Published online Dec 26, 2020. doi: 10.12998/wjcc.v8.i24.6229
Peer-review started: August 23, 2020
First decision: October 18, 2020
Revised: October 20, 2020
Accepted: November 4, 2020
Article in press: November 4, 2020
Published online: December 26, 2020
Processing time: 118 Days and 0.7 Hours
Conventional clinical guidelines recommend that at least 12 lymph nodes should be removed during radical rectal cancer surgery to achieve accurate staging. The current application of neoadjuvant therapy has changed the number of lymph node dissection.
To investigate factors affecting the number of lymph nodes dissected after neoadjuvant chemoradiotherapy in locally advanced rectal cancer and to evaluate the relationship of the total number of retrieved lymph nodes (TLN) with disease-free survival (DFS) and overall survival (OS).
A total of 231 patients with locally advanced rectal cancer from 2015 to 2017 were included in this study. According to the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) tumor-node-metastasis (TNM) classification system and the NCCN guidelines for rectal cancer, the patients were divided into two groups: group A (TLN ≥ 12, n = 177) and group B (TLN < 12, n = 54). Factors influencing lymph node retrieval were analyzed by univariate and binary logistic regression analysis. DFS and OS were evaluated by Kaplan-Meier curves and Cox regression models.
The median number of lymph nodes dissected was 18 (range, 12-45) in group A and 8 (range, 2-11) in group B. The lymph node ratio (number of positive lymph nodes/total number of lymph nodes) (P = 0.039) and the interval between neoadjuvant therapy and radical surgery (P = 0.002) were independent factors of the TLN. However,TLN was not associated with sex, age, ASA score, clinical T or N stage, pathological T stage, tumor response grade (Dworak), downstaging, pathological complete response, radiotherapy dose, preoperative concurrent chemotherapy regimen, tumor distance from anal verge, multivisceral resection, preoperative carcinoembryonic antigen level, perineural invasion, intravascular tumor embolus or degree of differentiation. The pathological T stage (P < 0.001) and TLN (P < 0.001) were independent factors of DFS, and pathological T stage (P = 0.011) and perineural invasion (P = 0.002) were independent factors of OS. In addition, the risk of distant recurrence was greater for TLN < 12 (P = 0.009).
A shorter interval to surgery after neoadjuvant chemoradiotherapy for rectal cancer under indications may cause increased number of lymph nodes harvested. Tumor shrinkage and more extensive lymph node retrieval may lead to a more favorable prognosis.
Core Tip: The number of lymph node retrieval and survival on surgery after neoadjuvant therapy in rectal cancer are still under debate. This study analyzes the effects of lymph node retrieval in rectal cancer after neoadjuvant therapy on patient survival. We concluded that a shorter interval to surgery after neoadjuvant chemoradiotherapy for rectal cancer under indications caused increased number of lymph nodes harvested. Tumor shrinkage and more extensive lymph node retrieval may lead to a more favorable prognosis.
- Citation: Mei SW, Liu Z, Wang Z, Pei W, Wei FZ, Chen JN, Wang ZJ, Shen HY, Li J, Zhao FQ, Wang XS, Liu Q. Impact factors of lymph node retrieval on survival in locally advanced rectal cancer with neoadjuvant therapy. World J Clin Cases 2020; 8(24): 6229-6242
- URL: https://www.wjgnet.com/2307-8960/full/v8/i24/6229.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i24.6229
The internationally recognized tumor-node-metastasis (TNM) classification system is widely practiced in the staging of clinical malignant tumors. Since first proposed by a Frenchman, Pierre Denoix, between 1943 and 1952, the American Joint Committee on Cancer (AJCC) and Union for International Cancer Control (UICC) have gradually established international classification standards, and in 1968, the first edition of the TNM classification of malignant tumors was published[1,2]. Currently, 8 editions of the TNM classification system for colorectal cancer have been developed, and the 8th edition has become a widely used reference for colorectal oncologists globally[3]. With social and economic development, colorectal cancer now ranks third in the world in terms of morbidity and mortality[4]. In China, colorectal cancer is also a serious threat to human health. Locally advanced rectal cancer accounts for the majority of colorectal cancer cases. Positive lymph nodes (pN1-2) suggest a risk of systemic metastasis and unfavorable prognosis[5]. The harvest of fewer than 12 lymph nodes has been identified as a predictor of unfavorable outcomes in rectal cancer according to the National Comprehensive Cancer Network (NCCN) guidelines, and this cut-off is regarded as a standard in surgical treatment[6,7]. With the widespread application of neoadjuvant therapy for rectal cancer, in general, the number of lymph nodes harvested is negatively correlated with tumor regression. The total number of retrieved lymph nodes (TLN) is often less than 12 after neoadjuvant therapy, which has a debatable effect on the rate of local recurrence and distant metastasis[8,9]. The lymph node ratio (LNR) is also a prognostic factor that is inversely correlated with survival outcomes[8,10,11]. Furthermore, accurate pathological staging plays an important role in determining the postoperative treatment regimen for rectal cancer, and more extensive lymph node retrieval can reduce local recurrence. There have been no large clinical trials investigating whether neoadjuvant chemoradiotherapy affects the TLN.
Various factors influencing lymph node retrieval during surgery make it difficult to determine the influence on the node stage (pN)[12]. Weight, sex, pelvic structure, specimen length and surgical procedure type are factors that may influence the retrieval of lymph nodes[13,14]. Interestingly, a lower N stage can be the result of lymph nodes not being sufficiently dissected by the surgeon or some of the lymph nodes being missed by the pathologist during specimen collection. This phenomenon is due to the neoadjuvant therapy which reduces the size of the tumor and the number of positive lymph nodes[15].
Anatomically, the circumferential margin of involvement of tumors in the rectum is similar to that of tumors in the colon invading the mesocolon, but the rectum is quite different from the colon in terms of the lymph node distribution. This difference leads to a higher recurrence rate of locally advanced rectal cancer (cN+). Therefore, preoperative neoadjuvant therapy plus radical total mesorectal excision (TME) as the standard therapy regimen has been applied in locally advanced rectal cancer[16]. Logically, fewer lymph nodes may be retrieved due to neoadjuvant chemoradiotherapy, and the TLN can thus be influenced by the chemotherapy regimen, radiotherapy dose and tumor response to chemoradiotherapy. A larger TLN may suggest a poorer prognosis.
In the present study, we examined the TLN as an indication for the most suitable neoadjuvant and surgical treatment regimen.
We analyzed the data of 231 patients who were clinically diagnosed with locally advanced rectal cancer. All patients were admitted to the Colorectal Surgery Department of the National Cancer Center/National Sciences Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, between November 2014 and August 2017 and enrolled in the treatment regimen, which was preoperative neoadjuvant therapy followed by laparoscopic TME[17]. The cohort was divided into two groups, with 12 lymph nodes as the boundary and the TLN determined by surgeons and pathologists. We investigated the factors that may affect the TLN, as well as factors associated with prognosis, including clinical characteristics, clinical T and N stage, pathological T and N stage, preoperative chemotherapy regimen, radiotherapy dose, TLN, LNR, surgical procedure, pathological outcomes and follow-up data (Table 1). This research was approved by the ethics committee of our institution, and informed consent to collect clinical data was obtained from each patient following the principles of the World Medical Association Declaration of Helsinki. We excluded patients who were diagnosed with clinical TNM stage I and IV disease, other carcinomas or lesions, obstruction, perforation, or bleeding, who were treated with palliative resection and who refused to accept chemoradiotherapy.
| Variable | TLN ≥ 12 (group A, n = 177) | TLN < 12 (group B, n = 54) | P value |
| Sex (n, %) | 0.591 | ||
| Male | 121 (68.4) | 39 (72.2) | |
| Female | 56 (31.6) | 15 (27.8) | |
| Age (yr) | 60 (51-65) | 59.5 (52.75-66.5) | 0.544 |
| ASA score (n, %) | 0.488 | ||
| 1 | 5 (2.8) | 2 (3.7) | |
| 2 | 139 (78.6) | 39 (72.2) | |
| 3 | 33 (18.6) | 13 (24.1) | |
| BMI (kg/m2) | 23.8 (21.9-26.4) | 23.4 (21.5-25.2) | 0.423 |
| cT, n (%) | 0.975 | ||
| 2 | 2 (1.1) | 0 (0) | |
| 3 | 137 (77.4) | 43 (79.6) | |
| 4 | 38 (21.5) | 11 (20.4) | |
| cN, n (%) | 0.109 | ||
| 0 | 44 (24.9) | 18 (33.3) | |
| 1 | 91 (51.4) | 28 (51.9) | |
| 2 | 42 (23.7) | 8 (14.8) | |
| Preoperative CEA (μg/L) | 2.99 (1.55-5.57) | 3 (1.68-5.57) | 0.729 |
| ypT, n (%) | 0.140 | ||
| 0 | 26 (14.7) | 14 (25.9) | |
| 1 | 4 (2.3) | 1 (1.9) | |
| 2 | 37 (20.9) | 11 (20.4) | |
| 3 | 97 (54.8) | 24 (44.4) | |
| 4 | 13 (7.3) | 4 (7.4) | |
| ypN, n (%) | 0.026 | ||
| 0 | 90 (50.8) | 38 (70.4) | |
| 1 | 64 (36.2) | 10 (18.5) | |
| 2 | 23 (13) | 6 (11.1) | |
| TRG, n (%) | 0.312 | ||
| 0 | 14 (7.9) | 4 (7.4) | |
| 1 | 26 (14.7) | 7 (13) | |
| 2 | 78 (44.1) | 22 (40.7) | |
| 3 | 33 (18.6) | 7 (13) | |
| 4 | 26 (14.7) | 14 (25.9) | |
| No downstaging, n (%) | 98 (55.4) | 20 (37) | 0.019 |
| Downstaging, n (%) | 56 (31.6) | 21 (38.9) | 0.324 |
| pCR, n (%) | 26 (14.7) | 14 (25.9) | 0.057 |
| Positive lymph nodes, n (%) | 0 (0-1) | 0 (0-0) | 0.003 |
| LNR | 0 (0-0.68) | 0 (0-0) | 0.012 |
| Radiotherapy dose, n (%) | 0.557 | ||
| 45-50.4 Gy/28 F | 125 (70.6) | 39 (72.2) | |
| < 45 Gy/25 F | 34 (19.2) | 5 (9.3) | |
| 25 Gy/5 F | 18 (10.2) | 10 (18.5) | |
| Preoperative concurrent chemotherapy regimen, n (%) | 0.376 | ||
| Capecitabine+ oxaliplatin | 54 (30.5) | 17 (31.5) | |
| Capecitabine, oral | 108 (61) | 37 (68.5) | |
| Fluorouracil union | 15 (8.5) | 0 (0) | |
| Tumor location, DAV (cm) | 5 (3-7) | 5 (3-7) | 0.605 |
| Interval, w (%) | 8 (6.5-11) | 10 (8-16) | 0.001 |
| Surgical procedure | 0.018 | ||
| Miles (n, %) | 74 (41.8) | 26 (48.2) | |
| Dixon (n, %) | 93 (52.5) | 20 (37) | |
| Hartmann (n, %) | 10 (5.6) | 8 (14.8) | |
| LLND (n, %) | 20 (11.3) | 1 (1.9) | 0.035 |
| Multivisceral resection (n, %) | 10 (5.6) | 2 (3.7) | 0.574 |
| PNI (n, %) | 0.819 | ||
| Yes | 42 (23.7) | 12 (22.2) | |
| No | 135 (76.3) | 42 (77.8) | |
| Intravascular tumor embolus (n, %) | 0.463 | ||
| Yes | 23 (13) | 5 (9.3) | |
| No | 154 (87) | 49 (90.7) | |
| Degree of differentiation, n (%) | 0.367 | ||
| Low and low-middle grades | 18 (10.2) | 9 (16.7) | |
| Middle, high-middle, and high grades | 132 (74.6) | 43 (79.6) | |
| Signet-ring and mucinous adenocarcinoma | 4 (2.3) | 2 (3.7) |
According to the AICC/UICC 8th edition TNM classification system[18], patients with stage II and III disease were treated with neoadjuvant chemoradiotherapy. The preoperative radiotherapy dose typically ranged from 45 Gy-50.4 Gy and was administered according to a long-course regimen; some patients were treated with a short-course radiotherapy regimen with a total dose of 25 Gy. Radiation was delivered to the pelvic cavity and tumor bed at an energy of 10 MV. The following chemotherapy regimens were administered concurrently with radiation: XELOX; oral capecitabine alone; and other regimens of fluorouracil combined with other agents.
All surgical specimens were sent for examination and fixation with paraffin in a timely manner after surgery. The pathologists were blinded to the patient information during analysis of the specimens. The final pathological TNM stage was classified using the AJCC/UICC 8th edition classification system. Our institution also uses the tumor regression grade (TRG, Dworak), ranging from 0 to 4: TRG0, the tumor tissue showed no significant regression macro- or microscopically; TRG1, the tumor tissue showed < 25% shrinkage microscopically; TRG2, the tumor tissue showed 25%-50% shrinkage microscopically; TRG3, the tumor tissue showed > 50% shrinkage microscopically; and TRG4, no residual tumor cells were observed microscopically. Perineural invasion (PNI) was defined as tumor cells invading any layer of the nerve sheath or tumor cells growing along the nerve wrapping more than 1/3 of the nerve circumference, and intravascular tumor embolus was defined by tumor cell invasion of the lymphatic vessels or vessels of the intestinal wall.
Follow-up was conducted in the outpatient clinic or by telephone or email. Clinical and chest, abdominal and pelvic enhanced computed tomography examinations were performed to detect recurrence. Patients were followed up every three months postoperatively for the first two years. If there was no recurrence after two years, patients were followed up every six months once a year. Colonoscopy was performed every six months during the first two years and once a year after the first two years. Disease-free survival (DFS) was analyzed from the day of surgery to the date of tumor-related recurrence. Overall survival (OS) was defined as the time between the date of surgery and cancer-related death. The last date to follow-up was March 22, 2020. The median follow-up was 41 mo.
The first part of our statistical analysis focused on factors affecting the TLN. Patient characteristics were analyzed with univariate and binary logistic regression analyses. On univariate analysis, we compared categorical variables with the Chi-squared test or Fisher’s exact test, and continuous variables that did not conform to the normal distribution were calculated by Mann-Whitney U test and were displayed as the median (range). Variables with P values less than 0.05 on univariate analysis were included in the binary logistic regression analysis.
The second part of the analysis examined whether DFS and OS were correlated with the TLN. Factors influencing the prognosis were analyzed by the Kaplan-Meier method and then included in Cox proportional hazards regression models with the aim of determining independent factors affecting DFS and OS.
All statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS) version 26.0 for Mac (IBM Corp, NY, United States).
Table 1 shows the clinicopathological features of the patients. There was no significant difference between the two groups except the operating time interval and the lymph node ratio, as shown in Table 2 by binary regression analysis. The median follow-up period was 41 mo (range, 2-62), with no difference between the groups. Fourteen (7.9%) patients in group A and 7 (13%) patients in group B experienced local recurrence (P = 0.259). The total number of deaths in the cohort was 27 (15.3%) in group A and 9 (16.7%) in group B (P = 0.803). Although there was no significant difference between the two groups, the local recurrence rate and death rate were higher in group B (TLN < 12) than in group A (TLN ≥ 12). More patients in group B than in group A experienced distant recurrence (group A, 19.8% vs group B, 37%, P = 0.009). Table 3 presents the tumor-related outcomes in both groups.
| P value | OR | 95%CI | |
| No downstaging | 0.640 | 0.797 | 0.309-2.061 |
| Positive lymph nodes | 0.152 | 0.873 | 0.725-1.051 |
| ypN0 vs ypN2 | 0.059 | 8.239 | 0.92-73.794 |
| ypN1 vs ypN2 | 0.442 | 2.228 | 0.289-17.163 |
| LNR | 0.039 | 66.666 | 1.239-3587.217 |
| Interval | 0.002 | 1.084 | 1.029-1.142 |
| LLND | 0.086 | 0.165 | 0.021-1.287 |
| Variable | TLN ≥ 12 (group A, n = 177) | TLN < 12 (group B, n = 54) | P value |
| Local recurrence, n (%) | 14 (7.9) | 7 (13) | 0.259 |
| Distant recurrence, n (%) | 35 (19.8) | 20 (37) | 0.009 |
| Death, n (%) | 27 (15.3) | 9 (16.7) | 0.803 |
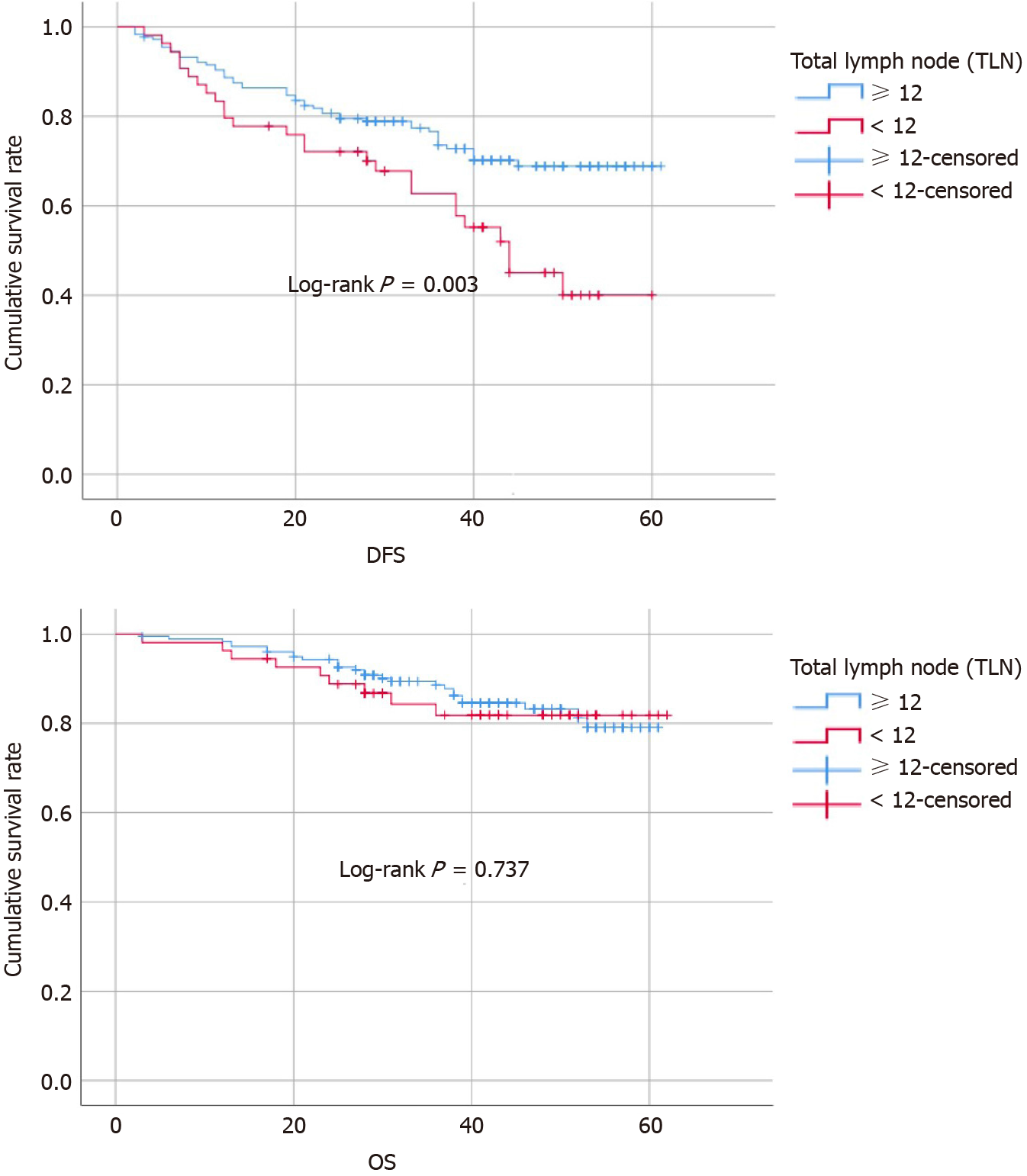
At the same time, the above potential prognostic factors were analyzed as categorical variables by the Kaplan-Meier method. The cumulative DFS rate, which was determined by the date of tumor recurrence, was higher in group A (TLN ≥ 12) than in group B (TLN < 12) (group A, 68.9% vs group, B 40%, P = 0.006), but there was no difference between the groups in OS (group A, 79% vs group B, 81%, P = 0.737) (Figure 1). Other factors leading to a significant difference in DFS included clinical N stage (P = 0.003), pathological T stage (P < 0.001) and N stage (P < 0.001), positive lymph nodes (P < 0.001), LNR (P < 0.001), interval (P = 0.044), multivisceral resection (P = 0.025), PNI (P < 0.001), intravascular tumor embolus (P < 0.001), and degree of differentiation (P = 0.028), and factors causing a significant difference in OS included pathological T stage (P < 0.001), PNI (P < 0.001), and intravascular tumor embolus (P = 0.004). All factors affecting survival were incorporated into the Cox proportional hazards regression model for further analysis. Table 4 presents the survival outcomes in both groups. We found that the TLN [P < 0.001, odds ratio (OR): 0.302, 95% confidence interval (CI): 0.172-0.531] and pathological T stage (P < 0.001) were independent predictors of DFS, while PNI (P = 0.002, OR: 3.74, 95%CI: 1.634-8.529) and pathological T stage (P = 0.002) were independent predictors of OS, as shown in Table 5.
| Variable | No. of cases | DFS P value | OS P value |
| Sex (n, %) | 0.708 | 0.655 | |
| Male | 160 (69.3) | ||
| Female | 71 (30.7) | ||
| Age (yr) | 0.86 | 0.968 | |
| < 60 | 111 (48.1) | ||
| ≥ 60 | 120 (51.9) | ||
| ASA score (n, %) | 0.912 | 0.951 | |
| 1 | 7 (3) | ||
| 2 | 178 (77) | ||
| 3 | 46 (19.9) | ||
| BMI (kg/m2) | 0.519 | 0.512 | |
| < 25 | 149 (64.5) | ||
| ≥ 25 | 82 (35.5) | ||
| cT, n (%) | 0.606 | 0.476 | |
| 2 | 2 (0.9) | ||
| 3 | 180 (77.9) | ||
| 4 | 49 (21.2) | ||
| cN, n (%) | 0.003 | 0.063 | |
| 0 | 79 (34.2) | ||
| 1 | 112 (48.5) | ||
| 2 | 40 (17.3) | ||
| ypT, n (%) | < 0.001 | < 0.001 | |
| 0 | 36 (15.6) | ||
| 1 | 6 (2.6) | ||
| 2 | 50 (21.6) | ||
| 3 | 122 (52.8) | ||
| 4 | 17 (7.4) | ||
| ypN, n (%) | < 0.001 | 0.097 | |
| 0 | 128 (55.4) | ||
| 1 | 74 (32) | ||
| 2 | 29 (12.6) | ||
| TLN, n (%) | 0.006 | 0.737 | |
| < 12 | 54 (23.4) | ||
| ≥ 12 | 177 (76.6) | ||
| Positive lymph nodes, n (%) | < 0.001 | 0.063 | |
| < 2 | 182 (78.8) | ||
| ≥ 2 | 49 (21.2) | ||
| LNR | < 0.001 | 0.058 | |
| ≤ 0.03 | |||
| > 0.03 | |||
| Preoperative concurrent chemotherapy regimen, n (%) | 0.148 | 0.561 | |
| Capecitabine+ oxaliplatin | 58 (25.1) | ||
| Capecitabine, oral | 140 (60.6) | ||
| Oxaliplatin combination | 22 (9.5) | ||
| Postoperative chemotherapy | 0.933 | 0.869 | |
| Yes | 153 (66.2) | ||
| No | 78 (33.8) | ||
| Interval, w (%) | 0.044 | 0.567 | |
| ≤ 8 wk | 139 (60.2) | ||
| > 8 wk | 92 (39.8) | ||
| Surgical procedure | 0.168 | 0.662 | |
| Miles (n, %) | 100 (43.3) | ||
| Dixon (n, %) | 113 (48.9) | ||
| Hartmann (n, %) | 18 (7.8) | ||
| LLND (n, %) | 21 (9.1) | 0.868 | 0.994 |
| Multivisceral resection (n, %) | 12 (5.2) | 0.025 | 0.948 |
| Preoperative CEA (μg/L) | 0.344 | 0.663 | |
| < 5 | 166 (71.9) | ||
| ≥ 5 | 65 (28.1) | ||
| PNI (n, %) | < 0.001 | < 0.001 | |
| Yes | 54 (23.4) | ||
| No | 177 (76.6) | ||
| Intravascular tumor embolus (n, %) | < 0.001 | 0.004 | |
| Yes | 28 (12.1) | ||
| No | 203 (87.9) | ||
| Degree of differentiation, n (%) | 0.028 | 0.152 | |
| Low and low-middle grades | 32 (13.9) | ||
| Middle, high-middle, and high grades | 193 (83.5) | ||
| Signet-ring and mucinous adenocarcinoma | 6 (2.6) |
| Disease-free survival | Overall survival | |||||
| P value | HR | 95%CI | P value | HR | 95%CI | |
| cN0 vs cN1 | 0.620 | 0.794 | 0.318-1.978 | 0.801 | 0.84 | 0.217-3.249 |
| cN0 vs cN2 | 0.496 | 1.281 | 0.628-2.610 | 0.296 | 1.712 | 0.625-4.693 |
| ypT0 vs ypT4 | < 0.001 | 0.074 | 0.019-0.288 | 0.011 | 0.11 | 0.02-0.607 |
| ypT1 vs ypT4 | 0.338 | 0.46 | 0.094-2.253 | 0.494 | 0.464 | 0.051-4.202 |
| ypT2 vs ypT4 | < 0.001 | 0.097 | 0.032-0.301 | 0.002 | 0.035 | 0.004-0.305 |
| ypT3 vs ypT4 | 0.014 | 0.418 | 0.209-0.837 | 0.011 | 0.309 | 0.125-0.761 |
| ypN0 vs ypN1 | 0.745 | 0.823 | 0.254-2.664 | 0.805 | 1.245 | 0.219-7.086 |
| ypN0 vs ypN2 | 0.706 | 1.178 | 0.504-2.756 | 0.816 | 1.156 | 0.341-3.92 |
| TLN (≥ 12 vs < 12 | < 0.001 | 0.302 | 0.172-0.531 | 0.606 | 0.8 | 0.342-1.871 |
| LNR (≤ 0.03 vs 0.03) | 0.840 | 0.914 | 0.379-2.20 | 0.768 | 1.228 | 0.314-4.799 |
| Positive lymph nodes (≥ 2 vs < 2) | 0.828 | 1.1 | 0.466-2.593 | 0.978 | 0.982 | 0.271-3.562 |
| Interval (≤ 8 wk vs > 8 wk) | 0.236 | 1.369 | 0.814-2.303 | 0.626 | 0.836 | 0.408-1.715 |
| Multivisceral resection | 0.119 | 1.875 | 0.851-4.13 | 0.792 | 0.816 | 0.181-3.69 |
| PNI | 0.051 | 1.8 | 0.998-3.248 | 0.002 | 3.747 | 1.634-8.592 |
| Intravascular tumor embolus | 0.244 | 1.479 | 0.766-2.858 | 0.584 | 1.292 | 0.517-3.23 |
| Degree of differentiation | ||||||
| Low and low-middle grades vs signet-ring and mucinous adenocarcinoma | 0.695 | 0.739 | 0.163-3.354 | 0.811 | 0.76 | 0.08-7.221 |
| Middle, high-middle, and high grades vs signet-ring and mucinous adenocarcinoma | 0.929 | 0.930 | 0.19-4.564 | 0.913 | 1.141 | 0.106-12.243 |
Comprehensive treatment regimens are constantly updated. Currently, the accepted standard treatment scheme is preoperative neoadjuvant chemoradiotherapy followed by radical surgery, but improvements in treatment are made with the ultimate aim of improving the long-term survival rate and quality of life of patients. The TNM classification system in the AJCC cancer staging manual (8th edition, 2017) is used as the current staging standard for rectal cancer; the TNM stage is not only the most important prognostic predictor in rectal cancer but also critical to the selection of a postoperative treatment regimen. There are few factors affecting the evaluation of the T and M stage, but the N stage is established based on the number of lymph nodes harvested, and both the TLN and number of positive lymph nodes are influenced by the surgical and pathological techniques[19]. In our study, the interval between neoadjuvant therapy and surgery was an independent factor of the number of lymph nodes harvested. This outcome is similar to that of a study performed by Sermier et al[20]; they found that the TLN in abdominoperineal resection was impacted by the interval between neoadjuvant therapy and surgery and the administration of neoadjuvant radiotherapy. Neoadjuvant therapy not only controlled the local recurrence rate after surgery but also reduced the TLN. There are various factors affecting the detection of lymph nodes in rectal cancer after neoadjuvant therapy. Prolonging the interval after neoadjuvant therapy can improve the local pathological response rate[21-23], and neoadjuvant therapy may play a paramount role in treating tumors over time. However, there seems to be a contradiction, as neoadjuvant therapy can also reduce the lymph node retrieval rate and the accuracy of TNM staging. Some studies have indicated that fewer lymph nodes are detected during surgery after neoadjuvant therapy[22,24-26]. Farinella et al[22] concluded that small lymph nodes (≤ 5 mm) impacted the N stage. We found that small lymph nodes (≤ 5 mm) accounted for 76.5% of lymph nodes retrieved in our study, which increased the difficulty in accurately identifying the N stage due to atrophy, fibrosis and even necrosis. In a total of 5647 patients who were enrolled in an analysis of the Surveillance, Epidemiology and End Results (SEER) database in 2005, the number of lymph nodes retrieved and the N stage were affected by the administration of neoadjuvant therapy[27]. We analyzed whether neoadjuvant chemoradiotherapy combined with chemoradiotherapy had a sensitization effect on radiotherapy. Theoretically, compared with preoperative radiotherapy, preoperative chemoradiotherapy should significantly reduce the TLN. In this regard, it has also been reported that preoperative chemo-radiotherapy has more advantages in terms of tumor shrinkage and tumor persistence than preoperative radiotherapy alone[28].
The effect of the minimum number of harvested lymph nodes on rectal cancer has been discussed between surgeons and pathologists. The effect of the number of lymph nodes harvested in rectal cancer on survival is also a controversial issue. With increasing lymph node dissection and detection, the stage determined according to the TNM classification system becomes increasingly accurate. Considering the effect of neoadjuvant therapy on the accuracy of the N stage, the LNR has been used as a prognostic indicator in some studies[29,30]. Ceelen et al[31] performed a systematic review and analysis of a total of 33 984 patients and found that the prognostic value of positive lymph nodes was inferior to that of the LNR. There was another interesting study on the prognostic value of the number of lymph nodes. Sun et al[32] reported that the number of negative lymph nodes for ypN+ after neoadjuvant therapy was a prognostic factor for DFS (number of negative lymph nodes ≥ 17, hazard ratio = 0.400, P = 0.022). Persiani et al[33] conducted a study involving 345 patients treated with neoadjuvant therapy followed by surgery, and they indicated that more lymph nodes were retrieved in cases of a less extensive tumor response and a shorter interval between neoadjuvant therapy and surgery. Hall et al[34] indicated that the TLN was strongly correlated with OS, with a cut-off value of 8 lymph nodes. Onitilo et al[35], in a retrospective cohort study of 708 patients with rectal cancer treated over the past 10 years, divided the 708 patients into the surgery alone group (279, SURG) and the neoadjuvant therapy plus surgery group (479, NEO) for comparison; the TLN showed a significant difference between the two groups (NEO 10.8 vs SURG 15.5, P < 0.001). In contrast, the disease-specific survival in the SURG group was not superior to that in the NEO group. The conclusion of this study explained that neoadjuvant therapy may eliminate the potential positive lymph nodes, leading to a decrease in the retrieval of lymph nodes after radical surgery, and that neoadjuvant therapy plays a concurrent role in controlling local recurrence. In our study, even with neoadjuvant therapy followed by radical resection for rectal cancer, TLN < 12 remained unfavorable in terms of DFS. Considering that the number of lymph nodes detected is affected by many factors, it is important to establish an effective system that re-evaluates the true number of positive lymph nodes harvested for accurate staging after neoadjuvant therapy. This is difficult because there is no method to evaluate the true rate of lymph node positivity. However, to deal with the issue of inaccurate N staging, two methods can be utilized. First, as more lymph nodes are harvested to be assessed, the accuracy of staging node-positive disease progressively increases[35-38]; thus, the rate of positive lymph node detection becomes infinitely closer to the true value. Additionally, DFS would seem to improve partly because of shift in staging. Second, a systematic review of many studies can be performed to identify factors affecting DFS on the basis of lymph node dissection. A study of the cohort of patients from the randomized EORTC trial 22921 indicated that DFS was not improved by postoperative adjuvant chemotherapy in patients treated with neoadjuvant chemoradiotherapy[39]. On the other hand, the EORTC trial suggested that the accuracy rate of N staging after neoadjuvant therapy followed by surgery may need to be improved to better plan for postoperative care.
Our study has several limitations. The first limitation is its retrospective nature. Second, there may be bias in the information collected, but the cohort showed acceptable homogeneity. A future multicenter, randomized controlled or cohort trial with a larger sample size may be needed to verify factors of lymph node retrieval after neoadjuvant therapy. Third, the radiotherapy dose administered to patients in our cohort varied because of discrepancies in clinical practice.
The TLN may help to predict the prognosis in colorectal cancer. Neoadjuvant therapy caused decreased number of lymph nodes detected, leading to inaccurate staging. The retrieval of more lymph nodes may improve the accuracy of TNM staging and result in a more favorable prognosis.
Surgery with total mesorectal excision following neoadjuvant therapy is a standard regime for locally advanced rectal cancer. The number of lymph node retrieval and survival on surgery after neoadjuvant therapy in rectal cancer are still under debate.
There is a lack of consensus concerning the actual number of lymph node retrieval in surgery after neoadjuvant therapy. Whether less or more 12 lymph nodes should be retrieved is controversial. Data are limited regarding outcomes of different number of lymph node retrieval.
The main aim of this study is to investigate whether different number of lymph node retrieval affects the rate of pathological complete response, preoperative outcomes and survival status.
This was a retrospective cohort study to collect the data of patients after neoadjuvant therapy for locally advanced rectal cancer. According to the clinicopathological characteristics and other data, the influence of neoadjuvant therapy on the number of lymph node dissection was analyzed.
A shorter interval to surgery after neoadjuvant chemoradiotherapy for rectal cancer under indications may cause increased number of lymph nodes harvested. Tumor shrinkage and more extensive lymph node retrieval may lead to a more favorable prognosis.
The TLN may help to predict the prognosis in colorectal cancer. Neoadjuvant therapy caused a decrease in the number of lymph nodes detected, leading to inaccurate staging. The retrieval of more lymph nodes may improve the accuracy of TNM staging and result in a more favorable prognosis.
Prospective randomized trials are required to evaluate the optimal number of lymph node retrieval that is needed to achieve minimum morbidity, and minimum disease recurrence.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Protopapas A S-Editor: Gao CC L-Editor: MedE-Ma JY P-Editor: Liu JH
| 1. | Doll R. The Pierre Denoix Memorial Lecture: nature and nurture in the control of cancer. Eur J Cancer. 1999;35:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Horiuchi J. [TNM classification of UICC (1974). Comparison between the old and new classifications for mouth and tongue neoplasms]. Rinsho Hoshasen. 1976;21:393-398. [PubMed] |
| 3. | Loughrey MB, Kent O, Moore M, Coghlin C, Kelly P, McVeigh G, Coleman HG. Impact on colorectal cancer pathology reporting practice of migration from TNM 5 to TNM 8. Histopathology. 2020;77:210-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3585] [Cited by in RCA: 4893] [Article Influence: 699.0] [Reference Citation Analysis (1)] |
| 5. | Ahmed M. Colon Cancer: A Clinician's Perspective in 2019. Gastroenterology Res. 2020;13:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 6. | Levine RA, Chawla B, Bergeron S, Wasvary H. Multidisciplinary management of colorectal cancer enhances access to multimodal therapy and compliance with National Comprehensive Cancer Network (NCCN) guidelines. Int J Colorectal Dis. 2012;27:1531-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Li Destri G, Di Carlo I, Scilletta R, Scilletta B, Puleo S. Colorectal cancer and lymph nodes: the obsession with the number 12. World J Gastroenterol. 2014;20:1951-1960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Isik A, Peker K, Firat D, Yilmaz B, Sayar I, Idiz O, Cakir C, Demiryilmaz I, Yilmaz I. Importance of metastatic lymph node ratio in non-metastatic, lymph node-invaded colon cancer: a clinical trial. Med Sci Monit. 2014;20:1369-1375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Chang GJ, Rodriguez-Bigas MA, Eng C, Skibber JM. Lymph node status after neoadjuvant radiotherapy for rectal cancer is a biologic predictor of outcome. Cancer. 2009;115:5432-5440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Pyo JS, Kim JH, Lee SY, Baek TH, Kang DW. Metastatic Lymph Node Ratio (mLNR) is a Useful Parameter in the Prognosis of Colorectal Cancer; A Meta-Analysis for the Prognostic Role of mLNR. Medicina (Kaunas). 2019;55:673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Ramos-Esquivel A, Juárez M, González I, Porras J, Rodriguez L. Prognosis impact of the lymph node ratio in patients with colon adenocarcinoma: a single-centre experience. J Gastrointest Cancer. 2014;45:133-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | He HF, Zhou MQ, Chen JQ, Tian W, Cai HK, Chen LR, Deng YC. Enhanced lymph node retrieval from colorectal cancer resections using a simple lymphatic staining method. Hepatogastroenterology. 2012;59:375-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Feng H, Zhao XW, Zhang Z, Han DP, Mao ZH, Lu AG, Thasler WE. Laparoscopic Complete Mesocolic Excision for Stage II/III Left-Sided Colon Cancers: A Prospective Study and Comparison with D3 Lymph Node Dissection. J Laparoendosc Adv Surg Tech A. 2016;26:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Thorn CC, Woodcock NP, Scott N, Verbeke C, Scott SB, Ambrose NS. What factors affect lymph node yield in surgery for rectal cancer? Colorectal Dis. 2004;6:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Marks JH, Valsdottir EB, Rather AA, Nweze IC, Newman DA, Chernick MR. Fewer than 12 Lymph nodes can be expected in a surgical specimen after high-dose chemoradiation therapy for rectal cancer. Dis Colon Rectum. 2010;53:1023-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ; Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3104] [Cited by in RCA: 3118] [Article Influence: 129.9] [Reference Citation Analysis (0)] |
| 17. | Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1867] [Cited by in RCA: 1914] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 18. | Weiser MR. AJCC 8th Edition: Colorectal Cancer. Ann Surg Oncol. 2018;25:1454-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 648] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 19. | Mechera R, Schuster T, Rosenberg R, Speich B. Lymph node yield after rectal resection in patients treated with neoadjuvant radiation for rectal cancer: A systematic review and meta-analysis. Eur J Cancer. 2017;72:84-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Sermier A, Gervaz P, Egger JF, Dao M, Allal AS, Bonet M, Morel P. Lymph node retrieval in abdominoperineal surgical specimen is radiation time-dependent. World J Surg Oncol. 2006;4:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Dias AR, Pereira MA, de Mello ES, Nahas SC, Cecconello I, Ribeiro U Jr. Lymph Node Yield After Neoadjuvant Chemoradiotherapy in Rectal Cancer Specimens: A Randomized Trial Comparing Two Fixatives. Dis Colon Rectum. 2018;61:888-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Farinella E, Viganò L, Fava MC, Mineccia M, Bertolino F, Capussotti L. In vivo lymph node mapping and pattern of metastasis spread in locally advanced mid/Low rectal cancer after neoadjuvant chemoradiotherapy. Int J Colorectal Dis. 2013;28:1523-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | de Campos-Lobato LF, Stocchi L, de Sousa JB, Buta M, Lavery IC, Fazio VW, Dietz DW, Kalady MF. Less than 12 nodes in the surgical specimen after total mesorectal excision following neoadjuvant chemoradiation: it means more than you think! Ann Surg Oncol. 2013;20:3398-3406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Wang BH, Zhang GN, Xiao Y, Wu B, Lin GL, Cui QC, Hu K, Zhong GX, Qiu HZ. [Impact of neoadjuvant therapy on lymph nodes retrieval in locally advanced mid-low rectal carcinoma]. Zhonghua Waike Zazhi. 2009;47:1779-1783. [PubMed] |
| 25. | Zhao YL, Cao DM, Zhou QC, Yang N, Yao HL. Accuracy of Endorectal Endoscopic Ultrasound (EUS) for Locally Advanced Rectal Cancer (LARC) Restaging After Neoadjuvant Chemoradiotherapy (NAT): A Meta-Analysis. Hepatogastroenterology. 2014;61:978-983. [PubMed] |
| 26. | Morcos B, Baker B, Al Masri M, Haddad H, Hashem S. Lymph node yield in rectal cancer surgery: effect of preoperative chemoradiotherapy. Eur J Surg Oncol. 2010;36:345-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 27. | Baxter NN, Morris AM, Rothenberger DA, Tepper JE. Impact of preoperative radiation for rectal cancer on subsequent lymph node evaluation: a population-based analysis. Int J Radiat Oncol Biol Phys. 2005;61:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC; EORTC Radiotherapy Group Trial 22921. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 2040] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 29. | Manilich EA, Kiran RP, Radivoyevitch T, Lavery I, Fazio VW, Remzi FH. A novel data-driven prognostic model for staging of colorectal cancer. J Am Coll Surg 2011; 213: 579-588, 588.e1-588. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Tong LL, Gao P, Wang ZN, Song YX, Xu YY, Sun Z, Xing CZ, Wang X, Xu HM. Can lymph node ratio take the place of pN categories in the UICC/AJCC TNM classification system for colorectal cancer? Ann Surg Oncol. 2011;18:2453-2460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Ceelen W, Van Nieuwenhove Y, Pattyn P. Prognostic value of the lymph node ratio in stage III colorectal cancer: a systematic review. Ann Surg Oncol. 2010;17:2847-2855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 32. | Sun Y, Zhang Y, Huang Z, Chi P. Prognostic Implication of Negative Lymph Node Count in ypN+ Rectal Cancer after Neoadjuvant Chemoradiotherapy and Construction of a Prediction Nomogram. J Gastrointest Surg. 2019;23:1006-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Persiani R, Biondi A, Gambacorta MA, Bertucci Zoccali M, Vecchio FM, Tufo A, Coco C, Valentini V, Doglietto GB, D'Ugo D. Prognostic implications of the lymph node count after neoadjuvant treatment for rectal cancer. Br J Surg. 2014;101:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Hall MD, Schultheiss TE, Smith DD, Fakih MG, Kim J, Wong JY, Chen YJ. Impact of Total Lymph Node Count on Staging and Survival After Neoadjuvant Chemoradiation Therapy for Rectal Cancer. Ann Surg Oncol. 2015;22 Suppl 3:S580-S587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Onitilo AA, Stankowski RV, Engel JM, Doi SA. Adequate lymph node recovery improves survival in colorectal cancer patients. J Surg Oncol. 2013;107:828-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Moro-Valdezate D, Pla-Martí V, Martín-Arévalo J, Belenguer-Rodrigo J, Aragó-Chofre P, Ruiz-Carmona MD, Checa-Ayet F. Factors related to lymph node harvest: does a recovery of more than 12 improve the outcome of colorectal cancer? Colorectal Dis. 2013;15:1257-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Fan L, Levy M, Aguilar CE, Mertens RB, Dhall D, Frishberg DP, Wang HL. Lymph node retrieval from colorectal resection specimens for adenocarcinoma: is it worth the extra effort to find at least 12 nodes? Colorectal Dis. 2011;13:1377-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Govindarajan A, Gönen M, Weiser MR, Shia J, Temple LK, Guillem JG, Paty PB, Nash GM. Challenging the feasibility and clinical significance of current guidelines on lymph node examination in rectal cancer in the era of neoadjuvant therapy. J Clin Oncol. 2011;29:4568-4573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 39. | Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, Bardet E, Beny A, Ollier JC, Bolla M, Marchal D, Van Laethem JL, Klein V, Giralt J, Clavère P, Glanzmann C, Cellier P, Collette L; EORTC Radiation Oncology Group. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 555] [Article Influence: 50.5] [Reference Citation Analysis (0)] |