Published online Nov 6, 2020. doi: 10.12998/wjcc.v8.i21.5221
Peer-review started: June 13, 2020
First decision: July 25, 2020
Revised: August 5, 2020
Accepted: September 28, 2020
Article in press: September 28, 2020
Published online: November 6, 2020
Processing time: 146 Days and 0.7 Hours
Wavelet index (WLi) and pain rating index (PRi) are new parameters for regulating general anesthesia depth based on wavelet analysis.
To investigate the safety and efficacy of using WLi or PRi in sevoflurane anesthesia.
This randomized controlled trial enrolled 66 patients scheduled for elective posterior lumbar interbody fusion surgery under sevoflurane anesthesia between September 2017 and February 2018. A random number generator was used to assign the eligible patients to three groups: Systolic blood pressure (SBP) monitoring group, WLi monitoring group, and PRi monitoring group. The main anesthesiologist was aware of the patient grouping and intervention used. The primary endpoint was anesthesia recovery time. Secondary endpoints included extubation time, sevoflurane consumption, number of unwanted events/ interventions, number of adverse events and postoperative visual analogue scale for pain.
A total of 62 patients were included in the final analysis (SBP group, n = 21; WLi group, n = 21; and PRi group, n = 20). There were no significant differences among the three groups in patient age, gender distribution, body mass index, American Society of Anesthesiologists class, duration of surgery, or duration of anesthesia. Anesthesia recovery time was shorter in the WLi and PRi groups than in the SBP group with no significant difference between the WLi and PRi groups. Extubation time was shorter in the WLi and PRi groups than in the SBP group. Sevoflurane consumption was lower in the WLi and PRi groups than in the SBP group. Nicardipine was more commonly needed to treat hypertension in the WLi and PRi groups than in the SBP group.
Regulation of sevoflurane anesthesia depth with WLi or PRi reduced anesthesia recovery time, extubation time and sevoflurane consumption without intraoperative unwanted events.
Core Tip: Anesthesia recovery time was shorter in the wavelet index (WLi) and pain rating index (PRi) groups than in the systolic blood pressure (SBP) group with no significant difference between the WLi and PRi groups. Extubation time was shorter in the WLi and PRi groups than in the SBP group. Sevoflurane consumption was lower in the WLi and PRi groups than in the SBP group. Nicardipine was more commonly needed to treat hypertension in the WLi and PRi groups than in the SBP group.
- Citation: Zhang JW, Lv ZG, Kong Y, Han CF, Wang BG. Wavelet and pain rating index for inhalation anesthesia: A randomized controlled trial. World J Clin Cases 2020; 8(21): 5221-5234
- URL: https://www.wjgnet.com/2307-8960/full/v8/i21/5221.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i21.5221
The ideal depth of anesthesia is that which achieves appropriate levels of hypnosis, analgesia, and muscle relaxation at the same time[1], but the depth of anesthesia must be carefully monitored during surgery to avoid physiologic responses to awareness such as tachycardia, hypertension, sweating, lacrimation, increased skeletal muscle tone, and spontaneous movement[2]. Traditional monitoring of anesthesia depth relies on clinical signs that represent the reactions of the body to noxious stimuli during surgery such as blood pressure or heart rate changes, body movement, sweating, lacrimation, eye movement, and pupillary reflex, but these indicators have poor specificity and can be influenced by many factors, including other drugs used in combination with general anesthetics. Thus, there is considerable interest in the development of better methods for monitoring the depth of anesthesia.
Classical electroencephalography (EEG) can be used to monitor the depth of anesthesia as well as EEG-based technologies[3]. The bispectral index (BIS) is recognized to accurately represent the sedative depth of anesthesia[4,5]. The BIS is derived from three parameters obtained from the EEG: The power spectrum, the phase spectrum, and the proportion of the signal that is isoelectric[6]. However, because the BIS only reflects the frequency domain of the EEG signal and not the time domain, the BIS does not demonstrate immediate changes in the EEG signal.
The wavelet index (WLi) is a new parameter that potentially could be used to regulate the sedative depth of anesthesia. WLi is a multiscale refined analysis of the original EEG wave achieved using scaling and translation functions. Similar to the BIS, the range of WLi values is 0–100, with a value of 0 indicating no EEG activity, a value of 100 indicating complete wakefulness, and a value of 40–60 taken to indicate an appropriate level of general anesthesia. A number of Chinese studies[7-10] showed that the WLi showed good correlation and consistency with the BIS and could be used as an index to monitor the sedative depth of anesthesia. Because wavelet analysis is a method that combines frequency and time domains, the information provided by the WLi is thought to be more accurate and comprehensive than that provided by the BIS[11].
The pain rating index (PRi) is an objective quantitative assessment indicator that was developed by Chinese researchers in 2016. Using wavelet analysis methods, the investigators extracted the repeatable regular metadata that change characteristically from the high-frequency rhythm (γ waveband, 40-100 Hz) and low-frequency rhythm (α and β wavebands, 8-30 Hz), which are highly associated with the intracerebral conduction of pain signals in EEG[12-14]. These data can be used to guide the doses and infusion velocities of the analgesics based on a multivariable regression model. The novel comprehensive EEG indicator can specifically reflect the tolerance degree of the cerebral cortex and subcortical center to pain stimuli, and the score ranges from 0 to 100. PRi of 50-70 means satisfactory analgesia, PRi < 50 means over analgesia, and PRi > 70 means insufficient analgesia.
We hypothesized that regulating the depth of general anesthesia using WLi or PRi as opposed to standard monitoring of systolic blood pressure (SBP) can shorten anesthesia recovery time and extubation time and reduce general anesthetic consumption in patients undergoing surgery. Therefore, the aim of this randomized controlled clinical trial was to investigate whether WLi and PRi could be used effectively and safely to regulate the depth of sevoflurane anesthesia in patients undergoing lumbar surgery.
This prospective, randomized controlled trial was conducted at the Department of Anesthesiology, Shanxi Dayi Hospital, Taiyuan, Shanxi, China between September 2017 and February 2018. The study was approved by the Shanxi Dayi Hospital Ethical Committee (No. YXLL-2017-005) and registered in the Chinese Clinical Trial Registry (registration number: ChiCTR-IPR-17012092; date of registration: July 23, 2017). Written informed consent was obtained from all patients.
Consecutive patients who met the following inclusion criteria were enrolled: (1) Age 40-60 years; (2) Body mass index 18-25 kg/m2; (3) American Society of Anesthesiologists grade I or II; (4) Diagnosis of lumbar spinal stenosis and/or lumbar disc herniation; (5) Scheduled for elective posterior lumbar interbody fusion under general anesthesia with tracheal intubation; and (6) Surgery was limited to a maximum of three lumbar segments. The exclusion criteria were: (1) History of central nervous system or respiratory system disease; (2) Misuse of alcohol or use of illicit drugs; (3) History of malignant hyperthermia; (4) History of severe psychiatric disorder; and (5) Refusal to provide informed written consent. Patients were also excluded from the final analysis if the following criteria were met: (1) Intraoperative WLi > 60 or PRi > 70 at a sevoflurane dosage of 4%; and (2) Surgical duration > 3 h (i.e. longer than that required for a routine operation). WLi 40-60 and PRi 50-70 are the indicators recommended by the manufacturers of the equipment for reflecting proper anesthesia depth. Sevoflurane was set at 4%, based on the minimum alveolar content (MAC) value of sevoflurane, which is about 2% when age is 40-60 years. As 1.3-1.6 MAC could meet the requirements of the operation, we set the sevoflurane at < 2 MAC in this study.
Conventional modulation of analgesia in clinical practices is based on the monitoring of blood pressure and heart rate. Therefore, SBP was selected for the control group. A random number generator was used to assign the eligible patients to three groups: SBP monitoring group, WLi monitoring group, and PRi monitoring group. The main anesthesiologist was aware of the patient grouping and intervention used. However, all patients and all staff who performed data collection and analysis were blinded to the patient grouping and intervention used.
Noninvasive blood pressure (NIBP), pulse oxygen saturation, heart rate (HR), and electrocardiogram were routinely monitored during surgery in all patients (IntelliVue MX700 bedside patient monitor; Philips, Amsterdam, The Netherlands). For the monitoring of WLi and PRi, the skin of the patient’s forehead and mastoid was degreased with alcohol, and the electrodes of the HXD-I multifunctional monitor (Beijing Easymonitor Technology, Co., Ltd., Beijing, China) were positioned as recommended by the manufacturer. The electrode impedance was kept below 7.5 kΩ as required by the manufacturer to ensure optimal contact. The baseline values for SBP, HR, WLi, and PRi were defined as the average of three consecutive measurements made soon after the patient’s arrival in the operating theater and before the induction of anesthesia. NIBP was measured every 3 min, while WLi and PRi were measured continuously.
During surgery, the anesthesiologist (who was aware of the patient grouping) managed the level of anesthesia based on the group to which the patient had been allocated. However, all investigators involved in the collection, recording, and analysis of data were blind to the patient grouping.
Only sevoflurane was enough for analgesia and sedation, which had high controllability, and could reduce the interactions among the compound anesthetics. No opioids were used during operation.
An intravenous catheter was inserted into a large forearm vein. After adequate preoxygenation with 100% inhaled oxygen, bolus intravenous injections of midazolam (0.05 mg/kg), sufentanil (0.5 µg/kg), and etomidate (0.3 mg/kg) were administered for induction of anesthesia followed by vecuronium (0.1 mg/kg) for muscle relaxation. The patient’s trachea was intubated and the lungs were ventilated mechanically with a tidal volume of 8-10 mL/kg, and the ventilatory rate was adjusted to maintain an end-tidal carbon dioxide partial pressure of 30-35 mmHg. Anesthesia induction, tracheal intubation, and adjustment of mechanical ventilation parameters were the same for all patients.
For all patients, the initial concentration (Cs) of inhaled sevoflurane (Maruishi Pharmaceutical Co., Ltd., Osaka, Japan) was 2 vol% with 2 L/min of fresh gas flow. During maintenance of anesthesia, the Cs range was limited to between 1.5 vol% and 4 vol%, and vecuronium (0.05 mg/kg) was administered every 40 min. The total sevoflurane consumption was calculated according to: Volume of sevoflurane consumption (mL) = sevoflurane Cs in the volatilization pot (%) × flow velocity (L/min) × lasting time at this flow velocity (min) × molecular weight of sevoflurane (200.06) ÷ 2412 ÷ relative density of sevoflurane (1.524)[15].
In the SBP group, Cs was adjusted to maintain the SBP at ± 20% of the baseline value. When SBP reached > 15% of the baseline value, Cs was increased by 0.5%-1%. When SBP reached < 15% of the baseline value, Cs was decreased by 0.5%-1%. In the WLi group, Cs was adjusted to maintain the WLi value between 40–60. When WLi reached < 45, Cs was decreased by 0.5%-1%. When WLi reached > 55, Cs was increased by 0.5%-1%. In the PRi group, Cs was adjusted to maintain the PRi value between 50-70. When PRi reached < 55, Cs was decreased by 0.5%-1%. When PRi reached > 65, Cs was increased by 0.5%-1%. In all cases, the interval between each adjustment was 3 min.
Nicardipine (0.3 mg) was administered intravenously for hypertension (SBP > 120% of baseline). Ephedrine (10 mg) was given intravenously for hypotension (SBP < 80% of baseline). Esmolol (10 mg) was administered intravenously for tachycardia (HR > 90 beats/min if baseline HR was ≤ 75 beats/min or HR > 120% of baseline if baseline HR was > 75 beats/min). Intravenous atropine (0.5 mg) was given for bradycardia (HR < 45 beats/min)[16-18].
At the end of surgery, sevoflurane was discontinued, and all patients received sufentanil (0.15 µg/kg) and tropisetron (5 mg). Extubation was performed when the patient had recovered respiration and consciousness. Anesthesia recovery time was defined as the time from discontinuation of sevoflurane to spontaneous opening of eyes. Extubation time was defined as the time from discontinuation of sevoflurane to extubation.
The primary endpoint was anesthesia recovery time. The secondary endpoints included: Sevoflurane consumption; extubation time; SBP, HR, WLi, and PRi values at major time points during general anesthesia; number of intraoperative unwanted events (hypertension, hypotension, tachycardia, and bradycardia); number of intraoperative interventions for unwanted events; number of intraoperative and postoperative adverse events (nausea, vomiting, agitation, respiratory depression, and awareness); and postoperative visual analogue scale (VAS) for pain.
The primary endpoint in this study was anesthesia recovery time. In accordance with the methods used in a previous randomized controlled trial[16], the sample size calculation was based on the results of a pilot study with 15 cases in each group. In the pilot study, anesthesia recovery time [mean ± standard deviation (SD)] was 25.6 ± 2.3 min in the SBP group, 18.4 ± 3.3 min in the WLi group, and 20.1 ± 3.3 min in the PRi group. Therefore, the effect size among the three groups was 0.53. On the assumption that the allocation ratio of the three groups was 1:1:1, the sample size for each group was calculated (F-test) to be 18 for a significance level of 0.05 and a power of 0.90. Considering a 20% dropout rate, the sample size for final enrollment was 22 in each group (a total of 66 patients).
Normally distributed measurement data are presented as mean ± SD and were analyzed using one-way analysis of variance and the least-significant difference test for multiple comparisons. Non-normally distributed data are expressed as medians (range) and were analyzed using the Brown-Forsythe test with Tamhane’s T2 test for multiple comparisons. Repeated measures analysis of variance was used for comparisons of SBP, HR, WLi, and PRi among time points during anesthesia. Categorical data are expressed as n (%) and were analyzed with the chi-squared test. A P value < 0.05 was considered statistically significant in all analyses. Statistical analyses were performed using SAS 9.4 statistical software (SAS Institute, Cary, NC, United States).
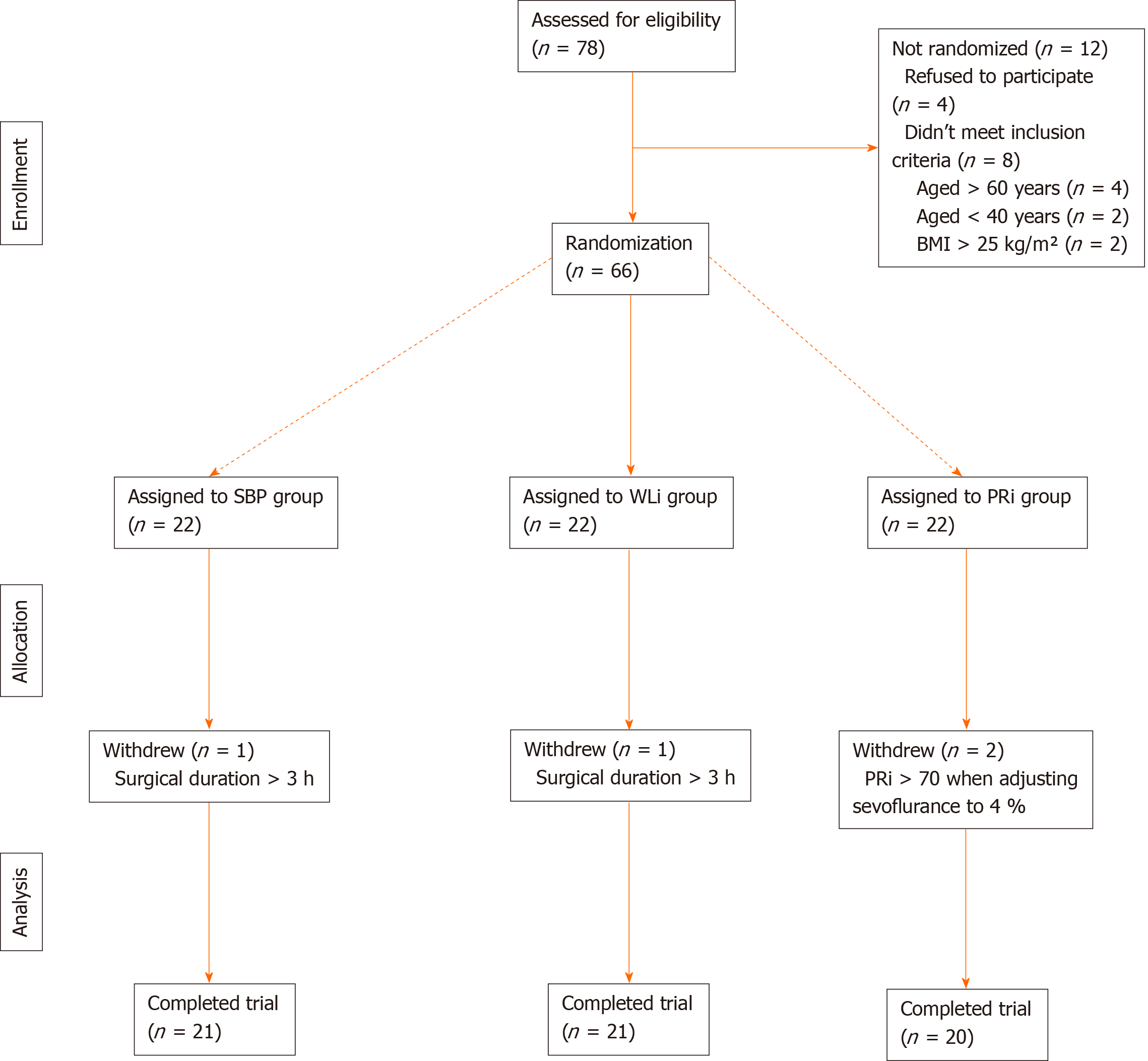
Among 78 patients assessed for eligibility, four patients refused to participate and eight patients did not meet the inclusion criteria (age > 60 years, n = 4; age < 40 years, n = 2; and body mass index > 25 kg/m2, n = 2). Therefore, 66 patients were initially enrolled in this study. A further four patients were excluded during the study (surgical duration > 3 h, n = 2; and PRi > 70 despite adjustment of sevoflurane Cs to 4%, n = 2). Therefore, a total of 62 patients were included in the final analysis (SBP group, n = 21; WLi group, n = 21; and PRi group, n = 20; Figure 1). There were no significant differences among the three groups in patient age, gender distribution, body mass index, American Society of Anesthesiologists class, duration of surgery, or duration of anesthesia (Table 1).
| SBP group, n = 21 | WLi group, n = 21 | PRi group, n = 20 | P value | |
| Sex, male/female | 13/8 | 12/9 | 10/10 | 0.742 |
| Age in yr | 54 ± 6 | 53 ± 5 | 52 ± 5 | 0.747 |
| BMI in kg/m2 | 24.5 ± 1.8 | 24.1 ± 1.6 | 24.0 ± 1.6 | 0.547 |
| ASA class, I/II | 16/5 | 17/4 | 15/5 | 0.891 |
| Duration of surgery in min | 134 ± 19 | 135 ± 12 | 137 ± 14 | 0.751 |
| Duration of anesthesia in min | 169 ± 18 | 170 ± 14 | 171 ± 17 | 0.920 |
Anesthesia recovery time was shorter in the WLi (19.7 ± 2.2 min) and PRi (20.6 ± 2.1 min) groups than in the SBP group (25.1 ± 2.2 min; P < 0.001). Extubation time was also shorter in the WLi (21.0 ± 2.2 min) and PRi (21.6 ± 2.6 min) groups than in the SBP group (26.6 ± 2.1 min; P < 0.001). Furthermore, sevoflurane consumption was lower in the WLi (26.7 ± 4.4 mL) and PRi (28.9 ± 3.5 mL) groups than in the SBP group (33.1 ± 2.1 ml; P < 0.001). There were no significant differences between the WLi and PRi groups in anesthesia recovery time, extubation time, or sevoflurane consumption (Table 2).
Trends in SBP, HR, WLi, and PRi values during general anesthesia are presented in Figure 2. There were no significant differences among groups in SBP (F = 0.031, P = 0.970) or HR (F = 0.720, P = 0.491) at any of the 13 major time points (Figure 2, Table 3). On the other hand, WLi (F = 10.924, P < 0.001) and PRi (F = 24.014, P < 0.001) did show some significant differences among groups (Figure 2, Table 4). At certain time points, the WLi and PRi values were higher in the WLi and PRi groups than in the SBP group (P < 0.05; Table 4). In addition, the PRi value was lower in the PRi group than in the WLi group at some time points during general anesthesia (P < 0.05; Table 4).
| Time point | Systolic blood pressure, mmHg | Heart rate, beats/min | ||||
| SBP group | WLi group | PRi group | SBP group | WLi group | PRi group | |
| Baseline | 125 ± 9 | 121 ± 8 | 122 ± 11 | 73 ± 9 | 71 ± 7 | 75 ± 9 |
| Five min after tracheal intubation | 109 ± 7 | 106 ± 4 | 107 ± 9 | 67 ± 10 | 66 ± 5 | 68 ± 10 |
| Placement in prone position | 110 ± 6 | 106 ± 4 | 109 ± 7 | 65 ± 10 | 65 ± 5 | 66 ± 7 |
| Incision | 112 ± 5 | 108 ± 5 | 112 ± 10 | 66 ± 7 | 64 ± 5 | 67 ± 8 |
| Vertebral plate exposure | 114 ± 6 | 115 ± 4 | 115 ± 12 | 67 ± 6 | 67 ± 6 | 68 ± 5 |
| Placement of pedicle screws | 118 ± 7 | 120 ± 8 | 118 ± 8 | 67 ± 8 | 69 ± 5 | 70 ± 5 |
| Spinal canal decompression | 132 ± 13 | 136 ± 9 | 134 ± 11 | 71 ± 97 | 73 ± 5 | 74 ± 5 |
| Interbody fusion | 126 ± 9 | 128 ± 10 | 127 ± 10 | 72 ± 12 | 72 ± 6 | 73 ± 6 |
| Placement of the fixed link | 121 ± 7 | 123 ± 9 | 123 ± 10 | 70 ± 12 | 71 ± 5 | 73 ± 6 |
| Placement of drainage tube | 119 ± 7 | 122 ± 7 | 122 ± 9 | 70 ± 11 | 71 ± 5 | 73 ± 6 |
| Skin closure | 126 ± 10 | 128 ± 9 | 128 ± 10 | 72 ± 11 | 73 ± 6 | 76 ± 6 |
| Placement in recovery position | 131 ± 7 | 130 ± 8 | 131 ± 10 | 76 ± 10 | 78 ± 4 | 80 ± 5 |
| Tracheal extubation | 132 ± 8 | 131 ± 9 | 131 ± 11 | 78 ± 10 | 79 ± 7 | 82 ± 6 |
| Time points | Wavelet index, 0-100 | Pain rating index, 0-100 | ||||
| SBP group | WLi group | PRi group | SBP group | WLi group | PRi group | |
| Baseline | 99.4 ± 1.2 | 99.5 ± 1.0 | 99.9 ± 0.7 | 85.9 ± 2.8 | 87.1 ± 1.8 | 87.5 ± 2.7 |
| Five min after tracheal intubation | 44.4 ± 3.9 | 44.4 ± 2.2 | 45.3 ± 3.2 | 57.0 ± 5.8 | 59.5 ± 4.6 | 58.9 ± 3.4 |
| Placement in prone position | 45.3 ± 2.2 | 46.1 ± 2.6 | 45.9 ± 2.3 | 55.6 ± 4.0 | 60.4 ± 3.6a | 59.2 ± 3.8a |
| Incision | 45.5 ± 2.3 | 46.7 ± 2.4 | 46.7 ± 1.4 | 55.6 ± 4.3 | 61.5 ± 3.6a | 59.5 ± 2.9a |
| Vertebral plate exposure | 46.2 ± 2.4 | 48.4 ± 4.3a | 48.5 ± 1.8a | 56.3 ± 4.2 | 62.3 ± 2.6a | 60.1 ± 3.9a |
| Placement of pedicle screws | 47.5 ± 2.4 | 50.0 ± 4.5a | 49.5 ± 2.7 | 56.1 ± 3.8 | 62.9 ± 2.3a | 60.8 ± 3.9a |
| Spinal canal decompression | 50.9 ± 4.4 | 55.0 ± 3.0a | 53.9 ± 1.7a | 60.8 ± 4.0 | 66.5 ± 1.8a | 63.9 ± 3.2a,b |
| Interbody fusion | 50.0 ± 4.1 | 52.3 ± 2.6a | 51.9 ± 2.1a | 59.7 ± 2.8 | 64.0 ± 3.6a | 61.7 ± 2.3a,b |
| Placement of the fixed link | 48.1 ± 2.9 | 51.5 ± 2.9a | 50.7 ± 1.9a | 58.8 ± 3.6 | 63.1 ± 3.0a | 60.2 ± 2.8b |
| Placement of drainage tube | 48.5 ± 3.2 | 51.8 ± 2.3a | 50.2 ± 2.3a | 58.1 ± 3.9 | 63.2 ± 3.4a | 60.3 ± 3.1b |
| Skin closure | 57.8 ± 4.9 | 60.0 ± 3.3 | 59.1 ± 1.2 | 65.9 ± 5.1 | 69.0 ± 1.8a | 68.6 ± 1.8a |
| Placement in recovery position | 83.4 ± 6.8 | 84.6 ± 4.3 | 86.4 ± 5.3 | 76.6 ± 5.0 | 78.1 ± 2.0 | 80.9 ± 2.7a,b |
| Tracheal extubation | 98.3 ± 1.5 | 97.2 ± 2.4 | 98.4 ± 2.6 | 87.0 ± 2.7 | 88.0 ± 2.9 | 87.9 ± 2.5 |
The total incidence of unwanted events and the incidence of each type of unwanted event (hypertension, hypotension, tachycardia, and bradycardia) did not differ significantly among the three groups (Table 5). The rates of intervention with esmolol, ephedrine, and atropine were also similar among groups (Table 5). On the other hand, the rate of intervention with nicardipine (for hypertension) was significantly higher in the WLi group (43%) and PRi group (60%) than in the SBP group (10%; P < 0.05; Table 5).
| SBP group, n = 21 | WLi group, n = 21 | PRi group, n = 20 | P value | |
| All unwanted events | 26 (1.24) | 16 (0.76) | 14 (0.70) | 0.101 |
| Hypertension | 14 (0.67) | 7 (0.33) | 9 (0.45) | 0.238 |
| Hypotension | 6 (0.29) | 3 (0.14) | 2 (0.10) | 0.267 |
| Tachycardia | 4 (0.19) | 3 (0.14) | 3 (0.15) | 0.905 |
| Bradycardia | 2 (0.10) | 3 (0.14) | 0 (0) | 0.239 |
| Nicardipine | 2 (0.10) | 9 (0.43)a | 12 (0.60)a | 0.024 |
| Esmolol | 4 (0.24) | 3 (0.14) | 4 (0.20) | 0.890 |
| Ephedrine | 2 (0.10) | 3 (0.14) | 2 (0.10) | 0.868 |
| Atropine | 4 (0.19) | 3 (0.14) | 0 (0) | 0.253 |
No patients reported awareness during anesthesia, and none experienced postoperative respiratory depression. There were no significant differences among groups in the incidences of postoperative nausea/vomiting or agitation (Table 6). Moreover, postoperative VAS for pain was also similar among the three groups (Table 6).
| SBP group, n = 21 | WLi group, n = 21 | PRi group, n = 20 | P value | |
| PONV | 4 (0.19) | 2 (0.10) | 3 (0.16) | 0.684 |
| Agitation | 3 (0.14) | 0 (0) | 2 (0.10) | 0.224 |
| Respiratory depression | 0 (0) | 0 (0) | 0 (0) | 1 |
| Intraoperative awareness | 0 (0) | 0 (0) | 0 (0) | 1 |
| Pain VAS0h | 0 (0-2) | 0 (0-2) | 0 (0-2) | 0.766 |
| Pain VAS1/2h | 2 (0-4) | 3 (0-4) | 2 (0-4) | 0.122 |
An important aim of modern anesthesiology is to optimize the use of anesthetic drugs by accurately regulating the depth of anesthesia while using the minimum effective dose of anesthetic agents. The accurate regulation of anesthesia depth enables maintenance of hemodynamic stability, improves the quality of anesthesia, and minimizes complications. The main findings of the present randomized controlled trial were that regulation of sevoflurane anesthesia depth with WLi or PRi in patients undergoing lumbar surgery shortened anesthesia recovery time and extubation time and reduced sevoflurane consumption as compared with regulation by monitoring of conventional clinical signs. Furthermore, the use of WLi or PRi to regulate anesthesia depth was not associated with any increases in intraoperative unwanted events or postoperative adverse events or any negative impacts on postoperative pain scores. Taken together, our data suggest that monitoring of WLi or PRi is an effective and safe method of regulating the depth of anesthesia and may have advantages over conventional monitoring of clinical signs.
Patients scheduled for lumbar spine surgery were selected for this study because the diagnosis of lumbar spinal stenosis or disc herniation can be made reliably and the surgical procedure is standardized, thereby minimizing variability among patients. Sevoflurane anesthesia was used because it has several advantageous characteristics: (1) It exerts dose-dependent sedative, analgesic, muscle relaxant, and autonomic reflex inhibitory actions; (2) It can be used to induce anesthesia; (3) It can be precisely controlled; (4) Recovery is rapid; and (5) It has a small impact on the cardiovascular system and fewer adverse effects on other organ systems compared with certain other general anesthetics[19,20].
The most notable findings of our study were that the use of WLi or PRi resulted in more accurate regulation of the depth of anesthesia than when monitoring SBP. This was evidenced by a shorter anesthesia recovery time, shorter extubation time, and lower sevoflurane consumption, with no increase in adverse events or postoperative pain. Previous research demonstrated that WLi correlated well with BIS and could be used to monitor the sedative depth of anesthesia[7-10]. Furthermore, the use of BIS to regulate anesthesia depth has been shown to have benefits over standard monitoring of clinical signs, including lower consumption of anesthetic drug, decreased risk of postoperative nausea and vomiting, and shorter times to extubation, orientation in time and place, and discharge from the operating room/postanesthetic care unit[4,5].
Importantly, WLi has several potential advantages over BIS. First, BIS references a patient’s forehead electromyogram signals, which are influenced by the degree of muscle relaxation[21-25]. Zhang et al[26] found that WLi was able to accurately reflect the recovery of consciousness in patients under muscle relaxation or with facial paralysis, whereas BIS was unable to reflect the recovery of consciousness with accuracy. This suggests that, unlike BIS, EEG signals extracted by WLi are not disturbed by muscle relaxants and forehead electromyogram signals. Second, BIS cannot regulate the sedation depth of anesthesia in real time because the EEG signals acquired by a BIS monitor have a delay of about 30 s. In contrast, Zikov et al[27] showed that WLi could reflect a patient’s EEG signals in real time. Third, BIS requires the use of special electrodes that are expensive, whereas WLi uses standard electrodes, which could reduce medical costs and the financial burden on the patient. However, the WLi has many instantaneous changes with large numerical changes leading to certain limitations in monitoring the depth of anesthesia. Besides, WLi was developed by Chinese researchers and mainly used in China. Meanwhile, the BIS was the only cerebral electrical parameter approved by the United States Food and Drug Administration for monitoring sedation depth of anesthesia and is more widely applied than the WLi.
Pain management is an important component of anesthesia for surgery. The sensation of pain during surgery is based on consciousness. During general anesthesia, the patient loses consciousness, and “pain” is mainly manifested as a nociceptive stress response. Thus, from the perspective of pain, the regulation of anesthesia depth essentially involves a balance between nociceptive stimulation during surgery and the antinociceptive effects of anesthesia. Accurate regulation of analgesia depth during general anesthesia can help guide the rational use of analgesic drugs and improve the quality of anesthesia. In recent years, the development of the surgical stress index[16,17,28-31], tip perfusion index[32-35], and analgesia nociception index[36-40] have greatly improved the regulation of analgesia depth. Nevertheless, the clinical application of these indexes is limited due to various complex factors. This study showed that compared with the SBP group, the WLi and PRi groups showed a faster recovery from anesthesia, a shorter time to extubation, and lower consumption of sevoflurane without compromising analgesia. Specifically, postoperative VAS scores for pain were not significantly different among the three groups, and there were also no differences in intraoperative unwanted events or postoperative adverse events. These data suggest that monitoring of PRi or WLi represents a safe and effective method for regulating the depth of sevoflurane anesthesia.
A notable observation of this study was that many of the patients in all three groups experienced a rise in blood pressure during vertebral plate exposure or spinal canal decompression. Although the incidence of hypertension did not differ significantly among the three groups, nicardipine was used significantly more often in the WLi and PRi groups than in the SBP group. A likely reason for this apparent discrepancy is that hypertension in the SBP group was often considered as a deficiency in sedation or analgesia and was managed by increasing the sevoflurane Cs, whereas hypertension in the WLi and PRi groups was controlled by nicardipine because the patients were considered to be at a suitable level of sedation (WLi 40-60) or analgesia (PRi 50-70). Thus, another potential advantage of monitoring WLi or PRi is that it may help identify episodes of hypertension that are not related to the level of anesthesia, thereby avoiding unnecessary increases in sevoflurane Cs and excessive use of general anesthetic agent. Nonetheless, it cannot be excluded that the administration of nicardipine may have affected the calculation of WLi or PRi values. Therefore, the effect of vasoactive drugs on WLi or PRi values should be examined in a future study.
This study has some limitations. First, this was a single center study with a small sample size, so the generalizability of the findings is unknown. Second, only patients undergoing lumbar surgery were included, and sevoflurane was the only general anesthetic used, so additional research is needed to explore the utility of WLi and PRi in patients undergoing other surgical procedures or administered other general anesthetics. Third, WLi and PRi values show some variability, and this may limit their ability to guide the regulation of anesthesia. Fourth, WLi and PRi values are susceptible to interference from the electrotome and postural changes. Fifth, the MAC was not measured in all patients and could not be analyzed. Sixth, even if all three groups showed similar occurrence of high SBP during surgery, the use of nicardipine was significantly different, which could have been influenced by the experience and unblinded state of the anesthesiologist. Seventh, no other index such as the BIS or the NarcoTrend index were used for comparison. Additional research is needed to refine the use of WLi and PRi in the regulation of anesthesia depth.
Monitoring of WLi or PRi to regulate the depth of sevoflurane anesthesia in patients undergoing lumbar surgery results in quicker recovery from anesthesia, shorter time to extubation, and lower consumption of sevoflurane as compared with monitoring of clinical signs. In addition, the use of WLi or PRi to regulate anesthesia depth is not associated with increases in intraoperative unwanted events or postoperative adverse events or negative impacts on postoperative pain scores. Therefore, monitoring of WLi and PRi may be an effective and safe technique for regulating the depth of sevoflurane anesthesia.
The wavelet index (WLi) and pain rating index (PRi) are new parameters developed by Chinese researchers that could be used to regulate depth of general anesthesia based on wavelet analysis.
The WLi and the PRi are currently understudied, although they could be more effective in regulating the depth of general anesthesia compared with standard monitoring of systolic blood pressure (SBP).
To investigate the safety and efficacy of using WLi and PRi in sevoflurane-based general anesthesia.
Patients scheduled for elective posterior lumbar interbody fusion surgery under sevoflurane anesthesia were assigned to the SBP, WLi, and PRi monitoring groups. The primary endpoint was anesthesia recovery time. Secondary endpoints included extubation time, sevoflurane consumption, number of unwanted events/interventions, number of adverse events, and postoperative visual analogue scale for pain.
The SBP, WLi, and PRi groups included 21, 21, and 20 patients, respectively. Anesthesia recovery time was shorter in the WLi and PRi groups than in the SBP group with no significant difference between the WLi and PRi groups. Extubation time was shorter in the WLi and PRi groups than in the SBP group. Sevoflurane consumption was lower in the WLi and PRi groups than in the SBP group. Nicardipine was more commonly needed to treat hypertension in the WLi and PRi groups than in the SBP group.
Regulating sevoflurane anesthesia depth with WLi or PRi reduced anesthesia recovery time, extubation time, and sevoflurane consumption without intraoperative unwanted events.
Large multicenter studies are needed to improve the generalizability of the current findings. Additional research is warranted to explore the utility of WLi and PRi in patients undergoing other surgical procedures or other administered general anesthetics. Further studies should be performed to reduce the susceptibility of WLi and PRi values to interference from the electrotome and postural changes. Such studies might promote the widespread use of these tools in the future.
The authors acknowledge and thank the anesthesiologists, surgeons, theatre staff, and recovery room staff of the Shanxi Dayi Hospital, Taiyuan, Shanxi, China for their assistance in this trial. The authors also thank Yongliang Feng, MD and Ruifeng Liang, MD of the Shanxi Medical University, Taiyuan, Shanxi, China for assistance with statistical analysis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nag DS S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wang LL
| 1. | Brown EN, Pavone KJ, Naranjo M. Multimodal General Anesthesia: Theory and Practice. Anesth Analg. 2018;127:1246-1258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 282] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 2. | Rani DD, Harsoor S. Depth of general anaesthesia monitors. Indian J Anaesth. 2012;56:437-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Kreuzer M. EEG Based Monitoring of General Anesthesia: Taking the Next Steps. Front Comput Neurosci. 2017;11:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Oliveira CR, Bernardo WM, Nunes VM. Benefit of general anesthesia monitored by bispectral index compared with monitoring guided only by clinical parameters. Systematic review and meta-analysis. Braz J Anesthesiol. 2017;67:72-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Park SW, Lee H, Ahn H. Bispectral Index Versus Standard Monitoring in Sedation for Endoscopic Procedures: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2016;61:814-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | March PA, Muir WW. Bispectral analysis of the electroencephalogram: a review of its development and use in anesthesia. Vet Anaesth Analg. 2005;32:241-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Xia C, Yue Y. Comparative investigation of wavelet index and bispectral index in monitoring depth of anesthesia. Linchuang Mzuixue Zazhi. 2010;26:836-839. |
| 8. | Yang N, Ge MF, Wang TL, Wu XG. [Feasibility analysis of wavelet index for monitoring the depth of anesthesia in patients undergoing general anesthesia]. Zhonghua Yi Xue Za Zhi. 2011;91:2849-2852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Zhang X, Xiong W, Wang B. Comparative investigation of the accuracy of wavelet index and bispectral index in mornitoring sedation depth of propofol. Zhongguo Yikan Zazhi. 2010;45:59-61. [DOI] [Full Text] |
| 10. | Xu L, Li Z, Tian G. Evaluation of wavelet index in monitoring sedation depth with dexmedetomidine under combined spinal-epidural anesthesia. Linchuang Mazuixue Zazhi. 2018;34:1202-1204. |
| 11. | Gramatikov B, Georgiev I. Wavelets as alternative to short-time Fourier transform in signal-averaged electrocardiography. Med Biol Eng Comput. 1995;33:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1118] [Cited by in RCA: 1193] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 13. | Morton DL, Sandhu JS, Jones AK. Brain imaging of pain: state of the art. J Pain Res. 2016;9:613-624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 14. | Ploner M, Sorg C, Gross J. Brain Rhythms of Pain. Trends Cogn Sci. 2017;21:100-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 278] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 15. | Dion P. The cost of anaesthetic vapours. Can J Anaesth. 1992;39:633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Won YJ, Lim BG, Lee SH, Park S, Kim H, Lee IO, Kong MH. Comparison of relative oxycodone consumption in surgical pleth index-guided analgesia vs conventional analgesia during sevoflurane anesthesia: A randomized controlled trial. Medicine (Baltimore). 2016;95:e4743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Chen X, Thee C, Gruenewald M, Wnent J, Illies C, Hoecker J, Hanss R, Steinfath M, Bein B. Comparison of surgical stress index-guided analgesia with standard clinical practice during routine general anesthesia: a pilot study. Anesthesiology. 2010;112:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Gan TJ, Glass PS, Windsor A, Payne F, Rosow C, Sebel P, Manberg P. Bispectral index monitoring allows faster emergence and improved recovery from propofol, alfentanil, and nitrous oxide anesthesia. BIS Utility Study Group. Anesthesiology. 1997;87:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 398] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 19. | Brioni JD, Varughese S, Ahmed R, Bein B. A clinical review of inhalation anesthesia with sevoflurane: from early research to emerging topics. J Anesth. 2017;31:764-778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 20. | De Hert S, Moerman A. Sevoflurane. F1000Res. 2015;4:626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Messner M, Beese U, Romstöck J, Dinkel M, Tschaikowsky K. The bispectral index declines during neuromuscular block in fully awake persons. Anesth Analg. 2003;97:488-491, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 161] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Nasraway SA SA Jr, Wu EC, Kelleher RM, Yasuda CM, Donnelly AM. How reliable is the Bispectral Index in critically ill patients? Crit Care Med. 2002;30:1483-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Vivien B, Di Maria S, Ouattara A, Langeron O, Coriat P, Riou B. Overestimation of Bispectral Index in sedated intensive care unit patients revealed by administration of muscle relaxant. Anesthesiology. 2003;99:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 192] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 24. | Dahaba AA, Mattweber M, Fuchs A, Zenz W, Rehak PH, List WF, Metzler H. The effect of different stages of neuromuscular block on the bispectral index and the bispectral index-XP under remifentanil/propofol anesthesia. Anesth Analg. 2004;99:781-787, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Panousis P, Heller AR, Burghardt M, Bleyl JU, Koch T. The effects of electromyographic activity on the accuracy of the Narcotrend monitor compared with the Bispectral Index during combined anaesthesia. Anaesthesia. 2007;62:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Zhang XT, Cheng H, Xiong W, Wang BG. Comparison of the ability of wavelet index and bispectral index for reflecting regain of consciousness in patients undergone surgery. Chin Med J (Engl). 2010;123:1520-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Zikov T, Bibian S, Dumont GA, Huzmezan M, Ries CR. Quantifying cortical activity during general anesthesia using wavelet analysis. IEEE Trans Biomed Eng. 2006;53:617-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Bonhomme V, Uutela K, Hans G, Maquoi I, Born JD, Brichant JF, Lamy M, Hans P. Comparison of the surgical Pleth Indexâ„¢ with haemodynamic variables to assess nociception-anti-nociception balance during general anaesthesia. Br J Anaesth. 2011;106:101-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Bergmann I, Göhner A, Crozier TA, Hesjedal B, Wiese CH, Popov AF, Bauer M, Hinz JM. Surgical pleth index-guided remifentanil administration reduces remifentanil and propofol consumption and shortens recovery times in outpatient anaesthesia. Br J Anaesth. 2013;110:622-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 30. | Höcker J, Broch O, Gräsner JT, Gruenewald M, Ilies C, Steinfath M, Bein B. Surgical stress index in response to pacemaker stimulation or atropine. Br J Anaesth. 2010;105:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Ledowski T, Burke J, Hruby J. Surgical pleth index: prediction of postoperative pain and influence of arousal. Br J Anaesth. 2016;117:371-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 32. | Nilsson L, Johansson A, Kalman S. Respiration can be monitored by photoplethysmography with high sensitivity and specificity regardless of anaesthesia and ventilatory mode. Acta Anaesthesiol Scand. 2005;49:1157-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Mowafi HA. The efficacy of plethysmographic pulse wave amplitude as an indicator for intravascular injection of epinephrine-containing epidural test dose in anesthetized adults. Anesth Analg. 2005;101:1506-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Klodell CT, Lobato EB, Willert JL, Gravenstein N. Oximetry-derived perfusion index for intraoperative identification of successful thoracic sympathectomy. Ann Thorac Surg. 2005;80:467-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Yamazaki H, Nishiyama J, Suzuki T. Use of perfusion index from pulse oximetry to determine efficacy of stellate ganglion block. Local Reg Anesth. 2012;5:9-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Gruenewald M, Ilies C, Herz J, Schoenherr T, Fudickar A, Höcker J, Bein B. Influence of nociceptive stimulation on analgesia nociception index (ANI) during propofol-remifentanil anaesthesia. Br J Anaesth. 2013;110:1024-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 37. | Boselli E, Daniela-Ionescu M, Bégou G, Bouvet L, Dabouz R, Magnin C, Allaouchiche B. Prospective observational study of the non-invasive assessment of immediate postoperative pain using the analgesia/nociception index (ANI). Br J Anaesth. 2013;111:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 38. | Boselli E, Bouvet L, Bégou G, Dabouz R, Davidson J, Deloste JY, Rahali N, Zadam A, Allaouchiche B. Prediction of immediate postoperative pain using the analgesia/nociception index: a prospective observational study. Br J Anaesth. 2014;112:715-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 39. | Szental JA, Webb A, Weeraratne C, Campbell A, Sivakumar H, Leong S. Postoperative pain after laparoscopic cholecystectomy is not reduced by intraoperative analgesia guided by analgesia nociception index (ANI®) monitoring: a randomized clinical trial. Br J Anaesth. 2015;114:640-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Turan G, Ar AY, Kuplay YY, Demiroluk O, Gazi M, Akgun N, Celikoglu E. [Analgesia Nociception Index for perioperative analgesia monitoring in spinal surgery]. Rev Bras Anestesiol. 2017;67:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |