Published online Dec 6, 2020. doi: 10.12998/wjcc.v8.i23.5926
Peer-review started: May 28, 2020
First decision: September 13, 2020
Revised: September 24, 2020
Accepted: October 26, 2020
Article in press: October 26, 2020
Published online: December 6, 2020
Processing time: 190 Days and 4.4 Hours
Assessment of the vascular status following limb fracture in children is important to evaluate the risk of compartment syndrome, which is an emergency condition.
To establish a simple and efficient grading scale of limb perfusion in children undergoing surgery for limb fracture.
This retrospective study included pediatric patients with a limb fracture and postoperative plaster fixation who were admitted at The Department of Pediatric Orthopedics of Xinhua Hospital between February 2017 and August 2017. The outcome was poor limb perfusion, which is defined as the postoperative use of mannitol. The children were divided into two groups: The normal perfusion group and the poor perfusion group. Key risk factors have been selected by univariable analyses to establish the Grading Scale for Vascular Status.
A total of 161 patients were included in the study: 85 in the normal perfusion group and 76 in the poor perfusion group. There were no significant differences in age, sex, body mass index, ethnicity, cause of fracture, fixation, or site of fracture between the two groups. After surgery, the skin temperature (P = 0.048) and skin color (P < 0.001) of the affected limb were significantly different between the two groups. The relative risk and 95% confidence interval for skin temperature of the affected limb, skin color, and range of motion of the affected limb are 2.18 (1.84-2.59), 2.89 (2.28-3.66), and 2.16 (1.83-2.56), respectively. The grading scale was established based on those three factors (score range: 0-3 points). Forty-one patients (32.5%) with score 0 had poor limb perfusion; all patients with scores 1 (n = 32) and 2 (n = 3) had poor limb perfusion (both 100%).
In children undergoing surgery for limb fracture, a higher Grading Scale for Vascular Status score is associated with a higher occurrence of poor limb perfusion. A prospective study is required for validation.
Core Tip: The assessment of the vascular status following limb fracture in children is important to evaluate the risk of compartment syndrome, which is an emergency condition. This study aims to establish a simple and efficient grading scale system of limb perfusion in children undergoing surgery for limb fracture, the Grading Scale for Vascular Status (GSVS). The results strongly suggest that in children undergoing surgery following limb fracture, a higher GSVS score is associated with a higher occurrence of poor limb perfusion. A prospective study is required for validation, but GSVS could help prevent compartment syndrome in children after limb fracture.
- Citation: Zhu T, Shi Y, Yu Q, Zhao YJ, Dai W, Chen Y, Zhang SS. Scoring system for poor limb perfusion after limb fracture in children. World J Clin Cases 2020; 8(23): 5926-5934
- URL: https://www.wjgnet.com/2307-8960/full/v8/i23/5926.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i23.5926
Fractures in children are common, with an incidence of about 137-201 per 10000 person-years[1-3]. The most common fracture sites are the limbs, and the most common mechanism is falling[1-3]. In addition, children are encouraged to practice physical activities to promote their health and normal development[4,5]. Many countries face epidemics of pediatric obesity and type 2 diabetes[6], and measures are being taken to encourage physical activity. This leads to an increase in the occurrence of fractures in children, and 39% of fractures in children are caused by sports and physical activities[1,7,8]. For example, the injury rate of soccer may reach 75.8 injuries per 1000 play hours[9]. Most fractures in children are treated at the outpatient clinic, but one in 18 cases is more serious and requires hospitalization[2]. Most fractures in children heal relatively fast and uneventfully[10].
Poor limb perfusion is a possible and severe complication following pediatric limb fracture[10,11]. Poor limb perfusion is caused by injured soft tissues that compress arteries and veins[12,13]. It is found in 20% of humeral fractures[12]. If not identified and not managed promptly, patients may experience hypoperfusion or venous return obstruction and present with a series of physiological or pathological changes, such as pain, swelling, and pale skin[10,11]. In severe cases, it may lead to compartment syndrome, acute avascular necrosis of the nerves or muscles and even deformity and disability[14,15]. Acute compartment syndrome is caused by increased pressure in compartments enclosed by fascia that give them a limited capacity to stretch before tissue injury ensues[14,15]. It is considered a surgical emergency[14,15]. Tibial fractures are the most common cause of compartment syndrome after fracture[14,15].
Mannitol is routinely used in patients with poor limb perfusion in China. The prompt use of mannitol reduces the risk of limb injury[16]. As children may have limited abilities of expression, the presence or absence of poor limb perfusion is judged by the caregiver (e.g., a physician, a nurse, or a family member) through close observation of the affected limb[15,17,18]. Currently, there is no grading scale for the caregiver to refer to when assessing the vascular status of a limb in children undergoing surgery for limb fracture.
Therefore, the aim of this retrospective study is to establish a simple and efficient grading scale of limb perfusion in children undergoing surgery for limb fracture. The scale could function as an early alert system for poor limb perfusion. The present study provides some clinical evidence for the use of this scale as an early observation system dedicated to the caregivers, facilitating decision-making and improving the prognosis of the injured children.
This retrospective study included pediatric patients with limb fractures who were admitted at the Department of Pediatric Orthopedics of Xinhua Hospital between February 2017 and August 2017. The study protocol was approved by the ethics committee of Xinhua Hospital. The need for individual consent was waived by the committee.
The inclusion criteria were: (1) 0-14 years of age; (2) Limb fracture confirmed by X-ray and managed by open reduction internal fixation (Kirschner wire or plate); (3) Postoperative cast fixation; and (4) Hospital stays > 3 d. The exclusion criteria were: (1) Confirmed diagnosis of nerve injury, already leading to sensory or motor dysfunction; (2) Non-limb fracture or any organic disease; or (3) Incomplete data.
Patients’ baseline data (including age, sex, fracture type, skin temperature, skin color, degree of swelling, arterial pulsation, laboratory results, imaging results, treatment methods, and fixation methods) and postoperative occurrence of poor limb perfusion (use of mannitol) were collected.
The skin temperature of the affected limb was measured at admission, every 1 h within 6 h after surgery, and daily from the first day after surgery. It was considered normal if it was similar to or 1-2 °C lower than the healthy limb. Skin temperature of the affected limb lower by ≥ 3 °C indicated poor perfusion. The normal color of the skin should be red, pink, or similar to that of the healthy limb. Skin color of the distal limb turning pale indicated an ischemic state, possibly due to arterial spasm or embolism. Arterial pulse and degree of swelling were judged by observation and experience. The limb arterial pulse could be normal or absent/weak. Swelling could be present or absent. The degree of self-perceived pain was evaluated.
In this study, the outcome was poor limb perfusion, which is defined as the postoperative use of mannitol and evaluated at admission, every 1 h within 6 h after surgery, and daily from the first day after surgery. The children were divided into the normal perfusion group and the poor perfusion group.
The continuous variables were tested for normal distribution using the Kolmogorov-Smirnov test. Continuous variables were presented as means ± standard deviation and analyzed using the Student t-test (normal distribution) or as medians (interquartile range) and analyzed using the Mann-Whitney U test (skewed distribution). Categorical variables were expressed as numbers (%) and analyzed using the chi-square test or Fisher’s exact test, as appropriate. The key risk factors have been selected by univariable analyses to establish the Grading Scale for Vascular Status (GCVS). P values ≤ 0.05 indicate statistically significant differences.
A total of 161 children were included: 85 in the normal perfusion group and 76 in the poor perfusion group. The median age of the normal and poor perfusion groups was 6.3 (3.9, 8.7) vs 6.7 (5.1, 9.0) years. There were 55 (52.9%) and 49 (47.4%) males in the normal and poor perfusion groups. There were no significant differences in age, sex, body mass index, ethnicity, cause of fracture, fixation, or site of fracture between the two groups (Table 1). After surgery, the skin temperature of the affected limb (P = 0.048) and skin color (P < 0.001) were significantly different between the two groups.
