Published online Nov 26, 2020. doi: 10.12998/wjcc.v8.i22.5744
Peer-review started: August 6, 2020
First decision: August 22, 2020
Revised: September 12, 2020
Accepted: September 23, 2020
Article in press: September 23, 2020
Published online: November 26, 2020
Processing time: 111 Days and 7.2 Hours
Gastric mixed adenoma-neuroendocrine tumors (NETs) are quite rare. In the 2019 world health organization classification of tumors of the digestive system, these were designated as a combination of grade 1 or grade 2 NETs and adenomas or tubular adenomas. There are no treatment guidelines for these tumors, and pathological and clinical studies are ongoing. Herein, we review previous case reports and present a case of gastric mixed adenoma-NET.
A 66-year-old man underwent gastrointestinal endoscopy for the evaluation of upper abdominal pain. Histopathological examination of the biopsy specimen indicated the possibility of gastric cancer. A histopathological examination by endoscopic submucosal dissection showed a mixed adenoma-NET that was completely excised by endoscopic submucosal dissection. No recurrence was observed on gastrointestinal endoscopy at the 6-mo follow-up.
Clinicians' awareness of this rare tumor is important for its timely diagnosis and treatment.
Core Tip: Neuroendocrine tumors are rare tumors, and analysis and classification of tumors have progressed due to recent advances in immunostaining and gene analysis. The mixture of gastric adenoma or adenocarcinoma with neuroendocrine tumor is quite rare, and it is interesting to investigate the mechanism of tumor development and differentiation. We experienced a case of mixed adenoma- neuroendocrine tumors and considered problems in diagnosis and treatment, so we will report it together with other reported cases.
- Citation: Kohno S, Aoki H, Kato M, Ogawa M, Yoshida K. Gastric mixed adenoma-neuroendocrine tumor: A case report. World J Clin Cases 2020; 8(22): 5744-5750
- URL: https://www.wjgnet.com/2307-8960/full/v8/i22/5744.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i22.5744
Neuroendocrine tumors (NETs) are a rare type of gastric tumor. Moreover, lesions with a combination of endocrine tumors and gastric adenomas have a very low incidence[1]. There are few case reports[1-8] of gastric mixed adenoma-NETs, and the clinical features are unknown. Advances in immunohistochemical staining and genetic analysis have led to their classification as mixed adenoma-NETs[9]. Gastric mixed adenoma-NETs are not commonly recognized by clinicians and are difficult to diagnose from a forceps biopsy specimen[1,7]. Herein, we report a case of gastric mixed adenoma-NET and review it together with 10 case reports.
A 66-year-old man presented with upper abdominal pain in September 2019.
The patient was receiving outpatient treatment to hypertension. In September 2019, he went to the clinic complaining intermittent epigastric pain and underwent gastrointestinal endoscopy.
The patient had a history of hypertension, hyperlipidemia, and colorectal polyps.
There is nothing special to mention in personal and family history.
Physical examination was normal.
Laboratory examinations are almost normal.
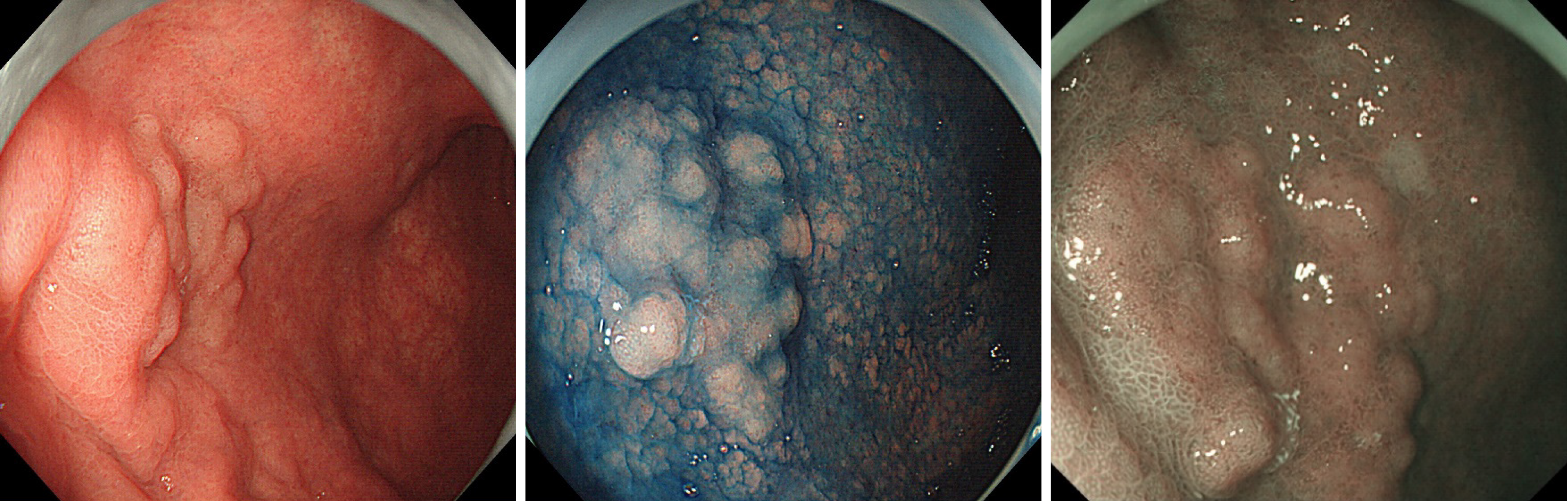
Endoscopy showed a superficial-elevated (0-IIa) granular lesion with a maximum diameter of 10 mm on the anterior wall of the gastric body (Figure 1).
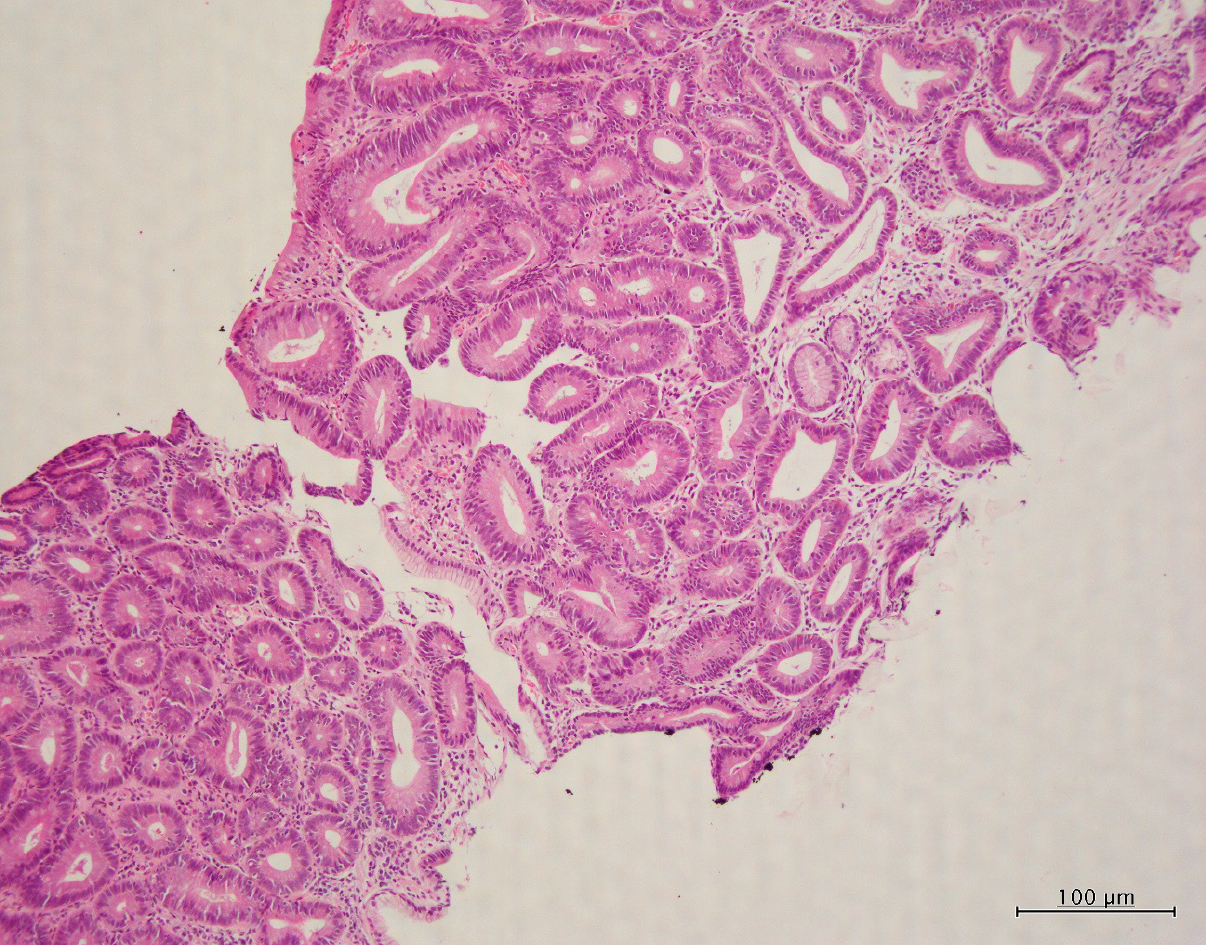
Histopathological examination revealed the proliferation of intestinal-type glands within the gastric mucosa and irregular branching in some of the glands. Despite mild nuclear stratification and enlargement, most of the visible glands were those of adenomas, although some had structurally atypical glands suggestive of a well-differentiated adenocarcinoma (Figure 2). The change in the surrounding mucosa was diagnosed as atrophic gastritis.
These cells resected by endoscopic submucosal dissection (ESD) were categorized as grade 1 (G1) NET cells. There was no submucosal invasion. Based on the histopathological features, the lesion was interpreted as a mixed adenoma-NET.
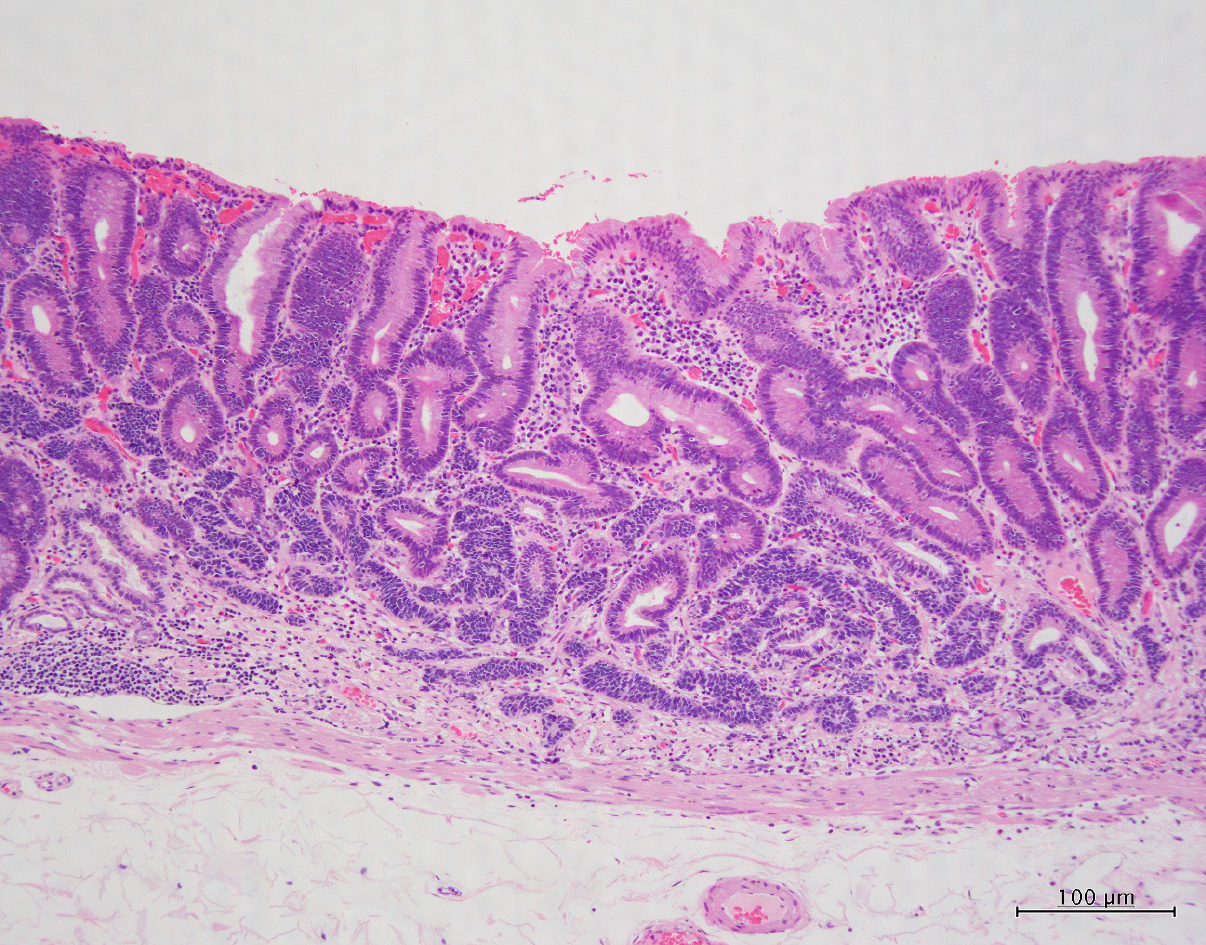
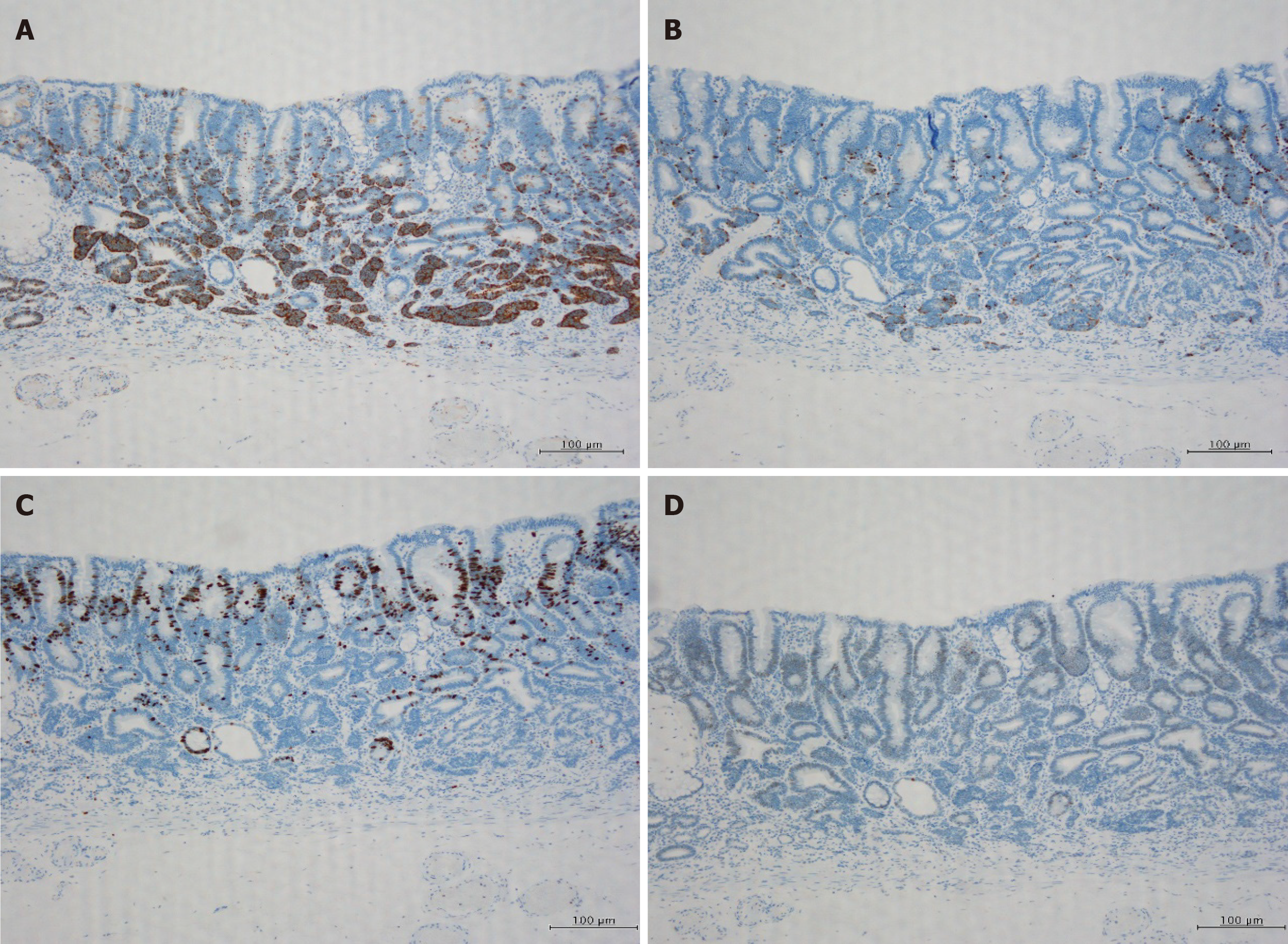
Therefore, the patient underwent ESD in November 2019. The ESD-resected specimen measured 30 mm × 30 mm, and hematoxylin and eosin staining showed slightly elevated lesions with many superficial tubular or dilated glands without fusing. The glands were lined by moderately atypical epithelial cells, indicating a diagnosis of tubular adenomas (Figure 3). Immunohistochemically, these atypical cells were weakly positive for p-53, and scattered neuroendocrine cells (synaptophysin + chromogranin A) were present in the deep adenoma. Many cords and sheets of small neuroendocrine-type cells that formed small clusters or sheets were observed. These cells were diffusely positive for synaptophysin and keratin. Staining for chromogranin A occasionally tested positive, whereas testing for Ki-67 was rarely positive (< 1%), and for p-53 was negative (Figure 4). No molecular pathological examination was performed.
After ESD, the patient was discharged from the hospital without complications. Endoscopic examination conducted 6 mo after ESD showed no tumor recurrence.
Composite glandular carcinoid tumors of the digestive tract was first reported in 1988 by Moyana et al[10] Owing to research progress, the definition of this lesion has changed over time. This lesion has received multiple names, including carcinoid tumor in adenoma, NET in adenoma, mixed adenoma well-differentiated neuroendocrine tumor, and mixed adenoma-NET[1-8]. Tumors with a mixture of glandular and neuroendocrine components in the gastrointestinal tract were classified as mixed neuroendocrine-non-neuroendocrine neoplasms (MiNEN) or mixed adenoma-NET following the 2019 World Health Organization (WHO) classification[9]. MiNEN includes mixed adenocarcinoma-neuroendocrine carcinoma or mixed adenoneuroendocrine carcinoma and mixed adenocarcinoma-NET. Mixed adenoma-NET has been classified separately from MiNEN in the 2019 WHO classification based on characteristic findings that were identified through advances in immunohistochemical staining and genetic testing[9]. Gastric mixed adenoma-NET is a rare tumor that is composed of G1 or grade 2 (G2) NETs as well as adenomas or tubulovillous adenomas. According to the 2019 WHO classification, neuroendocrine tumors (well-differentiated NETs with relatively low malignancy) are designated as G1 and G2 NETs. G1 and G2 are classified by the Ki-index[11,12]. As a typical pathological feature, the neuroendocrine component is generally located within the deep central portion of the polyp, whereas the adenomatous component occupies most of the periphery[1].
Although gastric lesions with mixed adenomas and NETs are rare, there are several reports showing similar cases[1-8]. 1 shows a report of gastric mixed adenoma-NETs. Eleven cases, including our case, have been reported. The average age was 65.1 years, and one of the cases was a female and the remaining cases were males. The average length of the tumor was 14.7 mm. The depth of invasion of the tumor was submucosal in 2 cases, mucosal in 8 cases, and unknown in 1 case. Treatment was ESD in 5 cases, polypectomy in 3 cases, gastrectomy in 2 cases, and unknown in the last case. No deaths were observed during the follow-up period. Endoscopic macroscopic findings included sessile polyps in 5 cases, 0-IIa type polyp in 1 case, the endoscopic findings were unknown in 5 cases. Gastric adenoma-NET was diagnosed by forceps biopsy in 3 cases and by evaluation of the pathological specimen obtained by polypectomy in 2 cases. One case was diagnosed as adenoma, another case was suspected to be an adenocarcinoma, and the diagnoses of the remaining four cases were unknown. According to Lee et al[7], who reported three cases among the unknown cases, the pathological diagnosis of mixed adenoma-NET by forceps biopsy was difficult (Table 1).
| No | Ref. | Age | Sex | Size (mm) | Location | Polyp type | Adenoma type | NET grade | Forceps biopsy diagnosis | Depth | Procedure | Follow-up |
| 1 | Lehtola et al[2] | NA | M | 5 | Body | 0-Is | TA, NA | NA | Gastric mixed adenoma-NET | NA | Polypectomy | AFD (1 yr) |
| 2 | Ito et al[3] | 54 | M | 20 | Body | 0-Is | TA, LG | NA | By polypectomy | MM | Subtotal Gx | AFD (19 yr) |
| 3 | Harada et al[4] | 72 | M | 10 | Body LC, GC | 0-Is | NA | NA | Gastric mixed adenoma-NET | LP | Bx→ESD | AFD (13 yr) |
| 4 | De Marco et al[5] | 76 | M | 10 | Body | 0-Is | TA, LG | G1 | By polypectomy | MM | Polypectomy | AFD (10 mo) |
| 5 | Coyne and O'Connor[6] | 68 | F | 11 | NA | NA | TA, LG | G1 | Gastric mixed adenoma-NET | SM | Bx→polypectomy | NA |
| 6 | Lee et al[7] | 64 | M | 6 | Body PW | NA | TA, NA | NA | NA | MM | ESD | AFD (2 yr) |
| 7 | Lee et al[7] | 63 | M | 40 | Antrum | NA | TA, NA | NA | NA | LP | ESD | AFD (2 yr) |
| 8 | Lee et al[7] | 52 | M | 18 | Body PW | NA | TA, NA | NA | NA | MM | ESD | AFD (2 yr) |
| 9 | La Rosa et al[1] | 55 | M | 15 | NA | NA | TA, HG | G1 | NA | MM | NA | AFD (27 yr) |
| 10 | Matsubara et al[8] | 69 | M | 5 | Body AW | 0-Is | NA | G2 | Adenoma | SM | ESD→proximal Gx | NA |
| 11 | Case | 66 | M | 22 | Body AW | 0-IIa | TA, HG | G1 | Adenocarcinoma susp | MM | ESD | AFD (6 mo) |
In our case, an early gastric cancer was suspected before ESD, but histopathological examination led to a final diagnosis of gastric mixed adenoma-NET. This lesion may be misdiagnosed as an adenocarcinoma due to the mixture and fusion of the gastric adenoma and neuroendocrine cells in some areas[1,7]. When an adenoma is investigated by endoscopy, malignant findings are often unevenly distributed and it is difficult to reliably diagnose the presence of malignancy or NET component by biopsy[1,7,13,14]. Localized endocrine neoplasia occurs frequently in both benign and malignant gastrointestinal tumors, whereas a truly mixed glandular endocrine neoplasia is a very rare tumor. Moreover, an accurate diagnosis based on histopathological examination of the biopsy specimen is difficult due to the presence of similar characteristics in MiNEN[15]. Mixed adenoma-NETs are considered difficult to diagnose by biopsy. To avoid a missed diagnosis, recognition of this disease is important. Consequently, diagnosis often requires histopathological examination by excision in addition to forceps biopsy[1].
The optimal treatment should be selected based on detailed results of the histopathological examination of the resected lesion. G1 NET is considered a low malignant potential tumor, although a few cases of lymph node metastasis in the same lesion have been reported, further research is required to ascertain the malignant potential of NETs[16,17].
To date, there are no therapeutic guidelines for mixed gastric adenoma and NET lesions. Hence, treatment is directed at the more aggressive and invasive lesions of the two components. Complete resection is the only treatment when the adenomatous part of the stomach is the more aggressive component. After the gastric cancer component is diagnosed as more aggressive, it is classified as MiNEN, and the treatment is performed following treatment for gastric cancer[18]. In cases where the NET component has a stronger or more aggressive malignant potential, we treated for NET following the recommended treatment methods[16,19]. Lymph node dissection is useful for lesions that are at high risk for lymph node metastasis[16]. Resection with lymph node dissection is recommended in accordance with the NET classification and tumor size[16]. In NETs, the possibility of lymph node metastasis is defined by the size and depth of invasion; therefore, preoperative diagnosis is essential for lymph node dissection during tumor resection. Among NETs < 1 cm that invade the lamina propria or submucosa, the incidence of lymph node metastasis is reported to be 3.4%. Endoscopic resection may be appropriate for intraepithelial tumors < 2 cm, and perhaps tumors < 1 cm invading into the lamina propria or submucosa[16]. Thus, we believe that a combined therapeutic strategy and the application of current knowledge would lead to better treatment. Given that this is a rare disease, accurate diagnosis through histopathological analysis of the lesions and scrutinization of recent information is important to determine the treatment plan.
Mixed adenoma-NET may be found in gastric polyps, and its recognition is necessary for diagnosis and treatment.
We would like to thank Dr. Nomura K in the Department of Pathology for his guidance on the diagnosis of pathological tissues and the interpretation of lesions.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yu C S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | La Rosa S, Uccella S, Molinari F, Savio A, Mete O, Vanoli A, Maragliano R, Frattini M, Mazzucchelli L, Sessa F, Bongiovanni M. Mixed Adenoma Well-differentiated Neuroendocrine Tumor (MANET) of the Digestive System: An Indolent Subtype of Mixed Neuroendocrine-NonNeuroendocrine Neoplasm (MiNEN). Am J Surg Pathol. 2018;42:1503-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Lehtola J, Karttunen T, Krekelä I, Niemelä S, Räsänen O. Gastric carcinoids with minimal or no macroscopic lesion in patients with pernicious anemia. Hepatogastroenterology. 1985;32:72-76. [PubMed] |
| 3. | Ito H, Ito M, Tahara E. Minute carcinoid arising in gastric tubular adenoma. Histopathology. 1989;15:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Harada T, Imura M, Masutani M, Konno S, Miyazaki H, Nomura A, Okushiba S, Katoh H, Itoh T, Shimizu M, Kawakami Y. Carcinoid tumor detected in gastric adenoma during long-term follow-up. Gastrointest Endosc. 2001;53:804-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | De Marco L, Carlinfante G, Botticelli L, Di Maira PV, Putrino I, Cavazza A. [Mixed neoplasia of the stomach: description of a case of tubular adenoma combined with carcinoid]. Pathologica. 2003;95:214-216. [PubMed] |
| 6. | Coyne JD, O'Connor B. Mixed adenoma-endocrine tumour of the stomach. Histopathology. 2010;57:492-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Lee SM, Ahn S, Lee YK, Jang KT, Park CK, Kim KM. Neuroendocrine tumor in gastric adenoma: a diagnostic pitfall mimicking invasive adenocarcinoma. Diagn Pathol. 2012;7:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Matsubara T, Hirahara N, Tabara H. A case report of a gastric neuroendocrine tumor arising from gastric adenoma. Int Surg. 2020;In press. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | WHO Classification of Tumors Editorial Board. WHO Classification of Tumors. Digestive System Tumors, 5th ed. International Agency for Research on Cancer (LARC): Lyon, France, 2019. |
| 10. | Moyana TN, Qizilbash AH, Murphy F. Composite glandular-carcinoid tumors of the colon and rectum. Report of two cases. Am J Surg Pathol. 1988;12:607-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | de Mestier L, Cros J, Neuzillet C, Hentic O, Egal A, Muller N, Bouché O, Cadiot G, Ruszniewski P, Couvelard A, Hammel P. Digestive System Mixed Neuroendocrine-Non-Neuroendocrine Neoplasms. Neuroendocrinology. 2017;105:412-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 12. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2442] [Article Influence: 488.4] [Reference Citation Analysis (3)] |
| 13. | Kasuga A, Yamamoto Y, Fujisaki J, Okada K, Omae M, Ishiyama A, Hirasawa T, Chino A, Tsuchida T, Igarashi M, Hoshino E, Yamamoto N, Kawaguchi M, Fujita R. Clinical characterization of gastric lesions initially diagnosed as low-grade adenomas on forceps biopsy. Dig Endosc. 2012;24:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Kim MS, Kim SG, Chung H, Kim J, Hong H, Lee HJ, Kim HJ, Kim MA, Kim WH, Jung HC. Clinical Implication and Risk Factors for Malignancy of Atypical Gastric Gland during Forceps Biopsy. Gut Liver. 2018;12:523-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Frizziero M, Chakrabarty B, Nagy B, Lamarca A, Hubner RA, Valle JW, McNamara MG. Mixed Neuroendocrine Non-Neuroendocrine Neoplasms: A Systematic Review of a Controversial and Underestimated Diagnosis. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (2)] |
| 16. | Saund MS, Al Natour RH, Sharma AM, Huang Q, Boosalis VA, Gold JS. Tumor size and depth predict rate of lymph node metastasis and utilization of lymph node sampling in surgically managed gastric carcinoids. Ann Surg Oncol. 2011;18:2826-2832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Soga J. Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer. 2005;103:1587-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 220] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1338] [Article Influence: 334.5] [Reference Citation Analysis (2)] |
| 19. | Shen C, Chen H, Chen H, Yin Y, Han L, Chen J, Tang S, Yin X, Zhou Z, Zhang B, Chen Z. Surgical treatment and prognosis of gastric neuroendocrine neoplasms: a single-center experience. BMC Gastroenterol. 2016;16:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |