Published online Nov 26, 2020. doi: 10.12998/wjcc.v8.i22.5737
Peer-review started: August 6, 2020
First decision: August 21, 2020
Revised: September 4, 2020
Accepted: September 18, 2020
Article in press: September 18, 2020
Published online: November 26, 2020
Processing time: 111 Days and 8.1 Hours
Turner syndrome (TS) has a variety of different karyotypes, with a wide range of phenotypic features, but the specific karyotype may not always predict the phenotype. TS with Y chromosome mosaicism may have mixed gonadal dysgenesis, and the mosaicism is related to the potential for gonadoblastoma.
In this case report, we report two cases of TS with different karyotypes and gonadal dysgenesis. Patient 1 had obvious virilization, and was positive for the SRY gene, but her karyotype in peripheral blood lymphocytes was 45X. Patient 2 had a mosaic karyotype, 45X/46X, dic (Y:Y) (p11.3:p11.2), and the proportion of Y-bearing cells was 50% in peripheral blood lymphocytes, but the patient had normal female external genitalia and streaky gonads, with no genital virilism. Different tissues in the same TS individual may exhibit different ratios of mosaicism. The gonadal determination and differentiation of mosaic TS are primarily dependent on the predominant cell line in the gonads.
In TS patients with virilization, it is necessary to test at least two to three tissues to search for cryptic Y material.
Core Tip: In this article, we report two cases of Turner syndrome (TS) with different karyotypes and gonadal dysgenesis. The results show the importance of testing at least two to three tissues to search for cryptic Y material in TS patients with virilization. Polymerase chain reaction and fluorescence in situ hybridization analyses can be used for enhanced screening of Y sequences in TS patients. Different tissues in the same TS individual may exhibit different ratios of mosaicism. The gonadal determination and differentiation of mosaic TS are primarily dependent on the predominant cell line in the gonads.
- Citation: Leng XF, Lei K, Li Y, Tian F, Yao Q, Zheng QM, Chen ZH. Gonadal dysgenesis in Turner syndrome with Y-chromosome mosaicism: Two case reports. World J Clin Cases 2020; 8(22): 5737-5743
- URL: https://www.wjgnet.com/2307-8960/full/v8/i22/5737.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i22.5737
Turner syndrome (TS) is considered to be one of the most common aneuploidies, with an incidence of 1:2500 live-born female infants[1]. This condition is caused by the complete or partial loss of one X chromosome in all or a portion of cells, resulting in a variety of different karyotypes, with a wide range of phenotypic features. However, it is difficult to carry out a comparative analysis of the karyotypes and phenotypes in TS, and the specific karyotype does not always predict the phenotype[2]. TS with Y chromosome mosaicism may have mixed gonadal dysgenesis, and the mosaicism is related to the potential for gonadoblastoma; thus, it is important to detect the mosaic Y material. In this article, we reported two girls with TS, who had different karyotypes and sexual phenotypes. We attempted to identify the associations between karyotype and sexual phenotype in TS in these cases, and discuss the detection of the Y chromosome mosaicism in TS. This information may aid physicians in making more precise judgements in the clinic.
Case 1: A 12-year-old girl was brought to the pediatric clinic in our hospital due to clitoromegaly since infancy and short stature.
Case 2: A 10-year-old girl was presented to our department with short stature.
Case 1: The girl was found to have an abnormal enlargement of the clitoris since birth and she grew slower than her peers at the same age.
Case 2: The girl was observed to grow slower than her peers at the same age since she was a baby.
Case 1: She had undergone repair of an interventricular septal defect and surgery for a right indirect inguinal hernia, at 1 year of age and 12 years of age, respectively.
Case 2: The patient had no previous medical history.
Case 1: She was the first child of non-consanguineous parents with an uneventful birth history. Mental development was in line with her peers, and menarche was absent.
Case 2: She was the only child of non-consanguineous parents with an uneventful birth history. Her mental development was in line with her peers, and menarche was absent.
Case 1: On examination, her height was 141 cm (< 3rd percentile), body weight was 36 kg (> 3rd percentile), the external genitalia exhibited clitoromegaly (phallic length: 2 cm), and after stimulation, the clitoris could become erect (phallic length: 3 cm). There was no deformity of the cubitus valgus, no webbed neck, and no other body deformities.
Case 2: Her height was 134 cm (10th percentile), and her body weight was 35 kg (50th percentile). She had cubitus valgus and webbed neck, with no other body deformities. Her breasts were Tanner 1 stage and she had no pubic hair or underarm hair. Examination of the external genitalia showed a normal female vulva, with separate urethral and vaginal orifices and no clitoromegaly.
Case 1: Laboratory findings were as follows: Testosterone (T; 4.29 nmol/L), progesterone (P; 1.53 nmol/L), estradiol (E2; 21.93 pmol/L), and follicle-stimulating hormone (FSH) 32.22 IU/L.
Case 2: Laboratory findings showed that serum T was < 0.1 ng/mL, E2 was < 5 pg/mL, and FSH was 70.61 IU/L.
Case 1: Pelvic ultrasonography demonstrated a primordial uterus with a patent vagina, but failed to identify the ovaries.
Case 2: Pelvic ultrasonography demonstrated a primordial uterus, and failed to identify the ovaries. Cardiac ultrasonography did not identify any heart malformations.
Case 1: Chromosomal analysis of peripheral blood lymphocytes revealed hypodiploidy, with only one X chromosome (45, X). Gene analysis, using peripheral blood lymphocytes and polymerase chain reaction (PCR) revealed that the patient was positive for the SRY gene. To screen for hidden Y-chromosome sequences, we used chromosomal microarray analysis (CMA) with the Cytoscan HD chip, which also did not detect any Y sequences.
Case 2: Chromosomal analysis of peripheral blood lymphocytes indicated that her karyotype was 45, X/46, X, dic (Y:Y) (p11.3:p11.2). CMA further revealed two populations of cells: One cell line (50%) showed 45, X monosomy; while the other cell line (50%) was diploid, with one X chromosome and a dicentric Y chromosome.
The final diagnosis of patient 1 was Turner syndrome with mixed gonadal dysgenesis, and patient 2 was also Turner syndrome, with a 45, X/46, XY karyotype.
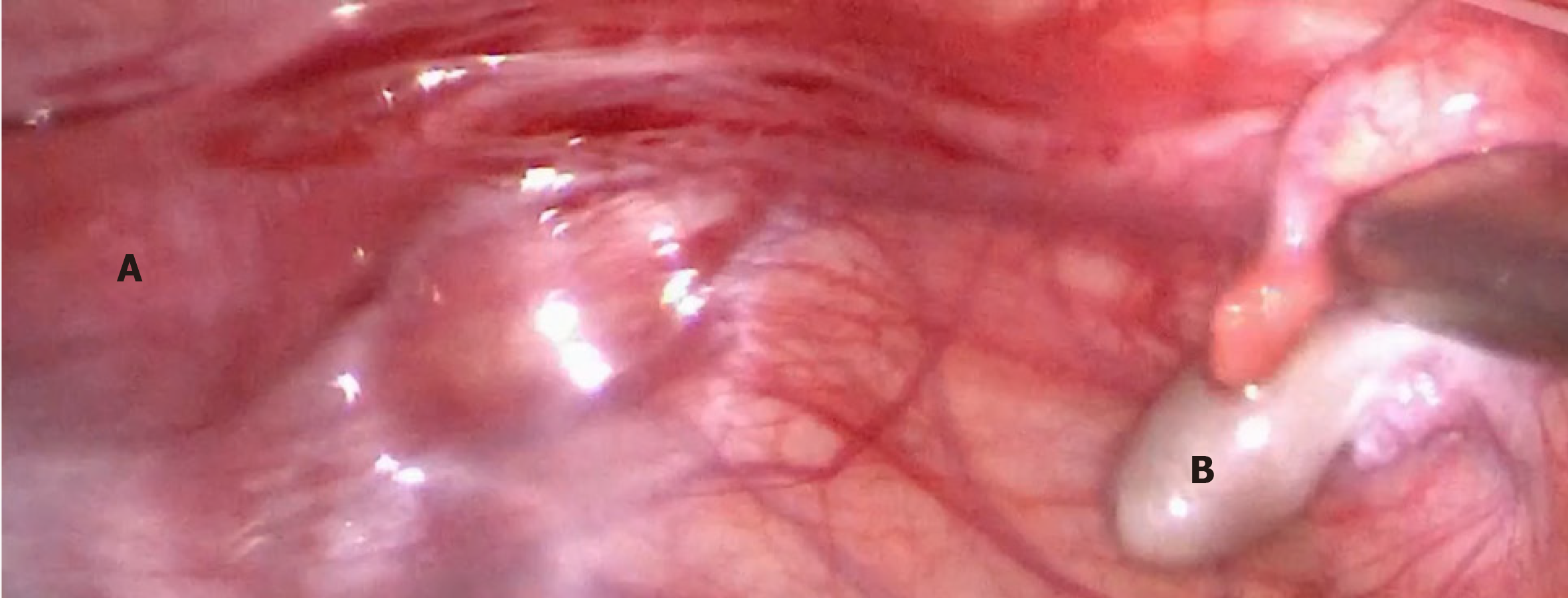
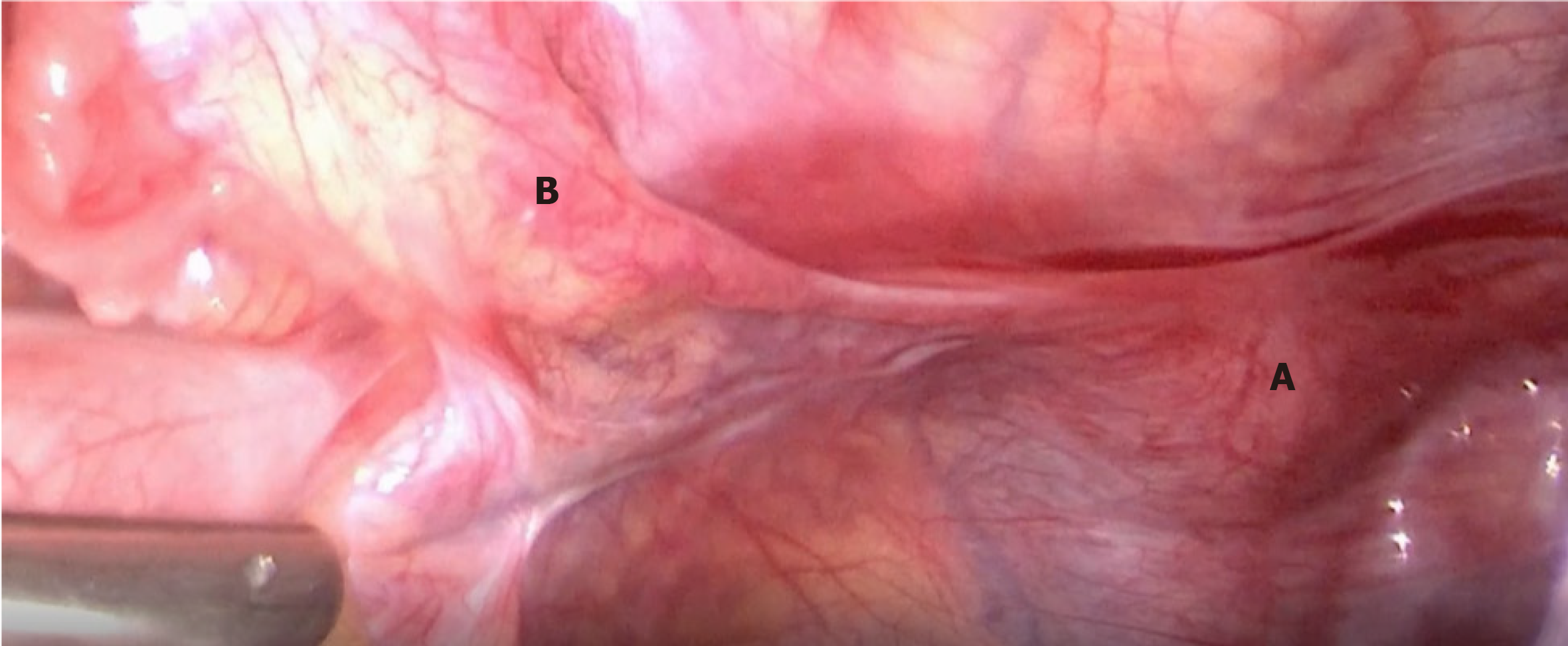
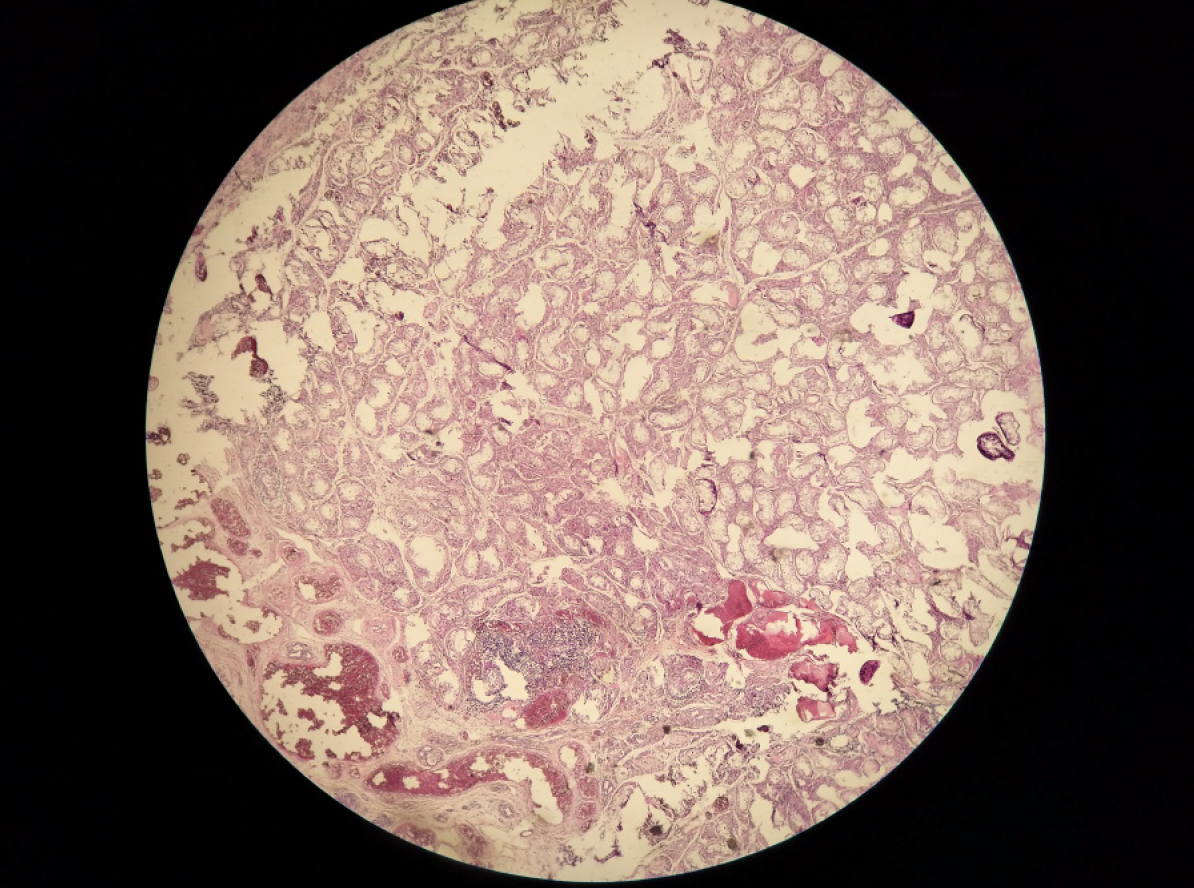
Considering the risk of gonadoblastoma in TS with a mosaic of Y sequences, it was suggested that the two patients undergo gonadectomy. The two patients, and their parents, provided consent. Patient 1 was then scheduled to undergo clitoroplasty and bilateral gonadectomy. On pelvic laparoscopy (Figures 1 and 2), the primordial uterus was seen, which was approximately 4 cm × 2 cm × 3 cm in size. Both fallopian tubes looked normal, although the left gonad looked streaky in appearance, and was approximately 0.5 cm × 0.3 cm in size. However, the right gonad looked similar to the testicular tissue, and was approximately 1 cm × 1 cm × 0.5 cm in size. Both adnexa were removed during surgery. Histopathology (Figures 3 and 4) showed that only a small amount of hyperplastic fibrous tissue and smooth muscle tissue were evident in the left dysgenetic gonad, and angiogenesis was observed. Neither ovarian stroma nor testicular tissue was evident. The right gonad mainly consisted of immature testicular tissue rather than ovarian elements. Only Sertoli cells could be seen in the seminiferous tubules of the right gonad; no germ cells were detected. Using PCR, we also detected Y mosaicism in two sides of the removed gonads. The left gonad, which had a streaky appearance, was negative for the SRY gene, while the right gonad looked similar to the testicular tissue and was positive for the SRY gene. Patient 2 also underwent bilateral gonadectomy. On pelvic laparoscopy, the primordial uterus could be seen, and the gonads on both sides looked streaky in appearance. Both adnexa were removed during surgery. Histopathology revealed that ovarian and fallopian tube tissue were present in the left and right adnexa. Furthermore, an epithelium inclusion, including endometrioid epithelium and mucous epithelium, was evident in the cortex of the ovary; no follicles were present. Following surgery, both patients commenced growth hormone (GH) treatment to increase levels to those normally attained during childhood. The dose of GH in both patients was initiated at 1.5 IU/kg/day.
Two days after surgery, the level of serum T in patient 1 was markedly reduced to less than 0.45 nmol/L. The rate of height growth in both patients after GH treatment was accelerated. Regular follow-up is still in progress, and no gonadoblastomas have been detected.
The most frequent karyotype in TS is 45, X monosomy, and accounts for 40%-50% of all karyotypes[2]. The common clinical manifestations of TS include a characteristic facial appearance; linear growth failure; ovarian insufficiency; early sensorineural hearing loss; distinctive congenital cardiovascular, skeletal, digital and renal anomalies; and a range of other disorders. Generally, patients with 45, X TS have gonads that are streaky in appearance or have immature ovaries. In this report, we describe the case of Patient 1, who had no ovarian tissue; instead, she had some immature testicular tissue containing Sertoli cells. This form of gonadal dysgenesis is typically observed in a mosaic 45, X/46, XY karyotype. However, standard cytogenetic analysis of peripheral blood lymphocytes, and even CMA, failed to detect any Y-chromosome sequences in Patient 1. Interestingly, PCR of the peripheral blood lymphocytes showed that this patient was positive for the SRY gene. This approach provided evidence of unrecognized Y sequences that were not found by classic karyotyping or CMA. In 2015, Jung et al[3] reported a similar case; the authors identified a TS patient (45, X) who was positive for the SRY gene. This patient had a normal female phenotype; however, pathological examination revealed a combination of ovarian tissue, including primordial follicles and testicular tissue, containing spermatogonia and seminiferous tubules. In 1992, Held et al[4] proposed that all female patients with TS and 45, X karyotypes carry a cell line containing two sex chromosomes at a low level of mosaicism, which is undetectable using standard cytogenetic analysis. This hypothesis was also mentioned in the European Clinical Practice Guideline in 2016, although this was not proven conclusively[2]. In view of this, we propose that Patient 1 carried a low level of Y mosaicism, which was undetectable using conventional karyotype analysis, even by CMA. PCR, with multiple Y-specific probes, is more sensitive for the detection of Y mosaicism in TS patients. In our cases, we used an SRY probe as a marker for the presence of Y sequences.
However, this still does not explain why Patient 1, with a 45, X karyotype, had more obvious virilization than Patient 2, who had normal female external genitalia and no genital virilism, while her karyotype was obviously mosaic with numerous Y sequences. Thus, it appears that the specific karyotype does not always predict the sexual phenotype. Therefore, which factors determine the sexual phenotype of TS patients? We know that the key to testicular formation from the undifferentiated embryonic gonad is the presence of the short arm of the Y chromosome, which contains the SRY gene. The SRY gene, located on Yp11.3, is a major molecular trigger of sex determination and differentiation. The presence of Y chromosome mosaicism in TS may determine modifications in the typical female phenotype, causing a wide range of degrees of virilization of the genital tract. However, this conclusion does not concur with the high proportion of Y-bearing cells in peripheral blood from Patient 2 (50%), whose gonads contained no sign of virilization.
Many recent studies have shown that the percentage of Y-bearing cell line in the peripheral blood remains an unreliable indicator of the sexual phenotype[5,6]. In these articles, the authors compared the ratio of cell lines in different tissues from TS patients including peripheral blood lymphocytes, buccal mucosa cells, skin fibroblasts, and gonadal tissue. They found that the ratio of cells showing 45, X monosomy, and cells with an abnormal Y chromosome, may differ from tissue to tissue. For example, Gole et al[5] analyzed a Turner phenotype with mixed gonadal dysgenesis, and karyotyped gonadal skin, the peripheral blood, and the right and left gonads, to determine the distribution of these two cell lines. The results showed that the patient’s right gonad, which was an ovotestis, had 31% of idic Y cells, while the left gonad, which was a streaky ovary, featured cells with a 45, X karyotype; the peripheral blood lymphocytes showed that 90% of cells had an idic Y chromosome. These studies suggest that the gonadal determination and differentiation of mosaic subjects is primarily dependent on the predominant cell line in the gonads. In other words, 45, X leads to the development of streaky gonad, while 46, X, idic (Y), or 46, XY, leads to a dysgenetic testis; the presence of both cell lines results in mixed gonadal dysgenesis[7]. This conclusion may support the discrepancy in our patients between their peripheral blood karyotypes, and their sexual phenotypes. In the majority of cells, the gonosomal karyotype in the gonadal tissue may represent the key factor responsible for the patient’s phenotypic characteristics[6]. Therefore, being able to identify the gonosomal karyotype is important, as it appears to influence the phenotypic presentation. One limitation of this study is that we could not further analyze the karyotypes of the gonadal tissue from these two patients.
The European Clinical Practice Guideline of 2016 reported that 45, X/46, XY accounted for 10%-12% of all karyotypes in TS[2]. However, another recent study reported that the frequency of Y-chromosome sequences in TS patients ranged from 4.6% to 60%[8]. These differences may be due to a range of factors including the criteria used to select patients, sample size, methodology, and the Y-chromosome markers used. When the analyzed tissue was peripheral blood alone, the prevalence of Y sequences ranged from 4.6% to 18.5%. However, in studies where more than one tissue was evaluated, the prevalence was greater. Y-chromosome sequences in TS patients raise concerns related to the potential for gonadoblastoma, a form of benign gonadal tumor with a high potential for malignant transformation. This tumor is mainly reported in females with gonadal dysgenesis; approximately 95% of these patients have Y sequences in their genomes[9]. Approximately 10% of TS patients with Y chromosome sequences may develop gonadoblastoma[2]. Therefore, when virilization is present in TS, it is necessary to test at least two to three tissues to search for cryptic Y material. Fluorescence in situ hybridization (FISH) or PCR with multiple Y-specific probes can increase the detection rate of Y-chromosomal material. Moreover, prophylactic gonadectomy is suggested in TS cases with Y chromosome material identified on standard karyotyping[2].
In conclusion, different tissues in the same TS individual may exhibit different ratios of mosaicism, and the gonadal determination and differentiation of mosaic TS are primarily dependent on the predominant cell line in the gonads. The mosaicism of Y chromosome sequences in TS is related to the potential for gonadoblastoma. Therefore, in TS patients with virilization, it is necessary to test at least two to three tissues to search for cryptic Y material, such as peripheral blood, buccal mucosa cells, skin fibroblasts, and especially gonadal tissue. However, conventional karyotype analysis is not sufficient. PCR or FISH analysis should be used to enhance the efficiency of screening for Y-chromosomal material in TS patients.
Manuscript source: Unsolicited manuscript
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang T S-Editor: Gong ZM L-Editor: Webster JR P-Editor: Li JH
| 1. | Sybert VP, McCauley E. Turner's syndrome. N Engl J Med. 2004;351:1227-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 518] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 2. | Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, Lin AE, Mauras N, Quigley CA, Rubin K, Sandberg DE, Sas TCJ, Silberbach M, Söderström-Anttila V, Stochholm K, van Alfen-van derVelden JA, Woelfle J, Backeljauw PF; International Turner Syndrome Consensus Group. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol. 2017;177:G1-G70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 664] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 3. | Jung JY, Yang S, Jeong EH, Lee HC, Lee YM, Han HS, Yi KH. Mixed gonadal dysgenesis in 45,X Turner syndrome with SRY gene. Ann Pediatr Endocrinol Metab. 2015;20:226-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Held KR, Kerber S, Kaminsky E, Singh S, Goetz P, Seemanova E, Goedde HW. Mosaicism in 45,X Turner syndrome: does survival in early pregnancy depend on the presence of two sex chromosomes? Hum Genet. 1992;88:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Gole LA, Lim J, Crolla JA, Loke KY. Gonadal mosaicism 45,X/46,X,psu dic(Y)(q11.2) resulting in a Turner phenotype with mixed gonadal dysgenesis. Singapore Med J. 2008;49:349-351. [PubMed] |
| 6. | Baer TG, Freeman CE, Cujar C, Mansukhani M, Singh B, Chen X, Abellar R, Oberfield SE, Levy B. Prevalence and Physical Distribution of SRY in the Gonads of a Woman with Turner Syndrome: Phenotypic Presentation, Tubal Formation, and Malignancy Risk. Horm Res Paediatr. 2017;88:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Shinawi M, Cain MP, Vanderbrink BA, Grignon DJ, Mensing D, Cooper ML, Bader P, Cheung SW. Mixed gonadal dysgenesis in a child with isodicentric Y chromosome: Does the relative proportion of the 45,X line really matter? Am J Med Genet A. 2010;152A:1832-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | de Marqui AB, da Silva-Grecco RL, Balarin MA. [Prevalence of Y-chromosome sequences and gonadoblastoma in Turner syndrome]. Rev Paul Pediatr. 2016;34:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Lipay MV, Bianco B, Verreschi IT. [Gonadal dysgenesis and tumors: genetic and clinical features]. Arq Bras Endocrinol Metabol. 2005;49:60-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |