fletcher JA, Bauer S, Rivera VM. Ponatinib inhibits polyclonal drug-resistant KIT oncoproteins and shows therapeutic potential in heavily pretreated gastrointestinal stromal tumor (GIST) patients. Clin Cancer Res. 2014;20:5745-5755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 73. | Heinrich MC, vonMehren M, Demetri GD, Fletcher JA, Sun J, Hodgson JG, Rivera VM, Turner CD, George S. A phase 2 study of ponatinib in patients (pts) with advanced gastrointestinal stromal tumors (GIST) after failure of tyrosine kinase inhibitor (TKI) therapy: Initial report. J Clin Oncol. 2014;32:10506. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Trent JC, Wathen K, Mehren Mv, Samuels BL, Staddon AP, Choy E, Butrynski JE, Chugh R, Chow WA, Rushing DA, Forscher CA, Baker LH, Schuetze S, Collaboration SAfRt. A phase II study of dasatinib for patients with imatinib-resistant gastrointestinal stromal tumor (GIST). J Clin Oncol. 2011;29:10006-10006. [RCA] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 75. | Farhat F, Othman A, El Baba G, Kattan J. Revealing a cancer diagnosis to patients: attitudes of patients, families, friends, nurses, and physicians in Lebanon-results of a cross-sectional study. Curr Oncol. 2015;22:e264-e272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Assi T, El Rassy E, Tabchi S, Ibrahim T, Moussa T, Chebib R, El Karak F, Farhat F, Chahine G, Nasr F, Ghosn M, Kattan J. Treatment of cancer patients in their last month of life: aimless chemotherapy. Support Care Cancer. 2016;24:1603-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 77. | Tabchi S, El Rassy E, Khazaka A, El Karak F, Kourie HR, Chebib R, Assi T, Ghor M, Naamani L, Richa S, Ghosn M, Kattan J. Validation of the EORTC QLQ-INFO 25 questionnaire in Lebanese cancer patients: Is ignorance a Bliss? Qual Life Res. 2016;25:1597-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Takeda. A Phase 2 trial of ponatinib in participants with metastatic and/or unresectable gastrointestinal stromal tumor (GIST). In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://ClinicalTrials.gov/show/NCT01874665 ClinicalTrials.gov Identifier: NCT01874665. |
| 79. | Bauer S. POETIG trial - POnatinib After rEsisTance to Imatinib in GIST (POETIG). In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://ClinicalTrials.gov/show/NCT03171389 ClinicalTrials.gov Identifier: NCT03171389. |
| 80. | Shoushtari AN, D'Angelo SP, Keohan ML, Dickson MA, Gounder MM, Abdullah AK, Erinjeri JP, Bluth MJ, Ustoyev Y, Condy MM, Streicher H, Takebe N, DeMatteo RP, Schwartz GK, Tap WD, Carvajal RD. Combined KIT and CTLA-4 blockade in patients with refractory GIST and other advanced sarcomas. J Clin Oncol. 2014;32:10521-10521. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 81. | Mehren Mv, Tetzlaff ED, Macaraeg M, Davis J, Agarwal V, Ramachandran A, Heinrich MC. Dose escalating study of crenolanib besylate in advanced GIST patients with PDGFRA D842V activating mutations. J Clin Oncol. 2016;34:11010-11010. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 82. | Blueprint Medicines Corporation. (NAVIGATOR) study of BLU-285 in patients with gastrointestinal stromal tumors (GIST) and other relapsed and refractory solid tumors. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://ClinicalTrials.gov/show/NCT02508532 ClinicalTrials.gov Identifier: NCT02508532. |
| 83. | Heinrich M, von Mehren M, Jones RL, Bauer S, Kang YK, Schoffski P, Eskens F, Serrano C, Cassier P, Mir O, Tap WD, Rutkowski P, Trent J, Patel S, Chowla SP, Zhou T, Lauz T, Schmidt-Kittler O, Mamlouk KK, Wolf BB, George S. Avapritinib is highly active and well-tolerated in patients (PTS) with advanced GIST driven by diverse variety of oncogenic mutations in KIT and PDGFRA. Connective Tissue Oncology Society. 2018;Annual Meeting; 2018 Nov 15; Rome, Italy. |
| 84. | George S. Heinrich M, Chi P, Razak A, von Mehren M, Gordon M, Ganjoo KN, Somaiah N, Trent JC, Rodon Ahnert J, Wolf J, Ruiz-Soto R, Rosen O, Janku F. Initial results of phase I study of DCC-2618, a broad-spectrum KIT and PDGFRa inhibitor, in patients (pts) with gastrointestinal stromal tumor (GIST) by number of prior regimens. ESMO Congress. 2018;Ann Oncol, 2018: viii576-viii595. |
| 85. | Novartis. A dose-finding study of a combination of imatinib and BYL719 in the treatment of 3rd line GIST patients. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://ClinicalTrials.gov/show/NCT01735968 ClinicalTrials.gov Identifier: NCT01735968. |
| 86. | Novartis. A dose-finding study of a combination of imatinib and BKM120 in the treatment of 3rd line GIST patients. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://ClinicalTrials.gov/show/NCT01468688 ClinicalTrials.gov Identifier: NCT01468688. |
| 87. | Blay J-Y, Labouret NH, Cropet C, Mazieres J, Nowak F, Bieche I, Troussard X, Lonchamp E, Charles J, Dalle S, Maubec E, Leboulleux S, Malka D, Arnulf B, Flechon A, Coquard IR, Pérol D, Pezzella V, Jimenez M, Buzyn A. Biomarker-driven access to vemurafenib in BRAF-positive cancers: Second study of the French National AcSé Program. J Clin Oncol. 2016;34:TPS11620-TPS11620. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 88. | Chi P, Qin L-X, D'Angelo SP, Dickson MA, Gounder MM, Keohan ML, Shoushtari AN, Condy MM, Konen T, Fruauff A, DeMatteo RP, Singer S, Hwang S, Antonescu CR, Tap WD. A phase Ib/II study of MEK162 (binimetinib [BINI]) in combination with imatinib in patients with advanced gastrointestinal stromal tumor (GIST). J Clin Oncol. 2015;33:10507-10507. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 89. | Nokihara H, Yamamoto N, Nakamichi S, Wakui H, Yamada Y, Nguyen L, Tamura T. O2–026A phase 1 study of cabozantinib in Japanese patients with advanced solid tumors: Anti-tumor activity in NSCLC and GIST. Ann Oncol. 2013;24:ix48. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 90. | European Organisation for Research and Treatment of Cancer – EORTC. Ph II CABOGIST in GIST. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://ClinicalTrials.gov/show/NCT02216578 ClinicalTrials.gov Identifier: NCT02216578. |
| 91. | Memorial Sloan Kettering Cancer Center. BGJ398 in combination with imatinib mesylate in patients with untreated advanced gastrointestinal stromal tumor (GIST). In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://ClinicalTrials.gov/show/NCT02257541 ClinicalTrials.gov Identifier: NCT02257541. |
| 92. | Mehren Mv, George S, Heinrich MC, Schuetze S, Belinsky MG, Janeway KA, Rink L, Ganjoo KN, Yu JQ, Yap JT, Wright JJ, Abbeele ADVD. Results of SARC 022, a phase II multicenter study of linsitinib in pediatric and adult wild-type (WT) gastrointestinal stromal tumors (GIST). J Clin Oncol. 2014;32:10507-10507. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 93. | Shapiro GI, Kwak E, Dezube BJ, Yule M, Ayrton J, Lyons J, Mahadevan D. First-in-human phase I dose escalation study of a second-generation non-ansamycin HSP90 inhibitor, AT13387, in patients with advanced solid tumors. Clin Cancer Res. 2015;21:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 94. | Astex Pharmaceuticals, Inc. A study to investigate the safety and efficacy of AT13387, alone or in combination with imatinib, in patients with GIST. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://ClinicalTrials.gov/show/NCT01294202 ClinicalTrials.gov Identifier: NCT01294202. |
| 95. | Reilley MJ, Bailey A, Subbiah V, Janku F, Naing A, Falchook G, Karp D, Piha-Paul S, Tsimberidou A, Fu S, Lim J, Bean S, Bass A, Montez S, Vence L, Sharma P, Allison J, Meric-Bernstam F, Hong DS. Phase I clinical trial of combination imatinib and ipilimumab in patients with advanced malignancies. J Immunother Cancer. 2017;5:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 96. | Bergonié I. Efficacy and safety of PD-0332991 in patients with advanced gastrointestinal stromal tumors refractory to imatinib and sunitinib. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://ClinicalTrials.gov/show/NCT01907607 ClinicalTrials.gov Identifier: NCT01907607. |