| Normal perfusion, n = 85 | Poor perfusion, n = 76 | P value | |
| Age in yr | 0.532 | ||
| mean ± SD | 6.7 ± 3.2 | 6.8 ± 2.9 | |
| Median (IQR) | 6.3 (3.9, 8.7) | 6.7 (5.1, 9.0) | |
| Age stratification in yr, n (%) | 0.898 | ||
| ≤ 3 | 12 (14.1) | 10 (13.2) | |
| 3-6 | 28 (32.9) | 23 (30.3) | |
| > 6 | 45 (53.0) | 43 (56.6) | |
| Sex, n (%) | 0.975 | ||
| Female | 30 (35.3) | 27 (35.5) | |
| Male | 55 (64.7) | 49 (64.5) | |
| BMI in kg/m2 | 0.978 | ||
| mean ± SD | 17.4 ± 3.3 | 17.5 ± 3.4 | |
| Median (IQR) | 16.6 (15.2, 18.8) | 16.5 (15.2, 18.8) | |
| BMI-for-age, z score | 0.939 | ||
| mean ± SD | 0.75 ± 1.79 | 0.64 ± 1.31 | |
| Median (IQR) | 0.49 (-0.37, 1.81) | 0.52 (-0.32, 1.58) | |
| ≥ 1, n (%) | 31 (36.5) | 28 (36.8) | 0.961 |
| < 1, n (%) | 54 (63.5) | 48 (63.2) | |
| Ethnicity, n (%) | 1 | ||
| Han | 84 (98.8%) | 75 (98.7%) | |
| Other | 1 (1.2%) | 1 (1.3%) | |
| Cause of fracture, n (%) | 0.422 | ||
| Fall | 82 (96.4) | 72 (94.7) | |
| Hit | 2 (2.4) | 4 (5.3) | |
| Falling | 1 (1.2) | 0 | |
| Method of postoperative fixation, n (%) | |||
| Tubular plaster | 83 (97.6) | 76 (100.0) | 0.498 |
| Non-tubular plaster | 2 (2.4) | 0 | |
| Site of fracture, n (%) | 0.26 | ||
| Radius or ulna | 21 (24.7) | 17 (22.4) | |
| Humerus | 57 (67.1) | 49 (64.5) | |
| Tibia or fibula | 5 (5.9) | 10 (13.2) | |
| Femur | 2 (2.4) | 0 | |
| Degree of self-perceived pain, score, n (%) | 1 | ||
| 1 | 3 (5.3) | 3 (5.2) | |
| 2 | 53 (93.0) | 54 (93.1) | |
| 3 | 1 (1.8) | 1 (1.7) | |
| Maximum oxygen saturation of the affected limb among the six follow-ups, n (%) | 0.248 | ||
| ≥ 95% | 82 (96.5) | 76 (100.0) | |
| 90%-95% | 3 (3.5) | 0 | |
| < 90% | 0 | 0 | |
| Skin temperature of the affected limb, n (%) | 0.048 | ||
| Like the healthy contralateral limb | 85 (100.0) | 72 (94.7) | |
| Different from the healthy contralateral limb | 0 | 4 (5.3) | |
| Skin color, n (%) | < 0.001 | ||
| 1 = Red | 85 (100.0) | 45 (59.2) | |
| 2 = Dark red | 0 | 31 (40.8) | |
| Degree of swelling of the affected limb, n (%) | 1 | ||
| 1 = No | 1 (1.2) | 0 | |
| 2 = Presence of mild skin texture | 84 (98.8) | 76 (100.0) | |
| Range of motion of the affected limb, n (%) | 0.103 | ||
| 2 = Mildly restrained | 85 (100.0) | 73 (96.1) | |
| 3 = Restrained with numbness | 0 | 3 (3.9) | |
| Arterial pulsation, n (%) | |||
| 1 = Present | 85 (100.0) | 76 (100.0) | |
| Degree of pain of the affected limb | |||
| 1 = Painless | 30 (35.3) | 19 (25.0) | |
| Capillary refill time, n (%) | |||
| 1 = 1-2 s | 85 (100.0) | 76 (100.0) | |
| 2 = < 1 s | 0 | 0 | |
| 3 = > 2-3 s | 0 | 0 | |
Based on their potential clinical value, three factors were selected (Table 2). The relative risk (RR) and 95% confidence intervals (CI) for skin temperature of the affected limb, skin color, and range of motion of the affected limb were 2.18 (1.84, 2.59), 2.89 (2.28, 3.66), and 2.16 (1.83, 2.56), respectively. A scoring system was built using those three factors (Table 3).
| RR | 95%CI | P value | |
| Skin temperature of the affected limb | 0.048 | ||
| Like the healthy contralateral limb | 1 | ||
| Different from the healthy contralateral limb | 2.181 | (1.840, 2.585) | |
| Skin color | < 0.001 | ||
| 1 = Red | 1 | ||
| 2 = Dark red | 2.889 | (2.281, 3.659) | |
| Range of motion of the affected limb | 0.103 | ||
| 2 = Mildly restrained | 1 | ||
| 3 = Restrained with numbness | 2.164 | (1.829, 2.561) |
| Factor | Score |
| Skin temperature | |
| Like the healthy contralateral limb | 1 |
| Different from the healthy contralateral limb | 0 |
| Skin color | |
| Red | 0 |
| A color other than red | 1 |
| Range of motion of the affected limb | |
| Restrained with numbness | 1 |
| Restrained without numbness | 0 |
The total GCVS of each patient was calculated. The range of the score was 0-3. There were 126 patients with score 0, 32 with score 1, and three with score 2. Among them, 41 (32.5%) with score 0 had poor limb perfusion; all patients with scores 1 and 2 had poor limb perfusion (both 100%, Table 4).
| Score | Occurrence of the outcome |
| 0 | 41/126 (32.5%) |
| 1 | 32/32 (100%) |
| 2 | 3/3 (100%) |
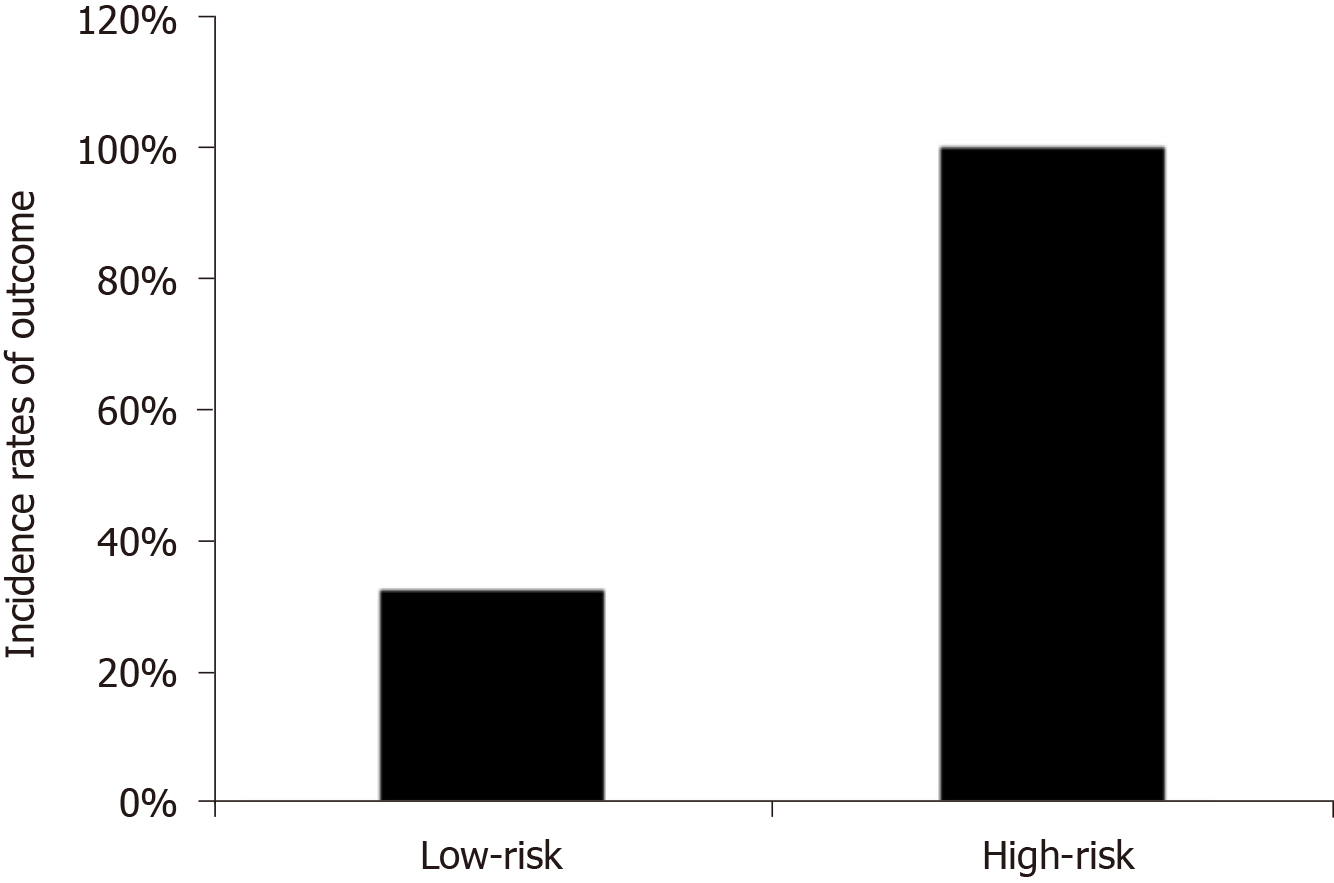
According to the results listed in Table 3, the scores were divided as low-risk (score: 0) and high-risk (score: ≥ 1). Poor limb perfusion is observed in 32.5% of the patients at low risk and in 100% of those at high risk (Figure 1).
Assessment of the vascular status following limb fracture in children is important to evaluate the risk of compartment syndrome, which is an emergency condition. Therefore, the present study aims to establish a simple and efficient grading scale of limb perfusion in children undergoing surgery for limb fracture. The results suggest that in children undergoing surgery for limb fracture, a higher GCVS score is associated with a higher occurrence of poor limb perfusion. A prospective study is required for validation.
If not diagnosed and managed in time, poor limb perfusion may lead to hypoperfusion or venous return obstruction and eventually to compartment syndrome, which may have dire consequences[10,11,14,15]. Poor limb perfusion has been reported in about 2%-20% of humeral fractures[12,19-21]. The occurrence of lower limb malperfusion appears to be lower (4%-7%)[22]. In the present study, poor limb perfusion was observed in 46% of the patients with humeral fractures, 45% of those with radius/ulna fractures and 66.7% of those with tibia/fibula fractures. Those rates are different from those reported in the literature, but the discrepancies could be due to a number of factors, including the criteria for malperfusion and local practice. At our center, mannitol is used to decrease tissue edema and alleviate reperfusion injury[23-25]. Because mannitol must be prescribed and is therefore indicated in the medical charts, it is a more reliable criterion than the physicians' and nurses' more or less objective descriptions.
The aim of the present study was indeed to determine an objective scoring system that could indicate the likeliness of developing poor limb perfusion after a pediatric fracture. In the present, a three-point score based on skin temperature of the affected limb, skin color, and range of motion of the affected limb showed that those with none of those factors had a lower occurrence of low limb perfusion (33%), while those with at least one of those factors had a 100% likelihood of having low limb perfusion. A recent review pointed out that there is currently no consensus about the symptoms and signs of poor limb perfusion[12]. Nevertheless, this review indicates that color, temperature, and edema should be assessed[12], while there is no evidence that the 2-s capillary refill is a valid assessment of perfusion[26]. Low skin temperature is thought to indicate poor limb perfusion[27]. Angiography is a gold standard for perfusion, but many authors consider that it will unnecessarily delay the definitive treatment[13,28]. Similar controversies exist for pulse oximetry and Doppler ultrasound[13]. Therefore, the scoring system suggested here could be of value for the evaluation of fractured limb in children because it can easily and rapidly be carried out on the bedside or in the evaluation room and identify patients with a higher risk of having low limb perfusion. The existing scoring systems evaluate the salvageability of limbs after trauma, but they are not specific to open fractures and to low limb perfusion[29]. The present study innovates by suggesting a scoring system for that specific situation in children.
Of course, the present study has limitations. It is a retrospective study, and the data that could be analyzed are limited to those available in the medical charts. In addition, the patients were from a single center, and the resulting sample size was small. The outcome was based on mannitol administration, not on a formal diagnosis. Finally, no validation of the scoring system was done, which will have to be carried out in future studies.
In conclusion, for children undergoing surgery for limb fracture, a higher GCVS score is associated with a higher occurrence of poor limb perfusion. A prospective study is required for validation.
Children are encouraged to practice physical activities to promote their health and normal development. Fractures in children are common, with an incidence of about 137-201 per 10000 person-years. Poor limb perfusion is a possible and severe complication following pediatric limb fracture. If not identified and not managed promptly, patients may experience hypoperfusion or venous return obstruction and present with a series of physiological or pathological changes such as pain, swelling, and pale skin. In severe cases, it may lead to compartment syndrome, acute avascular necrosis of the nerves or muscles, and even deformity and disability.
The assessment of the vascular status following limb fracture in children is important to evaluate the risk of compartment syndrome, which is an emergency condition.
To establish a simple and efficient grading scale of limb perfusion in children undergoing surgery for limb fracture.
This retrospective study included pediatric patients with a limb fracture and postoperative plaster fixation who were admitted at The Department of Pediatric Orthopedics of Xinhua Hospital between February 2017 and August 2017. The outcome is poor limb perfusion, which is defined as the postoperative use of mannitol. The children were divided into the normal perfusion group and the poor perfusion group. The key risk factors were selected by univariable analyses to establish the Grading Scale for Vascular Status (GCVS).
A total of 161 patients were included in the study: 85 in the normal perfusion group and 76 in the poor perfusion group. There were no significant differences in age, sex, body mass index, ethnicity, cause of fracture, fixation, or site of fracture between the two groups. After surgery, the skin temperature of the affected limb (P = 0.048) and skin color (P < 0.001) were significantly different between the two groups. The relative risk and 95% confidence interval for skin temperature of the affected limb, skin color, and range of motion of the affected limb were 2.18 (1.84-2.59), 2.89 (2.28-3.66), and 2.16 (1.83-2.56), respectively. The grading scale was established based on those three factors (score range: 0-3 points). Forty-one patients (32.5%) with score 0 had poor limb perfusion; all patients with scores 1 (n = 32) and 2 (n = 3) had poor limb perfusion (both 100%).
In children undergoing surgery for limb fracture, a higher GCVS score is associated with a higher occurrence of poor limb perfusion. A prospective study is required for validation.
The patients are from a single center, and the resulting sample size is small. The outcome is based on mannitol administration, not on a formal diagnosis. No validation of the scoring system was done. These issues will have to be addressed in future studies.
The authors acknowledge the help of Shen PQ from Xinhua Hospital. With his profound knowledge and rigorous academic attitude, Shen PQ offered numerous suggestions that enabled the smooth progress of the study. The authors also thank everyone who has offered help for the study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bolshakova GB, Iyngkaran P S-Editor: Huang P L-Editor: Filipodia P-Editor: Li JH
| 1. | Hedström EM, Svensson O, Bergström U, Michno P. Epidemiology of fractures in children and adolescents. Acta Orthop. 2010;81:148-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 347] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 2. | Naranje SM, Erali RA, Warner WC Jr, Sawyer JR, Kelly DM. Epidemiology of Pediatric Fractures Presenting to Emergency Departments in the United States. J Pediatr Orthop. 2016;36:e45-e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 240] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 3. | Moon RJ, Harvey NC, Curtis EM, de Vries F, van Staa T, Cooper C. Ethnic and geographic variations in the epidemiology of childhood fractures in the United Kingdom. Bone. 2016;85:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Hills AP, King NA, Armstrong TP. The contribution of physical activity and sedentary behaviours to the growth and development of children and adolescents: implications for overweight and obesity. Sports Med. 2007;37:533-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 210] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 5. | Strong WB, Malina RM, Blimkie CJ, Daniels SR, Dishman RK, Gutin B, Hergenroeder AC, Must A, Nixon PA, Pivarnik JM, Rowland T, Trost S, Trudeau F. Evidence based physical activity for school-age youth. J Pediatr. 2005;146:732-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2155] [Cited by in RCA: 1905] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 6. | Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6297] [Cited by in RCA: 5884] [Article Influence: 309.7] [Reference Citation Analysis (0)] |
| 7. | Randsborg PH, Gulbrandsen P, Saltytė Benth J, Sivertsen EA, Hammer OL, Fuglesang HF, Arøen A. Fractures in children: epidemiology and activity-specific fracture rates. J Bone Joint Surg Am. 2013;95:e42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Brudvik C, Hove LM. Childhood fractures in Bergen, Norway: identifying high-risk groups and activities. J Pediatr Orthop. 2003;23:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Hoff GL, Martin TA. Outdoor and indoor soccer: injuries among youth players. Am J Sports Med. 1986;14:231-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Mencio GA. Fractures and dislocations about the elbow. In: Mencio GA, Swiontkowski MF. Green’s skeletal trauma in children. 5th ed. Philadelphia: Elsevier Saunders; 2015: 182-245. |
| 11. | Hosseinzadeh P, Talwalkar VR. Compartment Syndrome in Children: Diagnosis and Management. Am J Orthop (Belle Mead NJ). 2016;45:19-22. [PubMed] |
| 12. | Badkoobehi H, Choi PD, Bae DS, Skaggs DL. Management of the pulseless pediatric supracondylar humeral fracture. J Bone Joint Surg Am. 2015;97:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Delniotis I, Delniotis A, Saloupis P, Gavriilidou A, Galanis N, Kyriakou A, Potoupnis M, Tsiridis E, Ktenidis K. Management of the Pediatric Pulseless Supracondylar Humeral Fracture: A Systematic Review and Comparison Study of "Watchful Expectancy Strategy" Versus Surgical Exploration of the Brachial Artery. Ann Vasc Surg. 2019;55:260-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Via AG, Oliva F, Spoliti M, Maffulli N. Acute compartment syndrome. Muscles Ligaments Tendons J. 2015;5:18-22. [PubMed] |
| 15. | Elliott KG, Johnstone AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg Br. 2003;85:625-632. [PubMed] |
| 16. | Shen RP, Hu JJ. Effect of mannitol combined with psycholigical intervention on postoperative swelling of extremitied fractures. Zhongguo Shenghua Yaowu Zazhi. 2017;37:33. |
| 17. | von Keudell AG, Weaver MJ, Appleton PT, Bae DS, Dyer GSM, Heng M, Jupiter JB, Vrahas MS. Diagnosis and treatment of acute extremity compartment syndrome. Lancet. 2015;386:1299-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 185] [Article Influence: 18.5] [Reference Citation Analysis (1)] |
| 18. | Bae DS, Kadiyala RK, Waters PM. Acute compartment syndrome in children: contemporary diagnosis, treatment, and outcome. J Pediatr Orthop. 2001;21:680-688. [PubMed] |
| 19. | Choi PD, Melikian R, Skaggs DL. Risk factors for vascular repair and compartment syndrome in the pulseless supracondylar humerus fracture in children. J Pediatr Orthop. 2010;30:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | White L, Mehlman CT, Crawford AH. Perfused, pulseless, and puzzling: a systematic review of vascular injuries in pediatric supracondylar humerus fractures and results of a POSNA questionnaire. J Pediatr Orthop. 2010;30:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Cambon-Binder A, Jehanno P, Tribout L, Valenti P, Simon AL, Ilharreborde B, Mazda K. Pulseless supracondylar humeral fractures in children: vascular complications in a ten year series. Int Orthop. 2018;42:891-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Waikakul S, Sakkarnkosol S, Vanadurongwan V. Vascular injuries in compound fractures of the leg with initially adequate circulation. J Bone Joint Surg Br. 1998;80:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Better OS, Zinman C, Reis DN, Har-Shai Y, Rubinstein I, Abassi Z. Hypertonic mannitol ameliorates intracompartmental tamponade in model compartment syndrome in the dog. Nephron. 1991;58:344-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Shah DM, Bock DE, Darling RC 3rd, Chang BB, Kupinski AM, Leather RP. Beneficial effects of hypertonic mannitol in acute ischemia--reperfusion injuries in humans. Cardiovasc Surg. 1996;4:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Daniels M, Reichman J, Brezis M. Mannitol treatment for acute compartment syndrome. Nephron. 1998;79:492-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Schriger DL, Baraff L. Defining normal capillary refill: variation with age, sex, and temperature. Ann Emerg Med. 1988;17:932-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 117] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Langer V. Management of major limb injuries. ScientificWorldJournal. 2014;2014:640430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Kumar R, Trikha V, Malhotra R. A study of vascular injuries in pediatric supracondylar humeral fractures. J Orthop Surg (Hong Kong). 2001;9:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Shanmuganathan R. The utility of scores in the decision to salvage or amputation in severely injured limbs. Indian J Orthop. 2008;42:368-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |