Published online Nov 6, 2020. doi: 10.12998/wjcc.v8.i21.5235
Peer-review started: May 28, 2020
First decision: July 29, 2020
Revised: September 11, 2020
Accepted: September 23, 2020
Article in press: September 23, 2020
Published online: November 6, 2020
Processing time: 162 Days and 1.4 Hours
Essential phospholipids (EPL) are used for the supportive treatment of non-alcoholic fatty liver disease (NAFLD), but data are mostly from small-scale studies.
To evaluate the efficacy of EPL treatment in adult patients with NAFLD and type 2 diabetes and/or obesity.
The MEDLINE, PubMed, Embase, and Cochrane databases were searched up to March 2019 for clinical trials and comparative observational studies. Eligible studies were those published in English or Chinese that enrolled adult patients (≥ 18 years) with NAFLD and type 2 diabetes mellitus and/or obesity receiving EPL as monotherapy or as add-on therapy to existing therapy, and that included at least one of the efficacy outcomes of interest. A variety of studies were identified; thus, direct, indirect and cohort meta-analyses were performed. Mean difference (MD) and 95% confidence interval (CI) were calculated for continuous variables, and relative risk with 95%CI for disease response and recovery. A random-effects model was used to address between-study heterogeneity.
Ten studies met the inclusion criteria (n = 22-324). EPL treatment duration ranged from 4 to 72 wk. In the direct meta-analysis (four randomized controlled trials), compared with antidiabetic therapy alone, EPL plus antidiabetic therapy was associated with a significantly greater reduction in [alanine aminotransferase (ALT); MD: 11.28 U/L (95%CI: -17.33, -5.23), P = 0.0003], triglyceride [MD: -49.33 mg/dL (95%CI: -66.43, -32.23), P < 0.0001] and total cholesterol levels [MD: -29.74 mg/dL (95%CI: -38.02, -21.45), P < 0.0001]. There was also a significant increase in the rate of overall improvement [relative risk 1.50 (95%CI: 1.26-1.79), P < 0.0001], and risk of no disease (P = 0.0091), and a reduction in moderate disease (P = 0.0187); there were no significant differences in severe disease, mild disease, or significant improvement. In the cohort meta-analysis of three non-randomized clinical trials, the MD in ALT levels was -16.71 U/L (95%CI: -24.94, -8.49) and 23% of patients had improved disease. In the cohort meta-analysis of five randomized trials, MD in ALT levels was –28.53 U/L (95%CI: -35.42, -21.65), and 87% (95%CI: 81%, 93%) and 58% (95%CI: 46%, 70%) of patients showed clinical improvement and significant clinical improvement.
This analysis provides evidence for a benefit of EPL in patients with NAFLD and diabetes and/or obesity. Further large-scale trials are warranted.
Core Tip: Essential phospholipids (EPL) are used for the supportive treatment of non-alcoholic fatty liver disease, but the data are mostly from small-scale studies. Thus, we used meta-analytical techniques to assess the efficacy of EPL in patients with non-alcoholic fatty liver disease and type 2 diabetes and/or obesity. Our results indicate EPL provides benefit in this patient population, reducing alanine aminotransferase, triglyceride and cholesterol levels, and improving disease severity (as measured by ultrasonography). Larger-scale trials are warranted to confirm these findings.
- Citation: Dajani AI, Popovic B. Essential phospholipids for nonalcoholic fatty liver disease associated with metabolic syndrome: A systematic review and network meta-analysis. World J Clin Cases 2020; 8(21): 5235-5249
- URL: https://www.wjgnet.com/2307-8960/full/v8/i21/5235.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i21.5235
Non-alcoholic fatty liver disease (NAFLD) is one of the most common liver diseases worldwide, with an estimated global prevalence of 25%[1]. However, the prevalence is markedly higher in individuals with type 2 diabetes, obesity, or hyperlipidemia[2]. While NAFLD is generally asymptomatic, it may progress from steatosis to inflammation, fibrosis, and cirrhosis, and potentially lead to end-stage liver complications, such as hepatocellular carcinoma[3]. NAFLD is now considered to be the hepatic manifestation of metabolic syndrome – the cluster of risk factors and conditions that encompasses central adiposity/obesity, insulin resistance/diabetes, hypertension, and dyslipidemia[4]. Patients with metabolic syndrome have a 4-fold higher risk of developing NAFLD than patients without metabolic syndrome[5]. In addition, metabolically healthy people with NAFLD are more likely to develop traits of metabolic syndrome than people without NAFLD[6], and growing evidence suggests that NAFLD may also be considered as an independent risk factor for metabolic syndrome[7].
NAFLD is thought to develop in genetically predisposed individuals as a result of multiple pathogenic “hits”, whereby the accumulation of triglycerides in the liver causes lipotoxicity, mitochondrial dysfunction, oxidative stress, and inflammation, eventually causing apoptosis and fibrosis[8,9]. This accumulation of triglycerides occurs as a result of obesity, dyslipidemia, and insulin resistance, and is exacerbated by alterations to the gut microbiome[8]. Therefore, it is not surprising that patients with NAFLD have an increased risk of cardiovascular mortality compared with patients without NAFLD, and the risk increases with worsening severity of hepatic pathology[10,11]. In a large outpatient cohort study of patients with type 2 diabetes[12], the prevalence of coronary, cerebrovascular, and peripheral vascular disease was greater among those with NAFLD than those without; this association was independent of medication use, traditional risk factors for cardiovascular disease, and diabetes-related variables.
Current treatment recommendations for NAFLD focus on lifestyle interventions (weight loss, diet, and exercise), as there are few if any accepted pharmacologic therapies[13]. However, weight loss of up to 10% may be needed to affect hepatic pathology, and lifestyle changes are difficult to implement and maintain long term[10,14]. Essential phospholipids (EPL) are used for the supportive treatment of liver diseases, including NAFLD, but data are mostly from small-scale studies conducted in a range of countries[15]. The aim of the current analysis was to apply meta-analytic techniques to data from clinical trials of EPL in high-risk NAFLD patients, specifically those with features of metabolic syndrome. Because of the variety of studies identified, a range of techniques were used: Direct meta-analysis, indirect meta-analysis and cohort meta-analysis.
This analysis was conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Eligible studies included randomized controlled clinical trials, non-randomized clinical trials, and comparative observational studies in which adult patients (≥ 18 years old) with NAFLD and type 2 diabetes and/or obesity received treatment with EPL as monotherapy or as add-on therapy to existing treatment. Eligible trials included data for at least one of the following efficacy outcomes: Alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, serum triglycerides, disease response, and disease severity (based on ultrasonography). Conference abstracts from the past 2 years were included and case studies were excluded. There were no restrictions applied to study duration, settings, or comparators. Only studies published in English or Chinese were included.
To identify potentially eligible trials, a literature search of the MEDLINE, PubMed, Embase, and Cochrane Central Register of Controlled trials databases (from database inception to March 2019) was conducted by an expert medical librarian. The literature search strategy used a range of Medical Subject Headings, EMTREE, and free-text terms based on the Population, Intervention, Comparator, and Outcome framework. Additionally, a manual search was performed on the reference lists of identified eligible studies and relevant systematic reviews. A copy of the PubMed search strategy is provided in the Supplementary material.
The web-based platform DOC Library (Doctor Evidence. 2018. DOC Library, Version 2.0. Santa Monica, CA: Doctor Evidence, LLC) was used for selection and screening. Screening was performed in two steps: (1) Title/abstract screening (conducted by a single screener, and checked by a second team member); and (2) Full-text screening (conducted by two independent screeners). Reasons for exclusion were documented at each step and discrepancies between the screeners were identified and resolved by an independent third reviewer.
For each trial, patient, treatment, and study characteristics, and efficacy/effectiveness outcomes were extracted. For continuous variables, the appropriate estimate measures and dispersion (mean, median, standard deviation, and range) were recorded, while for dichotomous and categorical variables, the number and/or proportion of patients were extracted. Data were obtained from the text and tables manually, and digitizer software was used to capture relevant data points from figures and charts.
Two reviewers assessed the risk of bias using the Cochrane Collaborations tool for clinical trials[16] and the Newcastle–Ottawa scale for non-randomized controlled studies. Discrepancies were addressed by an independent reviewer.
Due to the small number of studies forming a network, the indirect comparison analysis used frequentist network meta-analysis. Separate cohort meta-analyses were performed for the EPL arms in the randomized controlled trials and in the non-randomized trials.
For continuous variables, derived mean difference and 95% confidence interval (CI) were derived as measures of treatment effect. For disease response and recovery, relative risk (RR) with 95%CI were calculated.
A random-effects model was used to address between-study heterogeneity. To assess sources of heterogeneity, subgroup sensitivity analysis was performed based on population size (< 100 patients or ≥ 100 patients). There is a difference between subgroup estimates, but in general, one study represented one subgroup.
For the indirect meta-analysis, netmeta v1.0.1 software was used and for the cohort and direct meta-analysis, metaphor v2.0.0 was used.
The statistical methods of this study were reviewed by Tobias Sayre from Doctor Evidence, Santa Monica, CA, United States.
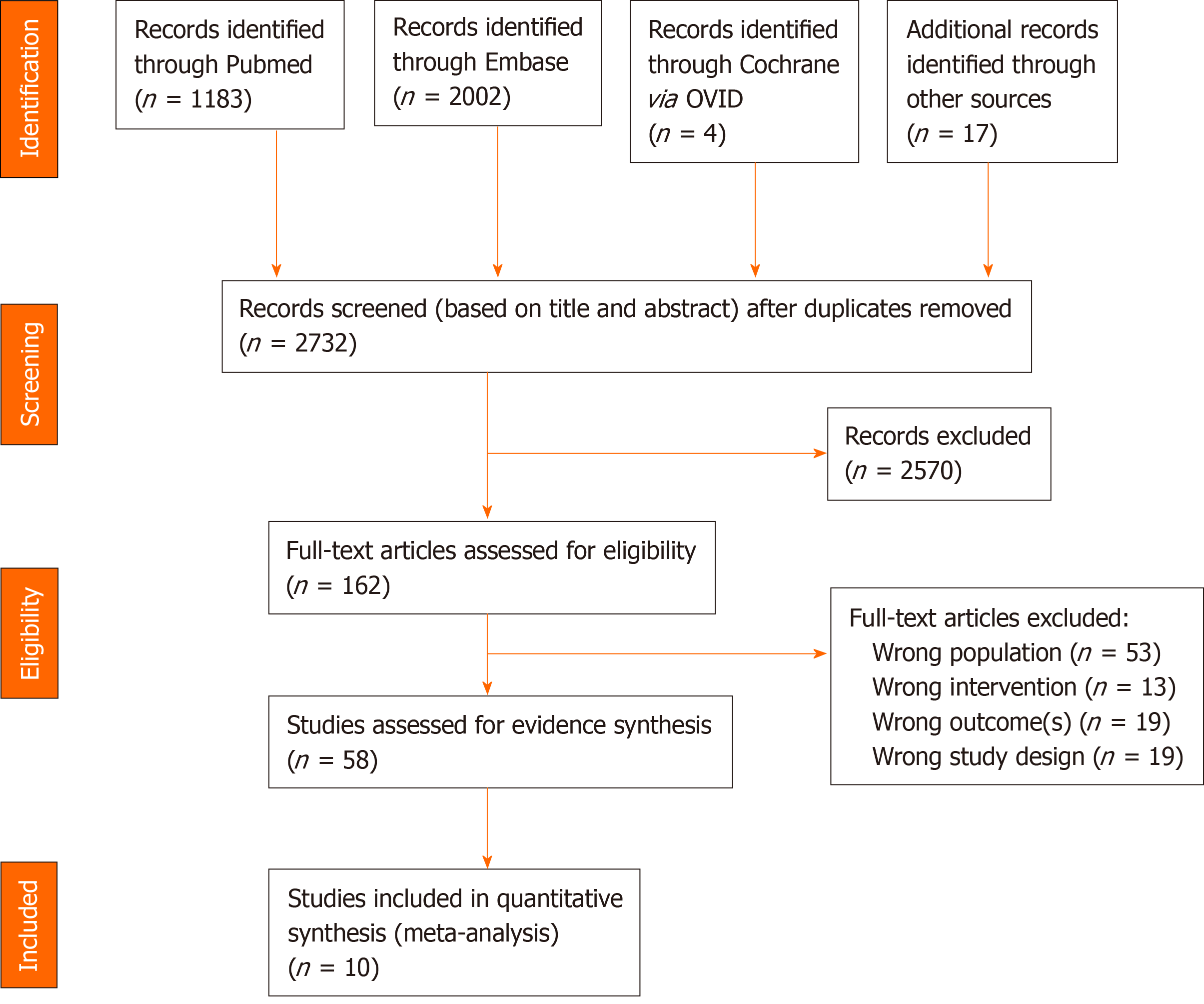
The search identified 2732 potential articles, of which 162 were potentially relevant based on the title and abstract (Figure 1). Of these, 104 did not meet eligibility criteria and 58 were considered for inclusion (Figure 1). Ten of these 58 studies were identified for inclusion in this analysis: Seven randomized controlled trials[17-23], two open-label studies[24,25], and one non-randomized controlled trial[26] (Table 1). These studies included between 22 and 324 patients (mean ages 41-59 years). Duration of EPL treatment ranged from 4 to 72 wk (Table 1). In five studies[18,19,21-23], EPL was given in combination with antidiabetic drugs.
| Ref. | Design | Patient type | Treatments | N | M/F | Age, yr, range (mean) | BMI, kg/m2 | Duration |
| Yin et al[23], 2000 | Randomized, OL | NAFLD + diabetes | PPC + ADs vs ADs | 125 | 73/52 | 42–78 (59) | N/A | 84 d |
| Poongothai et al[25], 2005 | Prospective, OL | NAFLD + type 2 diabetes | PPC + ADs | 22 | 11/11 | (41) | 28.2 | 6 mo |
| Arvind et al[26], 2006 | Prospective, non-randomized, DB | NAFLD + diabetes or obesity | PPC vs ursodeoxycholic acid | 40 | NA | NA | NA | 3 mo |
| Sun et al[21], 2008 | Prospective, randomized, OL | NAFLD + type 2 diabetes | PPC + metformin vs metformin | 74 | 40/34 | 28–60 (42) | NA | 12 wk |
| Wu et al[22], 2009 | Prospective, randomized, OL | NAFLD + type 2 diabetes | PPC + usual care vs usual care | 100 | 64/36 | (55) | NA | 1 mo |
| Li et al[18], 2013 | Prospective, randomized, OL | NAFLD + diabetes | PPC + metformin vs metformin | 86 | 57/29 | (51) | NA | 6 wk |
| Sas et al[19], 2013 | Prospective, randomized, OL | NASH + type 2 diabetes | PPC + metformin vs metformin | 189 | NA | NA | NA | 6 mo |
| Dajani et al[24], 2015 | Prospective, OL | NAFLD ± type 2 diabetes or hyperlipidemia | Essential phospholipid | 324 | 176/148 | 21–69 (43.5) | 29.3 | 72 wk |
| Shan et al[20], 2015 | Prospective, randomized, OL | NASH + obesity | PPC vs Chinese medicine (Quzhi hepatoprotection recipe) | 60 | 38/22 | 18-55 (41.3) | NA | 8 wk |
| Li et al[17], 2017 | Prospective, randomized, OL | NAFLD + diabetes | PPC + Chinese medicine (Shugan Huazhuo recipe) vs PPC | 80 | 44/36 | 29-63 (48) | NA | 3 mo |
Bias risk assessment results are shown in Table 2 and Supplementary Figure 1. Overall, studies were mainly of low or unclear risk across the domains of bias. In general, most studies did not specify the method of randomization or method of allocation concealment. The three non-randomized studies[24-26] had a high risk of selection bias attributable to their non-randomized nature, and two of these[24,25] had high risk of performance and detection bias due to their open-label design. The three non-randomized studies were analyzed separately. The distribution of risk of bias across the domains was similar between the remaining seven randomized studies (Supplementary Figure 1), indicating these studies could be combined in a meta-analysis assuming all other assumptions could be met.
| Ref. | Selection bias (sequence generation) | Selection bias (allocation concealment) | Performance bias (blinding) | Detection bias | Attrition bias (incomplete outcomes data) | Reporting bias (selective outcome reporting) | Other bias |
| Yin et al[23], 2000 | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk |
| Poongothai et al[25], 2005 | High risk | High risk | High risk | High risk | Low risk | Low risk | Low risk |
| Arvind et al[26], 2006 | High risk | High risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk |
| Sun et al[21], 2008 | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk |
| Wu et al[22], 2009 | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk |
| Li et al[18], 2013 | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk |
| Sas et al[19], 2013 | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk |
| Dajani et al[24], 2015 | High risk | High risk | High risk | High risk | Low risk | Unclear risk | Low risk |
| Shan et al[20], 2015 | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk |
| Li et al[17], 2017 | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk |
Four randomized studies[18,21-23] were suitable for direct meta-analysis; these studies compared EPL plus antidiabetic therapy vs antidiabetic therapy alone, and assessed effects of EPL on liver function parameters, lipid levels, recovery, and disease severity measured by ultrasonography. While a significant effect was seen with EPL plus antidiabetic therapy vs antidiabetic therapy alone, many of the comparisons showed significant heterogeneity.
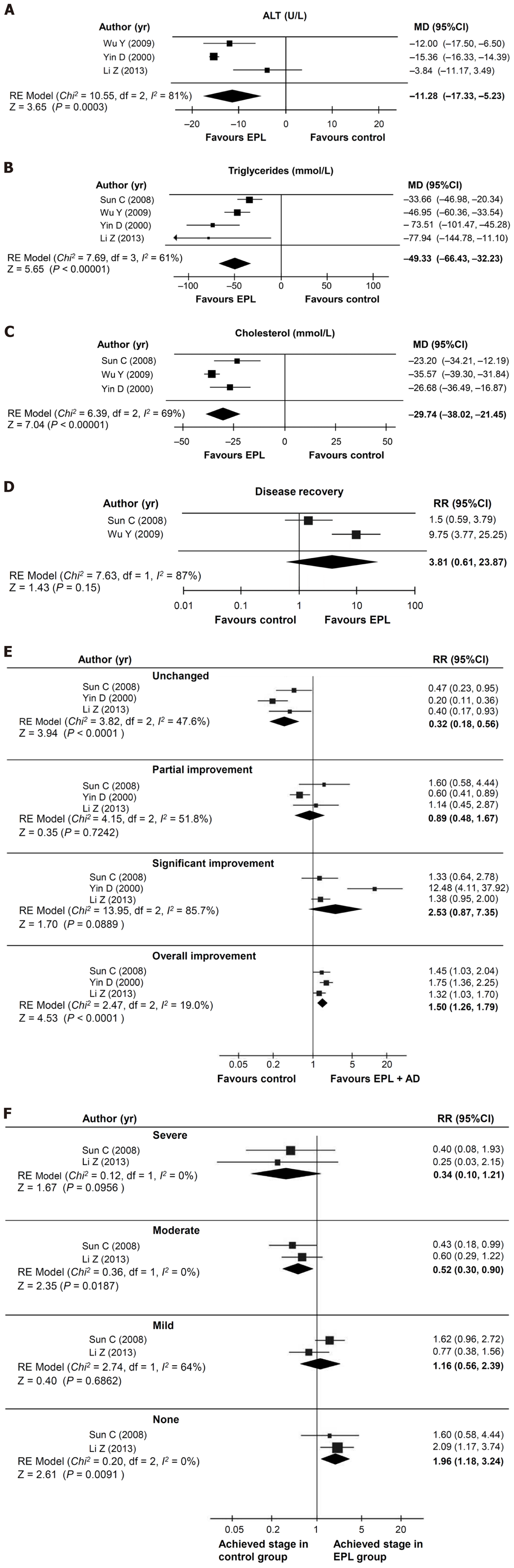
Three of the four studies (total n = 371)[18,22,23] could be analyzed for the effect on ALT levels, over a mean duration of 1.97 mo. This analysis showed that a significantly greater reduction in ALT levels was achieved with EPL plus antidiabetic therapy compared with antidiabetic therapy alone [mean difference of -11.28 U/L (95%CI: -17.33, -5.23); P = 0.0003; I2 = 81.0%; Figure 2A].
All four studies (total n = 445)[18,21-23] could be analyzed for the effect on triglyceride levels, over a mean of 2.1 mo. In this analysis, EPL plus antidiabetic therapy was associated with a significantly greater reduction in triglyceride levels compared with antidiabetic therapy alone [mean difference of -49.33 mg/dL (95%CI: -66.43, -32.23); P < 0.00001; I2 = 61.0%; Figure 2B]. Data for total cholesterol levels were available in three studies (total n = 359; mean of 2.27 mo)[21-23]. There was a significantly greater reduction in the total cholesterol level with EPL plus antidiabetic therapy compared with antidiabetic therapy alone [mean difference of -29.74 mg/dL (95%CI: -38.02, -21.45); P < 0.0001; I2 = 68.8%; Figure 2C].
Two studies (total n = 174)[21,22] could be analyzed for the effect on disease recovery over a mean of 1.75 mo. Although there was a trend towards an improved rate of disease recovery in patients receiving EPL plus antidiabetic therapy vs antidiabetic therapy alone, the effect size was not statistically significant and there was significant heterogeneity [RR: 3.81 (95%CI: 0.61, 23.87); P = 0.15; I2 = 86.9%; Figure 2D]. Disease response results were presented in three studies (total n = 345, mean treatment duration 2.42 mo)[18,21,23]. Compared with patients taking antidiabetic therapy alone, those receiving EPL plus antidiabetic therapy were more likely to achieve an overall improvement [RR: 1.50 (95%CI: 1.26, 1.79); P < 0.0001; I2 = 19.0%] and less likely to have no change in their disease [RR: 0.32 (95%CI: 0.18, 0.56); P < 0.0001; I2 = 47.6%; Figure 2E], and these analyses were not significantly heterogeneous. The likelihood of a partial improvement was similar in the two groups [RR: 0.89 (95%CI: 0.48, 1.67); P = 0.7242; I2 = 51.8%], but there was a trend towards a higher rate of significant improvement with EPL plus antidiabetic therapy vs antidiabetic therapy alone [RR: 2.53 (95%CI: 0.87, 7.35); P = 0.0889; I2 = 85.7%; Figure 2E]. Disease severity was assessed in two studies (total n = 160)[18,21] over a mean of 2.02 mo. Compared with antidiabetic therapy alone, the use of EPL plus antidiabetic therapy significantly reduced the likelihood of having moderate disease [RR: 0.52 (95%CI: 0.30, 0.90); P = 0.0187; I2 = 0.0%; Figure 2F] and showed a trend towards a reduced risk of severe disease [RR: 0.34 (95%CI: 0.10, 1.21); P = 0.0956; I2 = 0.0%], but no impact on the incidence of mild disease [RR: 1.16 (95%CI: 0.56, 2.39); P = 0.6862; I2 = 63.5%; Figure 2F). EPL plus antidiabetic therapy was also associated with an increased likelihood of having no disease compared with antidiabetic therapy alone [RR: 1.96 (95%CI: 1.18, 3.24); P = 0.0091; I2 = 0.0%].
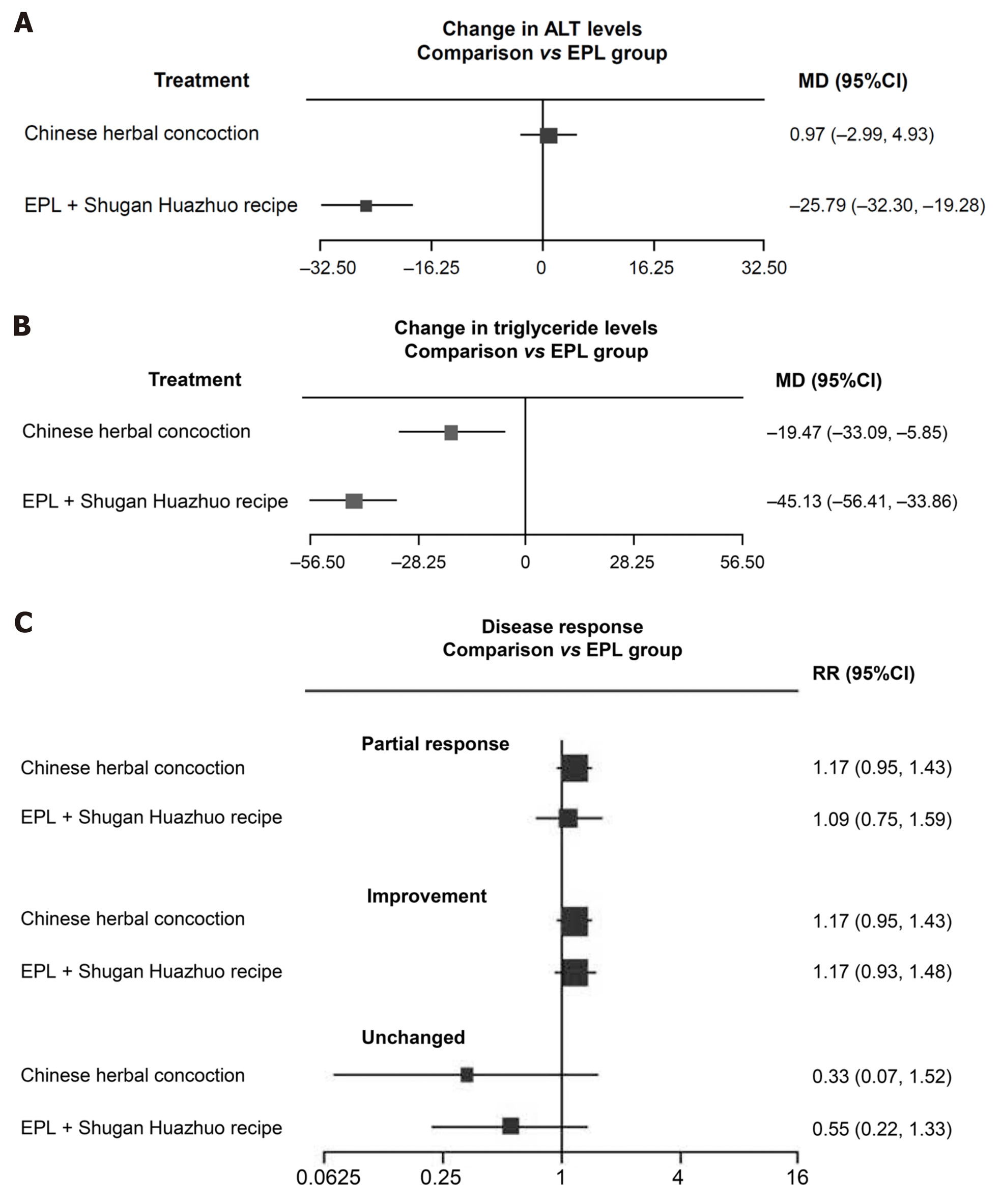
Two studies[17,20] were suitable for indirect meta-analysis, and could be used to evaluate changes in ALT and triglyceride levels, as well as disease response (see Supplementary Figure 2 for network plot). In one of these trials[20], 60 patients with non-alcoholic steatohepatitis and obesity received either EPL or Chinese medicine (Quzhi hepatoprotection recipe) for 8 wk, and in the other[17], 80 patients with NAFLD and diabetes received either EPL monotherapy or EPL plus Chinese medicine (Shugan Huazhuo Recipe) for 3 mo.
The mean difference in ALT levels was not significantly different between patients receiving a Chinese herbal concoction and those receiving EPL, but the combination of EPL and a Chinese herbal compound resulted in a significant greater decrease in ALT levels than EPL monotherapy (mean difference of -25.79 U/L; Figure 3A). The Chinese herbal concoction and the combination of EPL with a Chinese herbal compound both significantly reduced triglyceride levels in patients with NAFLD and diabetes or obesity (Figure 3B). There was a trend towards improved disease response with the Chinese herbal concoction or the combination of EPL plus a Chinese herbal compound compared with EPL alone, but the differences did not reach statistical significance (Figure 3C).
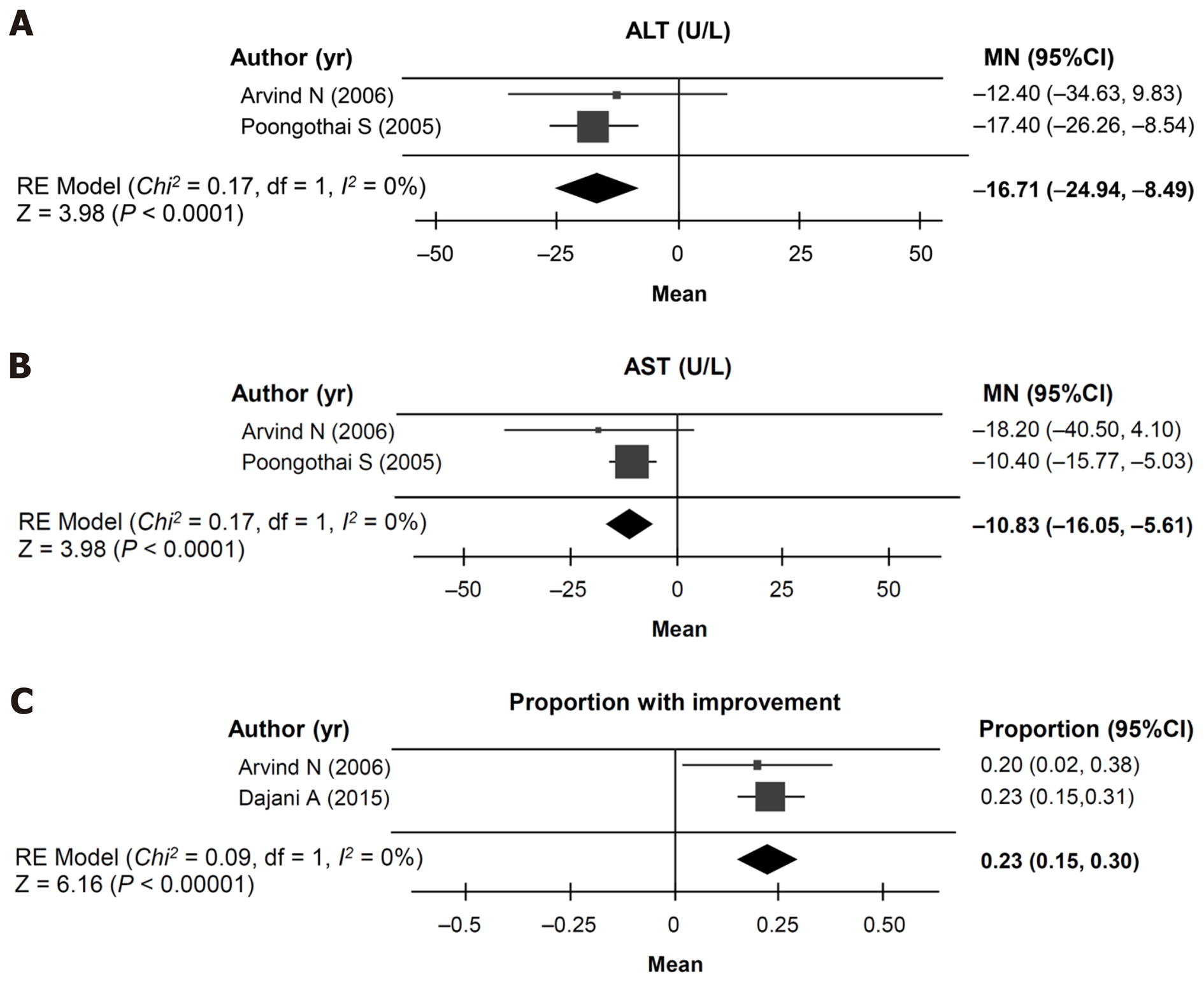
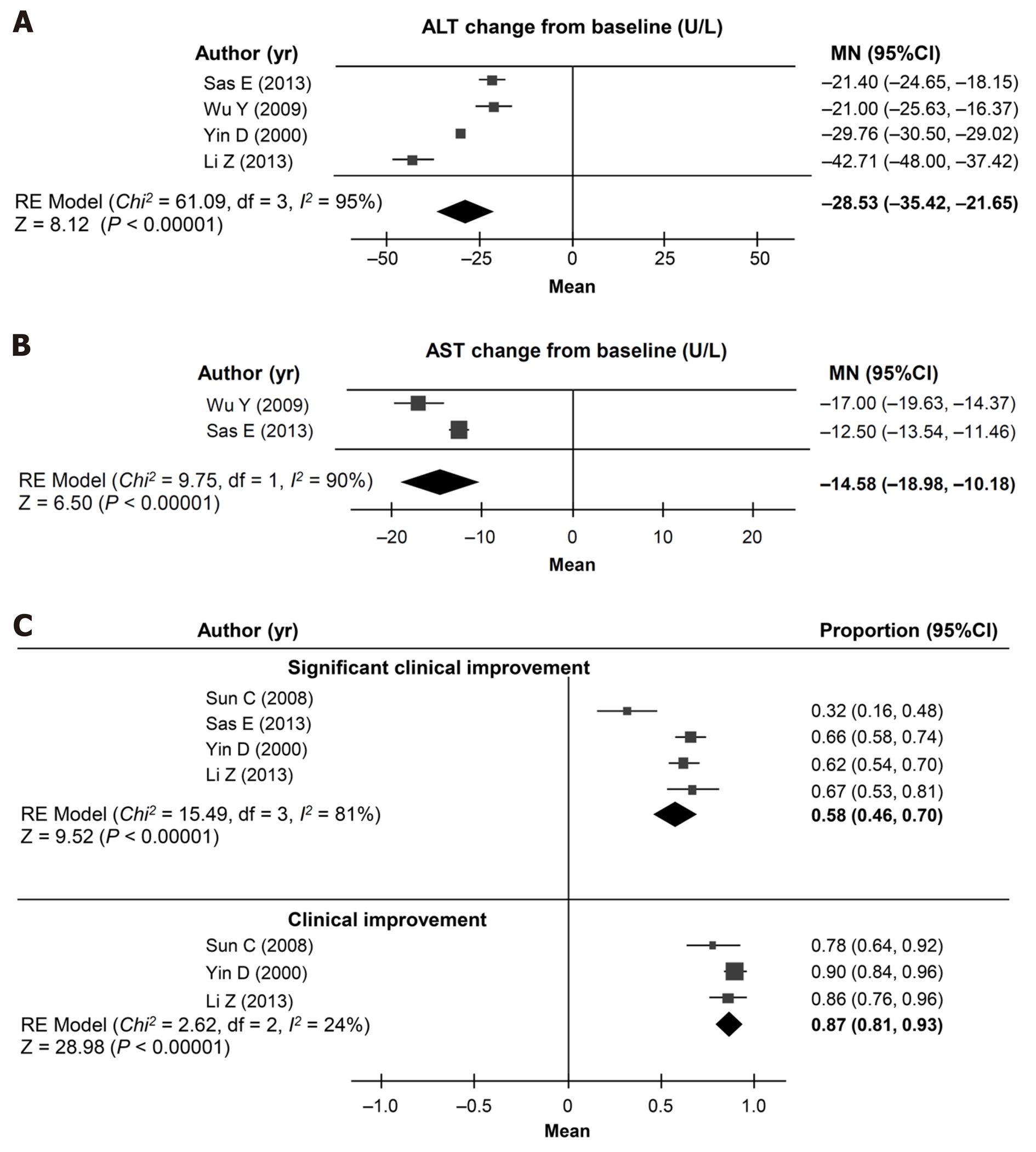
A cohort meta-analysis of ALT, AST, or clinical improvement could be undertaken using data from three non-randomized studies[24-26] for patients receiving EPL-containing treatment. ALT and AST could be analyzed in two studies (total n = 42)[25,26] with a mean duration of 2.02 mo. The mean change in ALT levels was –16.71 U/L (95%CI: -24.94, -8.49; Figure 4A) and the mean change in AST levels was –10.83 U/L (95%CI: -16.05, -5.61; Figure 4B). Disease improvement could be assessed in two studies (total n = 127)[24,26] with a mean study duration of 1.2 years, and showed that the proportion of patients in the EPL treatment groups with an improvement from baseline was 23% (95%CI: 15%, 30%; Figure 4C).
Cohort meta-analyses of ALT, AST, or clinical improvement could be undertaken using five randomized controlled studies[18,19,21-23], using the data for the patient groups who received EPL with antidiabetic drugs.
The change from baseline in ALT levels was assessed using data from four studies (n = 370; mean duration of treatment 3.69 mo)[18,19,22,23]. The pooled analysis showed a mean change in ALT levels of -28.53 U/L (95%CI: -35.42, -21.65; I2 = 95%; Figure 5A). Pooled change from baseline in AST levels could be calculated from two studies (n = 202; mean duration 4.76 mo)[19,22], and was –14.58 mg/dL (95%CI: -18.98, -10.18; I2 = 89.8%; Figure 5B).
The pooled estimate of the proportion of patients showing clinical improvement was 87% (95%CI: 81%, 93%; I2= 24%), based on data from three studies (n = 205)[18,21,23] over a mean of 2.47 mo (Figure 5C). The pooled estimate of the proportion of patients showing significant clinical improvement was 58% (95%CI: 46%, 70%; I2 = 81%), based on data from four studies (n = 357)[18,19,21,23] over a mean of 3.97 mo (Figure 5C).
Data from a randomized controlled study with < 100 patients[18] were compared with studies with ≥ 100 patients[22,23]. The effect of EPL on ALT levels was less marked in the study with < 100 patients [-3.84 U/L (95%CI: -11.17, + 3.49)] than in the studies with ≥ 100 patients [-14.82 U/L (95%CI: -17.24, -12.39); I2 = 28.05%; Supplementary Figure 3A, and there was a significant between-group difference (P = 0.005). EPL plus antidiabetic therapy significantly reduced triglyceride levels (Supplementary Figure 3B) and cholesterol levels (Supplementary Figure 3C) compared with antidiabetic therapy alone, and the magnitude of the effect was not significantly different in studies with < 100 patients or ≥ 100 patients.
One study each with < 100 and ≥ 100 patients[21,22] examined the rate of recovery with EPL plus antidiabetic drugs vs antidiabetic drugs alone. The risk ratio for recovery was higher in the study with ≥ 100 patients [RR: 9.75 (95%CI: 3.77, 25.25)] compared with the study in < 100 patients [RR: 1.50 (95%CI: 0.59, 3.79); Supplementary Figure 3D], with a significant between-group difference (P = 0.006). Similarly, the risk ratio for the effect of EPL on disease response of any magnitude (unchanged) was not significantly different between studies with < 100 patients and those with ≥ 100 patients (P = 0.0534; Supplementary Figure 3E), but the difference was significant for the endpoints of partial improvement (P = 0.0477; Supplementary Figure 3F) and significant improvement (P = 0.0002; Supplementary Figure 3G).
We used three different types of analysis (direct meta-analysis, indirect meta-analysis, and cohort meta-analysis) to investigate the effects of EPL treatment in patients with NAFLD and diabetes and/or obesity. EPL treatment was associated with a significant reduction in ALT levels in all three analyses, a significant reduction in triglycerides in the direct and indirect meta-analyses and a significant reduction in total cholesterol levels in the direct meta-analysis. EPL treatment was also associated with improved disease response, as measured by liver ultrasonography, in the direct meta-analysis and cohort analysis. To the best of our knowledge, this is the first meta-analysis to examine the effect of EPL in patients with NAFLD and diabetes or obesity, although the Cochrane group is planning to conduct a systematic analysis of EPL in patients with NAFLD[27].
The mechanism by which EPL may work to ameliorate NAFLD is likely to be multifactorial[28]. Phosphatidylcholine is an essential component of cell membranes and lipoproteins. When phosphatidylcholine is depleted, the liver secretes less very low density lipoprotein cholesterol, causing lipids to accumulate in hepatic cells[28]. Low levels of phosphatidylcholine may also stimulate de novo lipogenesis by activating sterol regulatory element-binding protein 1[28]. The ratio of phosphatidylcholine to phosphatidylethanolamine in hepatic cells appears to be a key determinant of NAFLD development[28]. Patients with NAFLD or non-alcoholic steatohepatitis have a lower ratio of phosphatidylcholine to phosphatidylethanolamine ratio than people without these conditions[29]. Therefore, the mechanism of EPL may involve restoring the optimal intrahepatic environment for normal lipid metabolism.
Another possible mechanism for the benefits of EPL in liver disorders is a reduction in oxidative stress. Studies in animal models of alcoholic[30] and nonalcoholic[31] fatty liver have demonstrated that EPL downregulate enzymes that produce reactive oxygen species, suppressing inflammation and fibrogenesis in the liver.
The results of the current analysis provide promising support for the use of EPL in patients with NAFLD. ALT levels were consistently and significantly reduced in all of the analyses, indicating improvement in hepatic function. However, not all patients with NAFLD have elevated ALT levels[9], and the American Association for the Study of Liver Diseases (AASLD) recommends that clinical trials in NAFLD use endpoints that assess the underlying severity of the hepatic pathology (steatosis and/or fibrosis)[32]. We found a trend towards an increased rate of recovery with EPL in the indirect meta-analysis, as well as a significant improvement in some parameters of disease severity in the direct meta-analysis. The overall effect of EPL on the number of patients with moderate or severe disease probably reflects an overall disease improvement with EPL use.
All the patients in our analysis had obesity or diabetes, supporting previous studies that indicate EPL may be a useful add-on treatment for patients with these conditions. As well as the data from the clinical studies in this meta-analysis showing that the addition of EPL enhances the efficacy of a range of standard treatment approaches, including standard diabetes treatment[22] and lifestyle intervention[23] in patients with diabetes and fatty liver, a separate study has shown that EPL with a vitamin B complex supplement + hypocaloric diet is beneficial in patients with obesity and hepatobiliary disorders caused by unhealthy diets[33].
In the studies included in our analysis, assessment of disease severity was based on ultrasonography; disease recovery was based on symptom improvement and biochemistry parameters in addition to ultrasonography. Ultrasonography can assess the degree of fatty infiltration in terms of echogenicity (where greater brightness indicates more infiltration), the hyperechoic pattern (diffuse or in patches), the extent of hepatic vasculature attenuation, the sharpness of the lower edge of the liver (where sharper indicates lower grade and blunter indicates higher grades of fatty infiltration), and the size of the liver (where normal is 14-16 cm, moderate enlargement is 16-20 cm, severe enlargement is > 20 cm). A change in the grade of fatty infiltration based on these ultrasonographic parameters indicates improvement or worsening or NAFLD. The AASLD recommendations for endpoints in clinical trials recommend liver biopsy and histological staging as the primary endpoint in studies of treatment for NAFLD[32]; however, liver biopsy has a number of important drawbacks including the invasiveness, pain, risk of complications, and the ability to sample only a small fraction of liver tissue[34]. In addition, because histological staging/grading includes a subjective element, there is the potential for interobserver variability in assessment[34]. For these reasons, noninvasive assessment by ultrasonography, computed to-mography and magnetic resonance elastography are increasingly used for clinical assessment[35]. These techniques are arguably better suited to clinical trials in which patients are regularly tested for change in steatosis severity, but have limited ability to identify inflammation, which is the more important predictor for progression to fibrosis and cirrhosis[35].
Our indirect and direct meta-analyses both showed that EPL was associated with a significant reduction in triglyceride levels. The direct meta-analysis was also able to assess the effect of EPL on total cholesterol, and found that this parameter was significantly reduced with EPL treatment. The effect of EPL on triglyceride levels may be clinically relevant since triglycerides have been shown to be an independent predictor of hepatic disease severity and disease progression in patients with NAFLD[36,37].
Our analysis has a number of limitations. Only 10 studies were eligible for inclusion, and these studies tended to be small. However, our sensitivity analysis found generally consistent results between studies with < 100 patients and those with ≥ 100 patients, suggesting that our analyses were robust. The studies were also of relatively short duration, and did not contain liver biopsy-based endpoints, as recommended by the AASLD[32]. The studies may have been too short to detect a difference in the histopathology of NAFLD. We also identified heterogeneity of different levels (varying from not important to considerable) between studies.
Although the data to date are limited and heterogeneous, the results of the current analyses provide evidence for a benefit of EPL in the high-risk groups of NAFLD patients with features of metabolic syndrome including type 2 diabetes and obesity. EPL warrant further investigation as a treatment of NAFLD patients, in large-scale studies with a long duration of follow-up.
Nonalcoholic fatty liver disease (NAFLD) is highly prevalent, particularly in patients with metabolic syndrome, but there are limited treatment options other than lifestyle changes. Preclinical data suggest that essential phospholipids (EPL) may inhibit the pathogenic processes that cause NAFLD. Therefore, EPL may be useful adjunctive therapy for patients with NAFLD in association with diabetes and/or obesity, but currently available data are limited.
Most of the data on the use of EPL in patients with NAFLD and diabetes and/or obesity are from small-scale studies, but we hypothesized that systematic evaluation and meta-analysis of these data may provide enough statistical power to assess the clinical effects of EPL in this population.
The aim of the current analysis was to apply meta-analytic techniques to data from clinical trials of EPL in high-risk NAFLD patients, specifically those with features of metabolic syndrome, to assess the effects of EPL, as monotherapy or as add-on therapy to existing treatment, on hepatic enzymes, lipid levels, and NAFLD severity.
We searched MEDLINE, PubMed, Embase, and Cochrane databases up to March 2019 for clinical trials and comparative observational studies published in English or Chinese that enrolled adult patients (≥ 18 years) with NAFLD and type 2 diabetes mellitus and/or obesity receiving EPL as monotherapy or as add-on therapy to existing therapy, and that included at least one of the efficacy outcomes of interest. Because a variety of studies were identified, we performed a range of analysis, i.e. direct, indirect and cohort meta-analyses. A random-effects model was used to address between-study heterogeneity.
Ten studies met the inclusion criteria (n = 22-324). EPL treatment duration ranged from 4 to 72 wk. In the direct meta-analysis (four randomized controlled trials), compared with antidiabetic therapy alone, EPL plus antidiabetic therapy was associated with a significantly greater reduction in alanine aminotransferase (ALT), triglyceride, and total cholesterol levels, and a significant increase in the rate of overall improvement. There was a significant increase in the risk of no disease (P = 0.0091), and a reduction in moderate disease (P = 0.0187), but no significant differences in severe disease, mild disease, or significant improvement. In the cohort meta-analysis of three non-randomized clinical trials, the mean difference in ALT levels was -16.71 U/L and 23% of patients had improved disease. In the cohort meta-analysis of five randomized trials, the mean difference in ALT levels was -28.53 U/L, and 87% of patients showed clinical improvement and 58% showed significant clinical improvement.
Although the data to date are limited and heterogeneous, the results of the current analyses provide evidence for a benefit of EPL in the high-risk groups of NAFLD patients with features of metabolic syndrome including type 2 diabetes and obesity.
EPL warrant further investigation as a treatment for NAFLD patients, in large-scale studies with a long duration of follow-up.
We thank the team of Dr. Evidence for performing the analysis, which was funded by Sanofi-Aventis; We would also like to thank Catherine Rees and Toni Dando, both of Springer Healthcare Communications who wrote the outline (CR) and first draft (TD) for this article; Tracy Harrison, also of Springer Healthcare Communications, provided additional medical writing assistance prior to submission. Medical writing was funded by Sanofi-Aventis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Jarcuska P, Plaza-Diaz J, Tanaka N S-Editor: Zhang L L-Editor: A P-Editor: Zhang YL
| 1. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7501] [Article Influence: 833.4] [Reference Citation Analysis (0)] |
| 2. | Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017;37 Suppl 1:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 437] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 3. | Lindenmeyer CC, McCullough AJ. The Natural History of Nonalcoholic Fatty Liver Disease-An Evolving View. Clin Liver Dis. 2018;22:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 204] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 4. | Dietrich P, Hellerbrand C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2014;28:637-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 317] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 5. | Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T, Sarui H, Shimazaki M, Kato T, Okuda J, Ida K. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 806] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 6. | Hwang YC, Ahn HY, Park CY. Association between Nonalcoholic Fatty Liver Disease and Future Deterioration of Metabolic Health: A Cohort Study. Obesity (Silver Spring). 2019;27:1360-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Yang KC, Hung HF, Lu CW, Chang HH, Lee LT, Huang KC. Association of Non-alcoholic Fatty Liver Disease with Metabolic Syndrome Independently of Central Obesity and Insulin Resistance. Sci Rep. 2016;6:27034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 2106] [Article Influence: 234.0] [Reference Citation Analysis (1)] |
| 9. | Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 934] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 10. | Li AA, Ahmed A, Kim D. Extrahepatic Manifestations of Nonalcoholic Fatty Liver Disease. Gut Liver. 2020;14:168-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 11. | Tana C, Ballestri S, Ricci F, Di Vincenzo A, Ticinesi A, Gallina S, Giamberardino MA, Cipollone F, Sutton R, Vettor R, Fedorowski A, Meschi T. Cardiovascular Risk in Non-Alcoholic Fatty Liver Disease: Mechanisms and Therapeutic Implications. Int J Environ Res Public Health. 2019;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 12. | Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 700] [Article Influence: 38.9] [Reference Citation Analysis (1)] |
| 13. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2611] [Article Influence: 200.8] [Reference Citation Analysis (1)] |
| 14. | Bril F, Cusi K. Management of Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes: A Call to Action. Diabetes Care. 2017;40:419-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 245] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 15. | Gundermann KJ, Gundermann S, Drozdzik M, Mohan Prasad VG. Essential phospholipids in fatty liver: a scientific update. Clin Exp Gastroenterol. 2016;9:105-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24695] [Article Influence: 1763.9] [Reference Citation Analysis (3)] |
| 17. | Li Y, Miao Q, Zhang W. Study on the clinical effect and mechanism of Shugan Huazhuo Decoction in treating diabetes mellitus complicated with nonalcoholic fatty liver disease. Zhongguo Yiyao Daobao. 2017; 14:119-124. |
| 18. | Li ZG. Efficacy of polyene for diabetes complicated with non-alcoholic fatty liver disease. Neimenggu Zhongyiyao. 2013;31:10-11. |
| 19. | Sas E, Grinevich V, Efimov O, Shcherbina N. Benefical influence of polyunsaturated phosphatidylcholine enhances functional liver condition and liver structure in patients with nonalcoholic steatohepatitis. Results of a prolonged randomized blinded prospective clinical study. J Hepatol Suppl. 2013;58 (Suppl 1):S549. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Shan L. Clinical study on the Quzhi hepatoprotection recipe in the treatment of non-alcoholic fatty liver concomitant with obesity. Yatai Chuantong Yiyao. 2015;11:123-125. |
| 21. | Sun C, Zheng X, Tan X, Cui F, Zhang R, Zhang H. Clinical observation on polyene phosphatidyl choline and metformin in the treatment of type 2 diabetes and non-alocholic fatty liver disease. Linchuang Huicui. 2008;23:1272-1273. |
| 22. | Wu Y. Efficacy analysis of polyene phosphatidylcholine for type 2 diabetes complicated with fatty liver. Hunan Zhong Yiyao Daxue Xuebao. 2009;29:41-42. |
| 23. | Yin DY, Kong LM. Observation for curative effect of Essentiale in treatment of fatty liver caused by diabetes mellitus. Qilu Yixue Zazhi. 2000;15:277-278. |
| 24. | Dajani AI, Abu Hammour AM, Zakaria MA, Al Jaberi MR, Nounou MA, Semrin AI. Essential phospholipids as a supportive adjunct in the management of patients with NAFLD. Arab J Gastroenterol. 2015;16:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Poongothai S, Karkuzhali K, Prakash GS, Sangeetha T, Saravanan G, Deepa R, Gopalkrishnan S, Mohan V. Effect of Essentiale in diabetic subjects with non-alcoholic fatty liver. Int J Diabetes Dev Countries. 2005;25:12-19. |
| 26. | Arvind N, Savaikar P, Rajkumar JS. Therapy for NAFLD - A comparative study of essential phospholipids vs ursodeoxycholic acid. Ind J Clin Pract. 2006;16:21-24. |
| 27. | Varganova DL, Pavlov CS, Casazza G, Nikolova D, Gluud C. Essential phospholipids for people with non-alcoholic fatty liver disease (protocol). Cochrane Database Syst Rev. 2019: CD013301. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | van der Veen JN, Kennelly JP, Wan S, Vance JE, Vance DE, Jacobs RL. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim Biophys Acta Biomembr. 2017;1859:1558-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 970] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 29. | Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, Sanyal AJ. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 914] [Cited by in RCA: 1039] [Article Influence: 57.7] [Reference Citation Analysis (1)] |
| 30. | Okiyama W, Tanaka N, Nakajima T, Tanaka E, Kiyosawa K, Gonzalez FJ, Aoyama T. Polyenephosphatidylcholine prevents alcoholic liver disease in PPARalpha-null mice through attenuation of increases in oxidative stress. J Hepatol. 2009;50:1236-1246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Cao M, Li X, Zhang B, Han S, Yang Y, Zhou B, Zhang Y. The effect of polyene phosphatidyl choline intervention on nonalcoholic steatohepatitis and related mechanism. Am J Transl Res. 2016;8:2325-2330. [PubMed] |
| 32. | Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, McCullough A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 579] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 33. | Cairella M, Callisto F, Godi R, Marchini G. [Polyunsaturated phosphatidylcholine combined with vitamin B complex in the treatment of patients with disorders of the hepatobiliary function caused by unbalanced nutrition]. Clin Ter. 1989;131:237-246. [PubMed] |
| 34. | Sanai FM, Keeffe EB. Liver biopsy for histological assessment: The case against. Saudi J Gastroenterol. 2010;16:124-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Li Q, Dhyani M, Grajo JR, Sirlin C, Samir AE. Current status of imaging in nonalcoholic fatty liver disease. World J Hepatol. 2018;10:530-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 170] [Article Influence: 24.3] [Reference Citation Analysis (3)] |
| 36. | Kim NH, Kim JH, Kim YJ, Yoo HJ, Kim HY, Seo JA, Kim NH, Choi KM, Baik SH, Choi DS, Kim SG. Clinical and metabolic factors associated with development and regression of nonalcoholic fatty liver disease in nonobese subjects. Liver Int. 2014;34:604-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Leung JC, Loong TC, Wei JL, Wong GL, Chan AW, Choi PC, Shu SS, Chim AM, Chan HL, Wong VW. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology. 2017;65:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 274] [Article Influence: 34.3] [Reference Citation Analysis (0)] |