Published online Nov 6, 2020. doi: 10.12998/wjcc.v8.i21.5446
Peer-review started: July 17, 2020
First decision: August 8, 2020
Revised: August 9, 2020
Accepted: September 23, 2020
Article in press: September 23, 2020
Published online: November 6, 2020
Processing time: 113 Days and 21.2 Hours
Cerebrotendinous xanthomatosis (CTX) is a treatable autosomal recessive inherited metabolic disorder. It results from a deficiency of sterol 27-hydroxylase (CYP27A1), which is a mitochondrial cytochrome P450 enzyme that catalyzes the hydroxylation of cholesterol and modulates cholesterol homeostasis. Patients with CYP27A1 deficiency show symptoms related to excessive accumulation of cholesterol and cholestanol in lipophilic tissues such as the brain, eyes, tendons, and vessels, resulting in juvenile cataracts, tendon xanthoma, chronic diarrhea, cognitive impairment, ataxia, spastic paraplegia, and peripheral neuropathy. CTX is underdiagnosed as knowledge of the disorder is mainly based on case reports.
A Chinese family with CTX consisting of one patient and four heterozygous carriers was studied. The patient is a 47-year-old male, who mainly had psychiatric signs but without some cardinal features of CTX such as cataracts, cerebellar ataxia, pyramidal signs and chronic diarrhea. There was a significant increase in the concentration of free fatty acid compared to normal range. Doppler ultrasound of the urinary system showed multiple left kidney stones, a right kidney cyst, and a hypoechoic area in the bladder, which could move with body position. Sagittal and axial magnetic resonance imaging (MRI) of the right ankle joint showed apparent enlargement of the right Achilles tendon and upper medial malleolus flexor tendon, abnormal thickening of the plantar fat, and a small amount of exudation around the fascia in front of the Achilles tendon. Cerebral MRI suggested white matter (WM) demyelination and slight cerebral atrophy. The diagnosis was confirmed by targeted sequencing, which identified compound heterozygous mutations in exon 2 and intron 7 of the CYP27A1 gene (c.435G>T, c.1263+1G>A). Treatment for 3 wk with a combination of lipid-lowering and antipsychotic therapy improved his psychiatric symptoms and normalized the levels of serum free fatty acid. Sediments in the bladder disappeared after therapy.
CYP27A1 genetic analysis should be the definitive method for CTX diagnosis. This case suggests that urinary system diseases may be neglected in CTX patients. The clinical, biological, radiological, and genetic characteristics of CTX are summarized to promote early diagnosis and treatment of this disease.
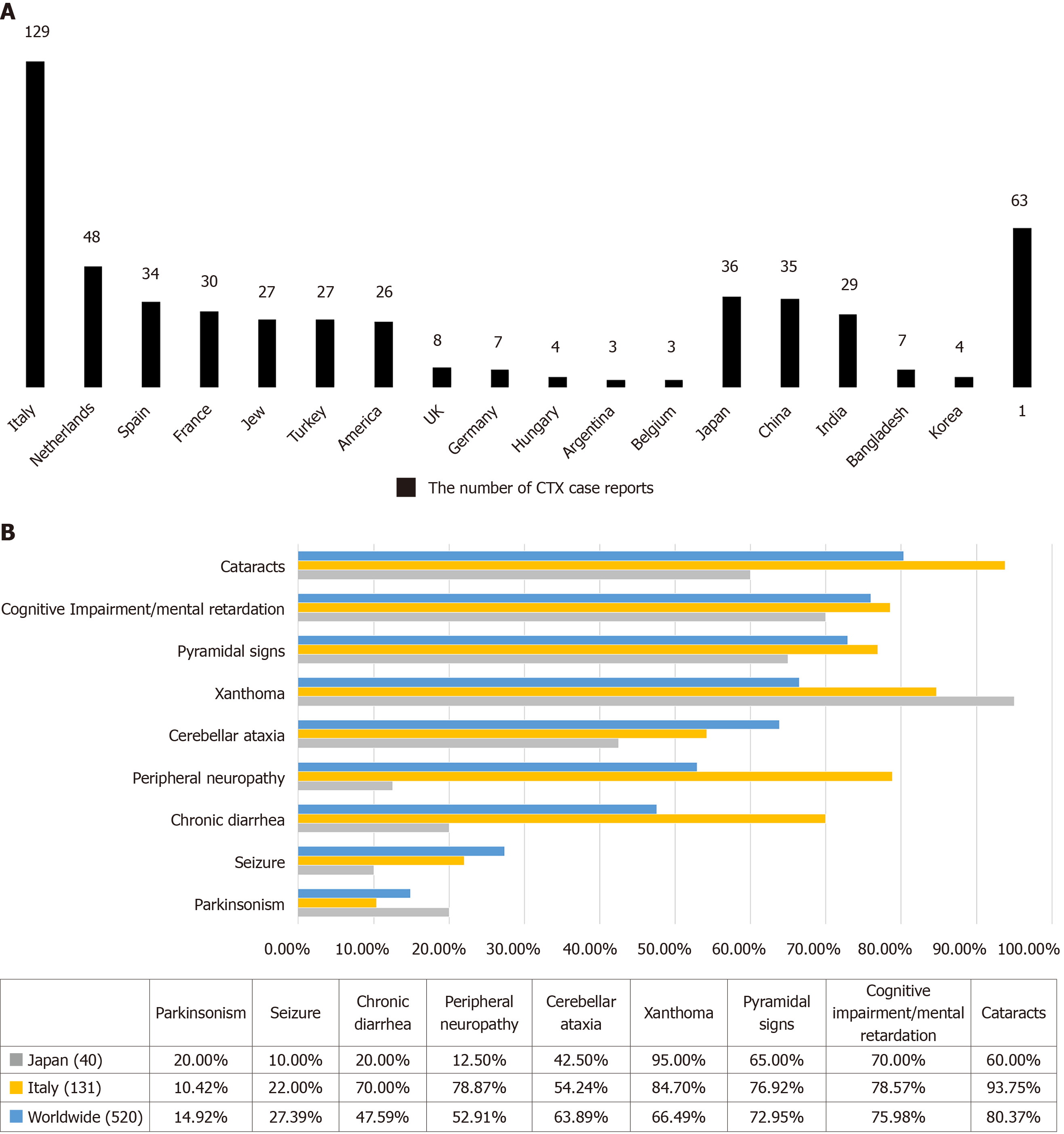
Core Tip: Cerebrotendinous xanthomatosis (CTX) is a metabolic disorder inherited in an autosomal recessive manner. A total of 520 CTX case reports published in the literature worldwide are reviewed to promote a better understanding of the clinical and genetic characteristics of CTX. Cataract is the most common symptom with a frequency of 80.37%, and xanthoma is the second most common systemic symptom of CTX. In all, 56.57% of patients showed electroencephalogram abnormalities including diffuse slowing, and 76.72% had abnormal magnetic resonance images. Bilateral hyperintensity of the dentate nuclei and surrounding white matter on T2 and fluid-attenuated inversion recovery sequences is considered a representative radiological feature of CTX.
- Citation: Cao LX, Yang M, Liu Y, Long WY, Zhao GH. Chinese patient with cerebrotendinous xanthomatosis confirmed by genetic testing: A case report and literature review . World J Clin Cases 2020; 8(21): 5446-5456
- URL: https://www.wjgnet.com/2307-8960/full/v8/i21/5446.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i21.5446
Cerebrotendinous xanthomatosis (CTX) is a metabolic disorder inherited in an autosomal recessive manner. It is caused by mutations of the CYP27A1 gene, which encodes sterol 27-hydroxylase[1]. Sterol 27-hydroxylase is a cytochrome P450 enzyme which converts cholesterol into polar metabolites that can leave cells rapidly, leading to excessive accumulation of cholesterol and cholestanol in lipophilic tissues like the brain, eyes, tendons and blood vessels, and also increasing the risk of premature atherosclerosis[2-4]. The core clinical manifestations are juvenile cataracts, tendon xanthoma, chronic diarrhea, and neurological symptoms such as intellectual disability, cognitive decline, ataxia, spastic paraplegia, peripheral neuropathy, Parkinsonism, and epilepsy. Other symptoms include psychiatric and behavioral disturbances, spinal xanthomatosis, oromandibular dystonia, osteoporosis with multiple fractures, neonatal cholestasis, pediatric hepatology, chronic renal failure and cardiovascular and cerebrovascular atherosclerosis[5-11]. CTX is underdiagnosed as knowledge of the disorder is mainly based on case reports, and although several hundred of these exist the actual number of patients is likely to be much greater. In addition, prevalence of CTX varies in different ethnic groups. In Caucasian populations, the rate has been found to range from 1/70000[12] to 5/100000[13]. A worldwide epidemiological study calculated allele frequency of the 57 CTX-causing mutations in an ExAC cohort of approximately 60000 unrelated adults, placing the incidence of CTX at 1/36072-1/75601 in South Asians, 1/64247–1/64712 in East Asians, 1/71677-1/148914 in Americans, 1/134970-1/461358 in Europeans, and 1/263222-1/468624 in Africans[14].
To date, at least 50 variants of the CYP27A1 gene have been identified[15]. Molecular analysis is a widely used tool for confirming a CTX diagnosis. However, it is difficult to establish the genotype and phenotype relationships of this disorder due to clinical heterogeneity. In one study, even twins with same pathogenic CYP27A1 mutations and similar lifestyles and diets presented with heterogeneous clinical features[16]. CTX is a treatable disease, but the effect of therapy is heavily dependent on the age of the patient receiving medication. There may be a delay of 20-25 years between the first symptoms in childhood (for instance, diarrhea, cataracts, intellectual disability and epilepsy) and later symptoms including apparent neurological disturbances such as walking difficulties and cognitive impairment[17]. The age at onset of symptoms is inevitably affected by recall bias and may not be accurate. Shortening the diagnostic delay should be given greater importance in clinical practice, not only for neurologists but also for pediatricians, psychiatrists and ophthalmologists.
Herein, we report the clinical, imaging and molecular characteristics of a Chinese patient with CTX confirmed by genetic testing. Meanwhile, 520 CTX case reports published in the past 30 years are reviewed to promote better understanding of the clinical and genetic characteristics of CTX.
A 47-year-old male was admitted to our institution with a history of intellectual disability for more than 30 years and behavioral abnormalities for the past 3 mo. He had a poor academic performance, having dropped out after completing elementary school. The patient could talk with others in daily life, but he was unable to do housework.
Three months ago, he underwent an operation for renal calculus, after which he showed progressive delusion (he believed unreasonably that he had a serious disease and was going to die), combined with slow response and speech reduction. At 1 wk before admission, he was unable to answer questions and developed an eating difficulty, incontinence, fever (T ≤ 38.2 °C) and severe weight loss. Chronic diarrhea was absent.
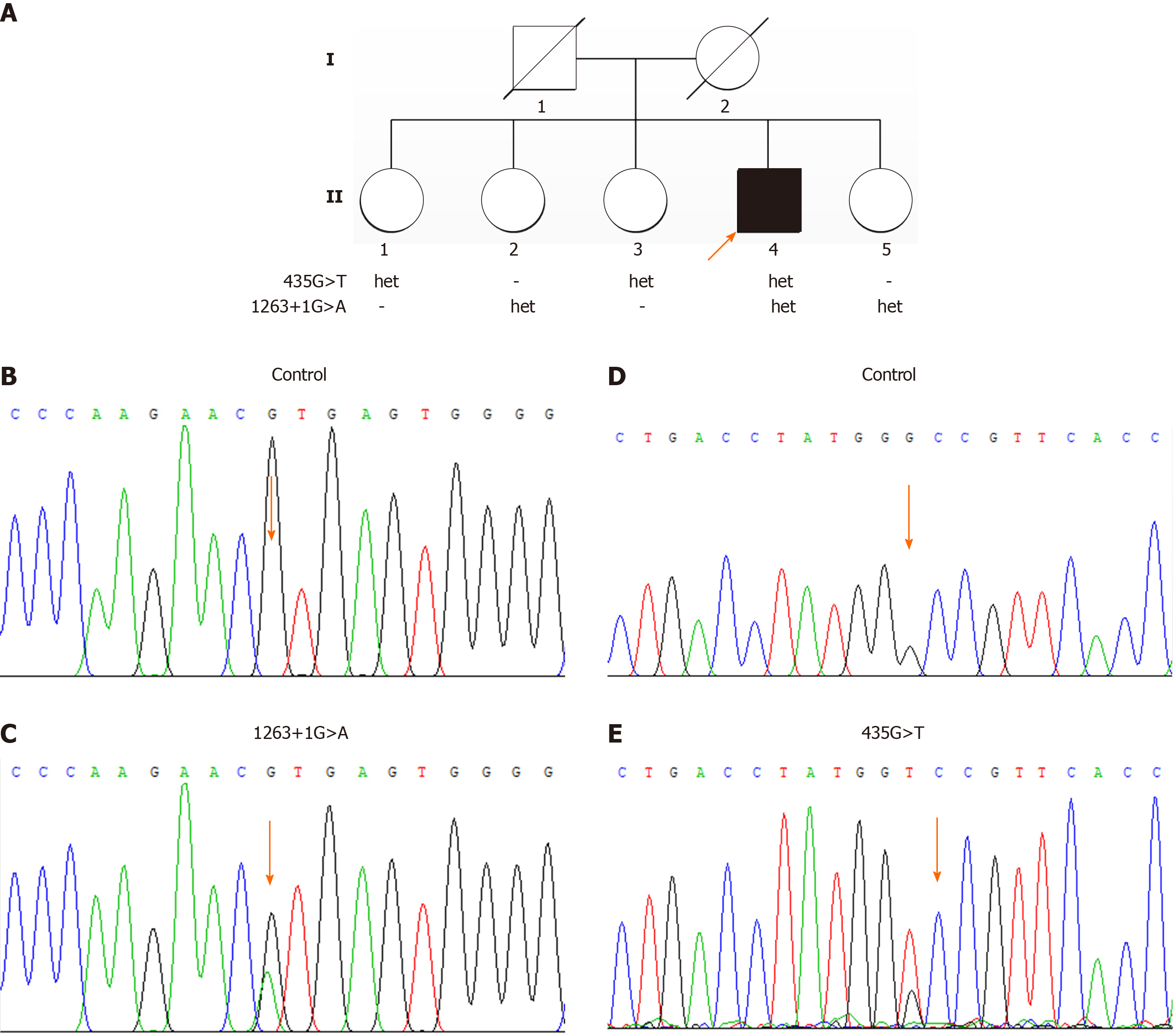
The proband is the 4th child of 5 in a non-consanguineous Chinese family. His family history was negative for symptoms related to neurological disorders. He was divorced twice and had no children, and did not have a history of smoking or drinking. The proband’s parents had died and his mother had a history of uremia. All of his four sisters are healthy (Figure 1A).
The patient had dark skin and a poor mental state (Figure 2A). Neurological examination indicated cervical rigidity and Kernig sign. Deep tendon reflexes and limb muscle strength were normal. Babinski sign was negative. Enlarged Achilles tendons and nodules on the bilateral tibial tubercles were observed (Figure 2B and C). There was no evidence of cataract or xanthomas at other sites such as the eyelids.
The laboratory results (Table 1) were as follows: Increased leukocyte count, neutrophil ratio and high erythrocyte sedimentation rate. There was also a significant increase in concentration of free fatty acid compared to normal range. In contrast, levels of high-density lipoprotein cholesterol were low. Serum cholesterol, glucose, electrolytes, and adrenocorticotropic hormone (ACTH) levels were all normal, as were the liver function tests. Doppler ultrasound of the urinary system showed multiple left kidney stones, a right kidney cyst, and a hypoechoic area in the bladder which could move with body position (Figure 2D). Cerebrospinal fluid protein was mildly elevated: 680.4 mg/L (normal range: 150-400 mg/L).
| Value | Normal range | Value | Normal range | Value | Normal range | |||
| Leukocyte count (109/L) | 10.7 ↑ | 4.0-10.0 | Follicle stimulating hormone (IU/L) | 19.75 ↑ | 1.27-19.26 | Intracranial pressure (mmH2O) | 170 | 70-180 |
| Neutrophil ratio (%) | 81.6 ↑ | 50.0-70.0 | Testosterone (nmol/L) | 4.53 ↓ | 6.07-27.10 | CSF Pandy test | + | - |
| Erythrocyte count (1012/L) | 6.06 ↑ | 4.09-5.74 | ACTH 12am (ng/L) | 9.49 | < 46 | CSF erythrocyte count (106/L) | 0 | 0-1 |
| Hb (g/L) | 126 ↓ | 131-172 | ACTH 8am (ng/L) | 43.64 | 7.2-63.0 | CSF karyocyte count | 2 | 6-10 |
| ESR (mm/h) | 26 ↑ | 0-15 | ACTH 4pm (ng/L) | 13.18 | < 46 | CSF protein (mg/L) | 680.4 ↑ | 150-450 |
| Free fatty acid (μmol/L) | 1127.7 ↑ | 100-760 | Serum cortisol 12am (μg/L) | 165.92 | None | CSF Cl- (mmol/L) | 133.6 ↑ | 112-120 |
| HDL-cholesterol (mmol/L) | 0.73 ↓ | 0.90-2.19 | Serum cortisol 8am (μg/L) | 267.83 | 52.7-224.5 | CSF glucose (mmol/L) | 4.32 | 1.5-4.5 |
| Prolactin (mIU/L) | 335 ↑ | 55.96-278.35 | Serum cortisol 4pm (μg/L) | 85.45 | 34.4-167.6 | CSF adenosine deaminase (U/L) | 1 | 0-5 |
| High sensitivity thyrotropin (mIU/L) | 0.24 ↓ | 0.38-5.58 |
X-ray of the lower limbs showed soft tissue swelling above the tibial tuberosities bilaterally (Figure 2E). Sagittal and axial magnetic resonance imaging (MRI) of the right ankle joint showed apparent enlargement of the right Achilles tendon and upper medial malleolus flexor tendon (consisting of low signal intensity on both T1- and T2-weighted images), abnormal thickening of the plantar fat, and a small amount of exudation around the fascia in front of the Achilles tendon (Figure 2F). The electroencephalogram (EEG) showed increased slow activities than normal and electrophysiological examination found mixed sensorimotor neuropathy of the upper and lower limbs. Cerebral MRI suggested white matter (WM) demyelination and slight cerebral atrophy (Figure 2G and H).
Genomic DNA was isolated from blood samples of the patient and his four healthy sisters. Mutation screening of all exons and flanking regions was performed on the patient’s sample by targeted sequencing as previously reported[18]. Targeted sequencing revealed that the proband had compound heterozygous mutations in the CYP27A1 gene and that his four sisters were mutation carriers (Figure 1B-E, and Table 2). Both of the variants found are known pathogenic mutations of CTX. The variant in exon 2 (c.435G>T, p.Gly145Gly), a functionally silent nucleotide substitution, has been reported to activate a 5’ splice site, leading to alternative pre-mRNA splicing[19]. The other variant (c.1263+1G>A) is a splice site mutation that disrupts normal mRNA splicing and results in exon 7 being skipped in the transcript[20]. In Italian and Japanese patients, the variant has been found to cause loss of a heme binding domain, a vital part of hydroxylase, and to damage bile acid synthesis[20,21].
On the basis of the clinical features and the results of supplemental studies, a diagnosis of CTX was the first conclusion. Addison’s disease, listed as a differential diagnosis due to his skin pigmentation and intellectual disability, was excluded due to the normal level of serum cortisol and ACTH. The diagnosis of CTX was confirmed by genetic analysis, which showed compound pathogenic heterozygous mutations in the CYP27A1 gene.
The patient was treated with a combination of 10 mg rosuvastatin once per night, 10 mg of ezetimibe once per night, and paliperidone (1 mg in the morning and 2 mg at night). He was provided with 0.25 g chenodeoxycholic acid (CDCA) three times per day in our hospital. He was also provided enteral nutritional support through a nasogastric feeding tube.
After a 3 wk treatment program, the patient’s skin became brighter, he showed an improvement of mental symptoms, and his psychiatric symptoms substantially improved. He could answer simple questions correctly and walk with help. The serum free fatty acid level decreased to less than half of the pretreatment level (from 1127.7 μmol/L to 523.1 μmol/L). The urinary system B-ultrasounds performed after treatment suggested sediments in the bladder had disappeared. After he was discharged, he persisted the medication of CDCA.
CTX, MIM# 213700, is a rare hereditary lipid storage disease whose precise epidemiological features are unknown because findings largely depend on case reports[11]. In the past 30 years, more than 500 CTX cases have been reported worldwide, with the highest frequencies reported in Italy, the Netherlands, Spain, Japan, and China (Figure 3). We have reviewed previous literature reporting CTX cases and summarize the clinical and genetic features of patients in Supplementary Table 1.
CTX patients from all over the world share certain clinical features, which are shown in Figure 3. Also shown are features in Italian and Japanese populations as representatives for Caucasians and Asians, to highlight characteristics from different origins.
In 520 global CTX cases (Figure 3, Supplementary Table 1), cataract is the most common symptom with a frequency of 80.37%, and it is usually diagnosed in the second decade of life[17]. However, its diagnostic role is less evident in Japan as only 60.00% of patients showed this sign[11]. Xanthoma is the second most common systemic symptom of CTX, and it is notable that it has a much higher frequency in Japanese patients (95.0%)[11]. Cognitive impairment is the most common neurological presentation, followed by pyramidal signs and cerebellar ataxia (72.95% and 63.89%, respectively). Peripheral neuropathy and chronic diarrhea are also very common, particularly in Italian patients, where the prevalence rates were 78.87% and 70.00%, respectively. Epilepsy is a relatively unusual presentation of CTX, but it sometimes appears as the initial symptom; thus CTX should be considered as a possible cause of seizure[11]. Parkinsonism is less frequent and is usually seen at an older age[11].
Awareness of psychiatric signs in CTX is growing in neurologists and psychiatrists, but these signs are rarely isolated in CTX[22], as seen in the present case. They are characterized by learning difficulties/cognitive impairment in childhood or adolescence; and behavioral disturbance, affective/mood disorders, personality changes and psychotic disorders in the advanced stage of the disease[23].
The multiple systematic features in patients indicate that almost every organ is involved in this disease. Other than the symptoms mentioned above, aberrant calcium deposits in the urinary system, nephrocalcinosis, nephrolithiasis and renal failure have been reported in CTX, although with a relatively low incidence[11,24]. The patient in this paper had a history of renal calculus and sediments in the bladder, which disappeared after treatment and might be associated with increased urinary bile alcohols. We cannot rule out the possibility that these urinary system diseases are neglected in CTX cases. More valuable clinical features are likely to be identified if complete examinations were performed in every CTX patient.
Phenotypes of CTX can vary considerably in intrafamilial cases, even in identical twins having similar lifestyles[16,25]. Different phenotypes may be associated with certain genetic modifiers or epigenetic changes that are undiscovered today[16]. Whether environmental factors play a role in the pathogenesis of CTX is still unclear. Mignarri et al[17] developed a simple and effective diagnostic tool named the suspicion index. Using this tool, our patient received a total score of 225, which is a very strong indicator for CTX. However, these authors also recommended that CYP27A1 genetic analysis should be the definitive method for diagnosis[17].
The CYP27A1 gene is located in chromosome locus 2q35 and consists of 9 exons and 8 introns that encode 531 amino acids. Known pathogenic mutations causing CTX are distributed in all exons and in introns 2, 4, 6, 7 and 8[26,27], but nearly half of all mutations are found in exons 6-8[26]. A genetic review in 2006 of 49 different mutations suggested that 45% are missense mutations, 20% are nonsense mutations, and 18% are splice site mutations. The remaining mutations were seven deletions (14%) and one insertion (2%)[1]. Missense mutations could impair the stability and catalytic efficacy of the enzyme; splice site mutations cause abnormal splicing and exon skipping; and deletion, insertion and nonsense mutations lead to a truncation of the mRNA and an incomplete enzyme structure[26]. It was reported that some mutations occurred more frequently in some ethnic groups, such as c.1214G>A (p.R405Q) in Japanese patients, c.1183C>T (p.R395C) in patients of Spanish origin and c.646G>C (p.A216P) in Italian patients[1,11,15].
No published case report has yet identified mutations in genes other than CYP27A1 in CTX patients[28]. It is not yet certain if there are any correlations between phenotypes and genotypes in this rare disease[1,29]. In 2018, Sekijima et al[11] discovered that three mutations were associated with certain phenotypes in Japanese patients: c.1421G>A (p.R474Q) was associated with the classical form; c.1214G>A (p.R405Q) with the spinal form; and c.435G>T (p.G145G) with the non-neurological CTX form. In Chinese population, the most frequent CTX-causing variants were c.410G>A (p.R137Q) and c.379C>T (p.R127W)[30,31]. CYP27A1 is also considered a causative gene of coronary artery disease, possibly related to its effect on reverse cholesterol transport[32].
The biochemical abnormalities detected in CTX are significant for diagnosis. The lack of sterol 27-hydroxylase inhibits the conversion of cholesterol into cholic and chenodeoxycholic acids in the liver[12]. The serum levels of cholesterol and triglyceride are usually normal while cholestanol levels are markedly high in the blood, bile and tendon xanthoma[12]. Since cholesterol synthesis is systematically decreased, cholesterol precursors increase substantially; this could also be regarded as a hallmark of CTX[33]. Although a high cholestanol level indicates CTX, it does not correlate with any symptoms or disability at diagnosis assessed by the Expanded Disability Status Scale[34].
Fast atom bombardment mass spectrometry is a semiquantitative technique which can be applied to analyze urinary bile acids. It will strongly support a diagnosis of CTX if increased bile alcohol glucuronides and reduced primary bile acid are detected[35]. However, when the urinary bile acid profile is consistent with CTX, propofol administration should be taken into account as another possible cause, to avoid misdiagnoses[36]. The combination of extremely low levels of 27-hydroxycholesterol (< 5 ng/mL or lower than the detection limit) and extremely high serum levels of 7 alpha-hydroxycholesterol (7α-OH-C) or 7 alpha-hydroxy-4-cholesten-3-one (7αC4) is a distinctive feature of CTX[36]. Both 7α-OH-C and 7αC4 have been suggested as ideal biomarkers for evaluating treatment effects[37].
With respect to auxiliary examinations, 56.57% of patients showed EEG abnormalities including diffuse slowing, which is a common feature of CTX patients. In addition, 76.72% had abnormal magnetic resonance (MR) images (Supplementary Table 1). The main brain MRI abnormalities include supratentorial and infratentorial atrophy, as well as T2W/FLAIR (fluid-attenuated inversion recovery) hyperintensity involving the subcortical, periventricular and cerebellar WM and the brainstem[38]. Bilateral hyperintensity of the dentate nuclei (DN) and surrounding WM on T2 and FLAIR sequences is considered a representative radiological feature of CTX[39,40]. DN plays a critical role in saccade motor planning within a network that involves the frontal cortex and basal ganglia[41]. Patients with DN lesions showed abnormal latency of reflexive and voluntary saccades, impaired precision and more uncorrected directional errors[41]. There is a strong correlation between DN hyperintensity in brain MRI and neurological manifestations including ataxia, spasticity and cognitive impairment[38]. Patients without DN hyperintensity are considered to have a better prognosis[38].
A 6-year MR follow-up study revealed that cerebellar hyperintensity in T1-weighted and FLAIR images tend to be replaced by hypointense areas, suggesting vacuolation[42]. Cerebellar hyperintensity on FLAIR images is probably due to lipid deposits, while hypointense areas may indicate cerebellar degeneration because of cholestanol-induced apoptosis or other mechanisms causing axonal injury[42,43]. Cerebellar vacuolation and calcification have been observed in a large number of patients. The former was positively related to the extent of DN hyperintensity in T1W and FLAIR images, and could be a useful indicator of disease progression and inadequate therapeutic response[38]. Additionally, cerebral micro- and macro-angiopathies such as micro-hemorrhages, vascular dilatation and calcification are common in CTX patients[44]. The xanthomas located within the ventricular region have also been observed in CTX cases[45].
The main components of xanthoma are connective tissue and foam cells (foamy macrophages) bathed in cholestanol, cholesterol, cholesterol esters, triglycerides and phospholipids[46]. The presence of Touton giant cells is a characteristic pathological finding[47]. The development of tendon xanthomas may be associated with low-grade tendon inflammation[48]. A tendon MRI study indicated that the enlargement of tendon xanthomas is primarily due to an increase in tendon water content, rather than in fat content[49]. Tendinous xanthomas have different appearances on MR depending on the proportions of the following components: cholesterol, which does not produce a measurable signal; triglycerides, which produce a high signal on T1-weighted images; and associated inflammation, which causes high signal intensity regions in contrast-enhanced T1-weighted images[46,50,51]. The diffuse reticulated pattern of xanthomas on axial images is a representative MRI feature of tendinous xanthoma[52]. This patient’s MRI showed enlargement of the Achilles tendon, which showed up as low signal intensity on both T1- and T2-weighted images. This fits well with previous reports and could be attributed to the low percentage of triglycerides[46].
In conclusion, we described a 47-year-old patient displaying psychiatric and behavioral disturbances, cognitive decline, a history of renal calculus, bilateral xanthomas of the Achilles tendon and tibial tubercles, peripheral neuropathy, high signal intensity in the WM and mild cerebral atrophy on brain MRI. The diagnosis was confirmed by compound heterozygous mutations in exon 2 (c.435G>T, p.Gly145Gly) and intron 7 (c.1263+1G>A) of the CYP27A1 gene. These lead to alternative pre-mRNA splicing and a skipping of exon 7 in the transcript, respectively. Three weeks′ treatment with a combination of lipid-lowering and antipsychotic therapy improved his psychiatric symptoms and normalized levels of serum free fatty acid. Sediments in the bladder disappeared after therapy.
CTX is an underdiagnosed inherited metabolic disease, and has a good biological and clinical response to the treatment of CDCA supplementation initiated at an early age. The screening methods for CTX in newborns need to be improved and performed in larger populations. We believe that our work will familiarize readers with the clinical, biological, radiological, and genetic features of CTX. In the future, as physicians become more familiar with this disease, and as testing and treatment methods evolve, an increasing number of CTX patients will benefit from earlier diagnosis and better clinical outcomes.
We appreciate the family members for participating in our study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wu ZY S-Editor: Huang P L-Editor: Filipodia P-Editor: Li X
| 1. | Gallus GN, Dotti MT, Federico A. Clinical and molecular diagnosis of cerebrotendinous xanthomatosis with a review of the mutations in the CYP27A1 gene. Neurol Sci. 2006;27:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | von Bahr S, Movin T, Papadogiannakis N, Pikuleva I, Rönnow P, Diczfalusy U, Björkhem I. Mechanism of accumulation of cholesterol and cholestanol in tendons and the role of sterol 27-hydroxylase (CYP27A1). Arterioscler Thromb Vasc Biol. 2002;22:1129-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Federico A, Dotti MT. Cerebrotendinous xanthomatosis: clinical manifestations, diagnostic criteria, pathogenesis, and therapy. J Child Neurol. 2003;18:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Kalidas K, Behrouz R. Inherited metabolic disorders and cerebral infarction. Expert Rev Neurother. 2008;8:1731-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Bartholdi D, Zumsteg D, Verrips A, Wevers RA, Sistermans E, Hess K, Jung HH. Spinal phenotype of cerebrotendinous xanthomatosis--a pitfall in the diagnosis of multiple sclerosis. J Neurol. 2004;251:105-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Valdivielso P, Calandra S, Durán JC, Garuti R, Herrera E, González P. Coronary heart disease in a patient with cerebrotendinous xanthomatosis. J Intern Med. 2004;255:680-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | von Bahr S, Björkhem I, Van't Hooft F, Alvelius G, Nemeth A, Sjövall J, Fischler B. Mutation in the sterol 27-hydroxylase gene associated with fatal cholestasis in infancy. J Pediatr Gastroenterol Nutr. 2005;40:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Kaneko H, Hirose M, Katada S, Takahashi T, Naruse S, Tsuchiya M, Yoshida T, Nakagawa M, Onodera O, Nishizawa M, Ikeuchi T. Novel GFAP mutation in patient with adult-onset Alexander disease presenting with spastic ataxia. Mov Disord. 2009;24:1393-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Gallus GN, Dotti MT, Mignarri A, Rufa A, Da Pozzo P, Cardaioli E, Federico A. Four novel CYP27A1 mutations in seven Italian patients with CTX. Eur J Neurol. 2010;17:1259-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Degos B, Nadjar Y, Amador Mdel M, Lamari F, Sedel F, Roze E, Couvert P, Mochel F. Natural history of cerebrotendinous xanthomatosis: a paediatric disease diagnosed in adulthood. Orphanet J Rare Dis. 2016;11:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Sekijima Y, Koyama S, Yoshinaga T, Koinuma M, Inaba Y. Nationwide survey on cerebrotendinous xanthomatosis in Japan. J Hum Genet. 2018;63:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Rafiq M, Sharrack N, Shaw PJ, Hadjivassiliou M. A neurological rarity not to be missed: cerebrotendinous xanthomatosis. Pract Neurol. 2011;11:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Lorincz MT, Rainier S, Thomas D, Fink JK. Cerebrotendinous xanthomatosis: possible higher prevalence than previously recognized. Arch Neurol. 2005;62:1459-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Appadurai V, DeBarber A, Chiang PW, Patel SB, Steiner RD, Tyler C, Bonnen PE. Apparent underdiagnosis of Cerebrotendinous Xanthomatosis revealed by analysis of ~60,000 human exomes. Mol Genet Metab. 2015;116:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Smalley SV, Preiss Y, Suazo J, Vega JA, Angellotti I, Lagos CF, Rivera E, Kleinsteuber K, Campion J, Martínez JA, Maiz A, Santos JL. Novel splice-affecting variants in CYP27A1 gene in two Chilean patients with Cerebrotendinous Xanthomatosis. Genet Mol Biol. 2015;38:30-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Zádori D, Szpisjak L, Madar L, Varga VE, Csányi B, Bencsik K, Balogh I, Harangi M, Kereszty É, Vécsei L, Klivényi P. Different phenotypes in identical twins with cerebrotendinous xanthomatosis: case series. Neurol Sci. 2017;38:481-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Mignarri A, Gallus GN, Dotti MT, Federico A. A suspicion index for early diagnosis and treatment of cerebrotendinous xanthomatosis. J Inherit Metab Dis. 2014;37:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 18. | Xu H, Zhang C, Cao L, Song J, Xu X, Zhang B, Chen B, Zhao G. ATL3 gene mutation in a Chinese family with hereditary sensory neuropathy type 1F. J Peripher Nerv Syst. 2019;24:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Chen W, Kubota S, Teramoto T, Nishimura Y, Yonemoto K, Seyama Y. Silent nucleotide substitution in the sterol 27-hydroxylase gene (CYP 27) leads to alternative pre-mRNA splicing by activating a cryptic 5' splice site at the mutant codon in cerebrotendinous xanthomatosis patients. Biochemistry. 1998;37:4420-4428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Shiga K, Fukuyama R, Kimura S, Nakajima K, Fushiki S. Mutation of the sterol 27-hydroxylase gene (CYP27) results in truncation of mRNA expressed in leucocytes in a Japanese family with cerebrotendinous xanthomatosis. J Neurol Neurosurg Psychiatry. 1999;67:675-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Garuti R, Lelli N, Barozzini M, Tiozzo R, Dotti MT, Federico A, Ottomano AM, Croce A, Bertolini S, Calandra S. Cerebrotendinous xanthomatosis caused by two new mutations of the sterol-27-hydroxylase gene that disrupt mRNA splicing. J Lipid Res. 1996;37:1459-1467. [PubMed] |
| 22. | Sedel F, Baumann N, Turpin JC, Lyon-Caen O, Saudubray JM, Cohen D. Psychiatric manifestations revealing inborn errors of metabolism in adolescents and adults. J Inherit Metab Dis. 2007;30:631-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Fraidakis MJ. Psychiatric manifestations in cerebrotendinous xanthomatosis. Transl Psychiatry. 2013;3:e302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Chang WN, Cheng YF. Nephrolithiasis and nephrocalcinosis in cerebrotendinous xanthomatosis: report of three siblings. Eur Neurol. 1995;35:55-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Verrips A, van Engelen BG, Wevers RA, van Geel BM, Cruysberg JR, van den Heuvel LP, Keyser A, Gabreëls FJ. Presence of diarrhea and absence of tendon xanthomas in patients with cerebrotendinous xanthomatosis. Arch Neurol. 2000;57:520-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Lorbek G, Lewinska M, Rozman D. Cytochrome P450s in the synthesis of cholesterol and bile acids--from mouse models to human diseases. FEBS J. 2012;279:1516-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 27. | Nie S, Chen G, Cao X, Zhang Y. Cerebrotendinous xanthomatosis: a comprehensive review of pathogenesis, clinical manifestations, diagnosis, and management. Orphanet J Rare Dis. 2014;9:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 204] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 28. | Jiao H, Olin M, Hansson M, Eggertsen G, Eriksson M, Angelin B, Björkhem I. Unique case of cerebrotendinous xanthomatosis revisited: All the mutations responsible for this disease are present in the CYP27A1 gene. J Intern Med. 2018;283:604-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Pilo-de-la-Fuente B, Jimenez-Escrig A, Lorenzo JR, Pardo J, Arias M, Ares-Luque A, Duarte J, Muñiz-Pérez S, Sobrido MJ. Cerebrotendinous xanthomatosis in Spain: clinical, prognostic, and genetic survey. Eur J Neurol. 2011;18:1203-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Chen C, Zhang Y, Wu H, Sun YM, Cai YH, Wu JJ, Wang J, Gong LY, Ding ZT. Clinical and molecular genetic features of cerebrotendinous xanthomatosis patients in Chinese families. Metab Brain Dis. 2017;32:1609-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Tao QQ, Zhang Y, Lin HX, Dong HL, Ni W, Wu ZY. Clinical and genetic characteristics of Chinese patients with cerebrotendinous xanthomatosis. Orphanet J Rare Dis. 2019;14:282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Inanloorahatloo K, Zand Parsa AF, Huse K, Rasooli P, Davaran S, Platzer M, Fan JB, Amini S, Steemers F, Elahi E. Mutation in CYP27A1 identified in family with coronary artery disease. Eur J Med Genet. 2013;56:655-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | de Sain-van der Velden MG, Verrips A, Prinsen BH, de Barse M, Berger R, Visser G. Elevated cholesterol precursors other than cholestanol can also be a hallmark for CTX. J Inherit Metab Dis. 2008;31 Suppl 2:S387-S393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Wallon D, Guyant-Maréchal L, Laquerrière A, Wevers RA, Martinaud O, Kluijtmans LA, Yntema HG, Saugier-Veber P, Hannequin D. Clinical imaging and neuropathological correlations in an unusual case of cerebrotendinous xanthomatosis. Clin Neuropathol. 2010;29:361-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Pierre G, Setchell K, Blyth J, Preece MA, Chakrapani A, McKiernan P. Prospective treatment of cerebrotendinous xanthomatosis with cholic acid therapy. J Inherit Metab Dis. 2008;31 Suppl 2:S241-S245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Claesen JLA, Koomen E, Schene IF, Jans JJM, Mast N, Pikuleva IA, van der Ham M, de Sain-van der Velden MGM, Fuchs SA. Misdiagnosis of CTX due to propofol: The interference of total intravenous propofol anaesthesia with bile acid profiling. J Inherit Metab Dis. 2020;43:843-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Lütjohann D, Stellaard F, Björkhem I. Levels of 7alpha-hydroxycholesterol and/or 7alpha-hydroxy-4-cholest-3-one are the optimal biochemical markers for the evaluation of treatment of cerebrotendinous xanthomatosis. J Neurol. 2020;267:572-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Mignarri A, Dotti MT, Federico A, De Stefano N, Battaglini M, Grazzini I, Galluzzi P, Monti L. The spectrum of magnetic resonance findings in cerebrotendinous xanthomatosis: redefinition and evidence of new markers of disease progression. J Neurol. 2017;264:862-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 39. | Barkhof F, Verrips A, Wesseling P, van Der Knaap MS, van Engelen BG, Gabreëls FJ, Keyser A, Wevers RA, Valk J. Cerebrotendinous xanthomatosis: the spectrum of imaging findings and the correlation with neuropathologic findings. Radiology. 2000;217:869-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | De Stefano N, Dotti MT, Mortilla M, Federico A. Magnetic resonance imaging and spectroscopic changes in brains of patients with cerebrotendinous xanthomatosis. Brain. 2001;124:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Rosini F, Pretegiani E, Mignarri A, Optican LM, Serchi V, De Stefano N, Battaglini M, Monti L, Dotti MT, Federico A, Rufa A. The role of dentate nuclei in human oculomotor control: insights from cerebrotendinous xanthomatosis. J Physiol. 2017;595:3607-3620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Mignarri A, Dotti MT, Del Puppo M, Gallus GN, Giorgio A, Cerase A, Monti L. Cerebrotendinous xanthomatosis with progressive cerebellar vacuolation : six-year MRI follow-up. Neuroradiology. 2012;54:649-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Inoue K, Kubota S, Seyama Y. Cholestanol induces apoptosis of cerebellar neuronal cells. Biochem Biophys Res Commun. 1999;256:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Androdias G, Vukusic S, Gignoux L, Boespflug-Tanguy O, Acquaviva C, Zabot MT, Couvert P, Carrie A, Confavreux C, Labauge P. Leukodystrophy with a cerebellar cystic aspect and intracranial atherosclerosis: an atypical presentation of cerebrotendinous xanthomatosis. J Neurol. 2012;259:364-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 45. | Brienza M, Fiermonte G, Cambieri C, Mignarri A, Dotti MT, Fiorelli M. Enlarging brain xanthomas in a patient with cerebrotendinous xanthomatosis. J Inherit Metab Dis. 2015;38:981-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Smithard A, Lamyman MJ, McCarthy CL, Gibbons CL, Cooke PJ, Athanasou N. Cerebrotendinous xanthomatosis presenting with bilateral Achilles tendon xanthomata. Skeletal Radiol. 2007;36:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Pudhiavan A, Agrawal A, Chaudhari S, Shukla A. Cerebrotendinous xanthomatosis--the spectrum of imaging findings. J Radiol Case Rep. 2013;7:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Abate M, Schiavone C, Salini V, Andia I. Occurrence of tendon pathologies in metabolic disorders. Rheumatology (Oxford). 2013;52:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 167] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 49. | Griffith JF, Hu M, Yeung DKW, Guo P, Lam SL, Xiao F, Wang D, Tomlinson B. Achilles Tendon Xanthomas: Fat-Water Separation at Baseline and after Treatment. Radiology. 2017;285:876-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Otake S, Imagumbai N, Tajima A, Ohba S. Unusual high signal intensity on MR images in a patient with multiple tendinous xanthomas. Eur Radiol. 1997;7:1025-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Liem MS, Leuven JA, Bloem JL, Schipper J. Magnetic resonance imaging of Achilles tendon xanthomas in familial hypercholesterolemia. Skeletal Radiol. 1992;21:453-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Bude RO, Adler RS, Bassett DR. Diagnosis of Achilles tendon xanthoma in patients with heterozygous familial hypercholesterolemia: MR vs sonography. AJR Am J Roentgenol. 1994;162:913-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |