Published online Nov 6, 2020. doi: 10.12998/wjcc.v8.i21.5172
Peer-review started: June 28, 2020
First decision: July 24, 2020
Revised: August 3, 2020
Accepted: September 25, 2020
Article in press: September 25, 2020
Published online: November 6, 2020
Processing time: 130 Days and 23.3 Hours
Previous reports have demonstrated that S-1 has remarkable effects in the maintenance treatment of advanced non-small-cell lung cancer (NSCLC), and has less toxic and side effects than conventional drugs.
To investigate the efficacy and safety of S-1 maintenance therapy in patients with advanced NSCLC.
Ninety-four patients with NSCLC admitted to our hospital from September 2015 to April 2018 were included in the study and divided into the S-1 group (47 cases) and the gemcitabine group (47 cases) by random digital table method. The S-1 group was treated with S-1, while the gemcitabine group received gemcitabine treatment. The clinical efficacy and quality of life of the patients after treatment in the two groups were evaluated.
There was no significant difference in the total effectiveness rate between the two groups (P = 0.519). The quality-of-life scores indicated that there was no significant difference between the two groups in terms of four dimensions of the GQOLI-74 questionnaire (P = 0.518, 0.094, 0.338, 0.418). The incidence of nausea and vomiting, granulocytopenia and diarrhea in the S-1 group was significantly lower than that in the gemcitabine group (P = 0.001, 0.001 and 0.001, respectively). There was no significant difference in the incidence of thrombocytopenia (P = 0.366), the progression-free survival (P = 0.064), and the survival between the two groups (P = 0.050).
S-1 maintenance therapy shows a significant therapeutic effect in patients with advanced NSCLC. It has the same clinical efficacy as gemcitabine, but with less toxic and side effects than conventional drugs.
Core Tip: A comparative study was conducted to observe the efficacy and safety of S-1 maintenance therapy in advanced non-small-cell lung cancer patients. S-1 maintenance therapy shows a significant therapeutic effect in patients with advanced non-small-cell lung cancer. It has the same clinical efficacy as gemcitabine, but with less toxic and side effects, and is more effective and safer than conventional drugs.
- Citation: Cheng XW, Leng WH, Mu CL. Efficacy and safety of S-1 maintenance therapy in advanced non-small-cell lung cancer patients. World J Clin Cases 2020; 8(21): 5172-5179
- URL: https://www.wjgnet.com/2307-8960/full/v8/i21/5172.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i21.5172
Lung cancer is one of the major fatal diseases in China, and the incidence and mortality of lung cancer have been on the rise over the last 50 years. Epidemiological statistics show that the mortality and morbidity of lung cancer in men are higher than in women, and the incidence of lung cancer in cities is lower than in rural areas[1]. Lung cancer is known to be related to long-term smoking, air pollution, and other factors, but its specific pathogenesis has still not been determined.
Non-small-cell lung cancer (NSCLC), the main type of lung cancer, accounts for up to 75%-80% of the total number of lung cancer, and NSCLC patients without symptoms at an early stage or without typical symptoms can hardly be diagnosed[2]. Although radical resection has an excellent therapeutic effect in the early-stage and middle-stage NSCLCs, a large number of NSCLC patients still need to receive other treatments because they have missed the opportunity of radical resection.
Chemotherapy is the predominant treatment for advanced NSCLC patients. Cisplatin, paclitaxel, and other commonly applied drugs can control the progress of the disease to a certain extent. After chemotherapy, drugs like gemcitabine and docetaxel are usually used to treat advanced NSCLC patients to improve their quality of life and extend their survival time[3]. In recent years, some reports have shown that S-1 has remarkable effects in the maintenance treatment of advanced NSCLC with less toxic and side effects than conventional drugs[4,5]. To verify this finding, a comparative study was conducted to observe the efficacy and safety of S-1 maintenance therapy in advanced NSCLC patients.
Ninety-four patients with NSCLC admitted to our hospital from September 2015 to April 2018 were included, who all met the diagnostic criteria for NSCLC in Clinical Guidelines for the Diagnosis and Treatment of Lung Cancer by the Chinese Medical Association (2018 Edition). Inclusion criteria: (1) Patients with tumor-node-metastasis (TNM) staging at phase IIIB-IV stage; (2) Patients with stable conditions after 4 cycles of combined chemotherapy with platinum-containing drugs; (3) Patients with no heart failure, respiratory failure, and other serious diseases; (4) Patients who were informed of the medication program and volunteered to participate; and (5) Patients with consciousness, who were willing to complete quality of life questionnaire. Exclusion criteria: (1) Patients who withdrew from the treatment plan halfway and were transferred to another hospital; (2) Patients would die 3 mo later according to pretreatment estimation; and (3) Patients with incomplete general data. As shown in Table 1, there was no significant difference in the clinical data between the two groups of patients. The study was approved by the Ethics Committee of our hospital.
| Clinical information | S-1 group (n = 47) | Gemcitabine group (n = 47) | χ2/t | P value |
| Gender | 0.092 | 0.761 | ||
| Male | 27 (57.45) | 26 (55.32) | ||
| Female | 20 (42.55) | 21 (44.68) | ||
| Types | 0.361 | 0.548 | ||
| Squamous carcinoma | 23 (48.94) | 25 (53.19) | ||
| Adenocarcinoma | 24 (51.06) | 22 (46.81) | ||
| TNM staging | 0.130 | 0.719 | ||
| Phase IIIB | 36 (76.60) | 37 (78.72) | ||
| Phase IV | 11 (23.40) | 10 (21.28) | ||
| Age (yr) | 56.95 (4.15) | 56.17 (4.01) | 0.927 | 0.357 |
| Weight (kg) | 53.94 (3.52) | 54.09 (3.61) | 0.204 | 0.839 |
| BMI (kg/m2) | 21.14 (3.21) | 21.51 (3.45) | 0.538 | 0.592 |
The drug was purchased from Broad Medicine Huangshi Feiyun Pharmaceutical Co., Ltd., Chinese Medicine Approval No. H20133194. It was delivered by intravenous drip at a dose of 1000 mg/m2 on d 1 and d 8. The regimen was repeated every 21 d until the patient's disease progressed.
The drug was purchased from Fuzhou Haiwangfu Pharmaceutical Co., Ltd., Chinese Medicine Approval No. H20140020. The drug was delivered orally at a dose of 80 mg/m2 per day on d 1 to d 14. The regimen was repeated every 21 d for 1 cycle until the patient's disease progressed.
Curative effect evaluation: Complete remission: The patient's target lesion and clinical symptoms basically disappeared. Remission: The target lesion reduced by ≥ 30%, and the clinical symptoms improved significantly after treatment. Controlled: The target lesion reduced by < 30% or by < 20% after treatment. Progressed: New lesions appeared, the total diameter of the lesion increased by ≥ 20%, and the total effectiveness rate of treatment = (total remission cases + remission cases)/total number of cases × 100%.
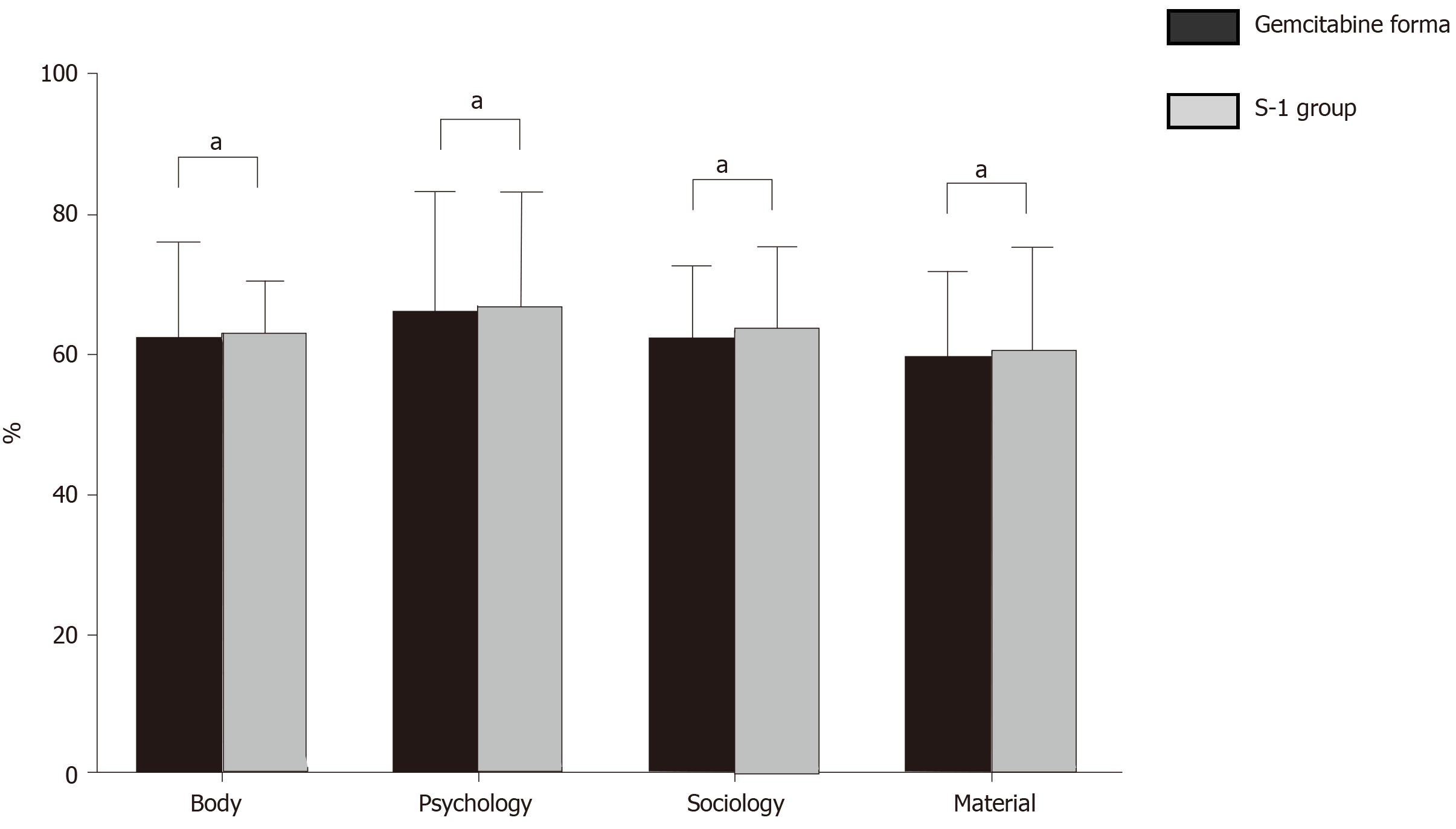
The life quality of patients was assessed by the GQOLI-74 questionnaire, which covered four dimensions, namely, material, society psychology and body. A higher score indicated the higher quality of life.
The incidence of toxic and side effects such as thrombocytopenia, nausea and vomiting, granulocytopenia, and diarrhea in the two groups during the treatment period was analyzed.
The patients were followed up, whose progression-free survival and survival were analyzed. The progression-free survival was defined as the period from the startof maintenance treatment to the cease of disease progression, and the survival was defined as the period from the start of maintenance treatment to death.
The observation data such as quality-of-life score and incidence of toxic and side effects were analyzed and processed using the SPSS statistical software. The measurement data (± s) were subjected to independent sample t test if the normal distribution was satisfied, and the non-normal data indicated count data with Median (IQR) and percentage (%) and were subjected to χ2 test. P < 0.05 indicated significant differences between groups.
There was no significant difference in the total effectiveness rate of treatment between the S-1 group and the gemcitabine group (P = 0.519) (Table 2).
| Curative effect | S-1 group (n = 47) | Gemcitabine group (n = 47) | χ2/t | P value |
| Complete remission | 3 (6.38) | 2 (4.26) | 0.446 | 0.504 |
| Remission | 13 (27.66) | 12 (25.53) | 0.116 | 0.733 |
| Controlled | 17 (36.17) | 18 (38.30) | 0.097 | 0.755 |
| Progressed | 14 (29.79) | 15 (31.91) | 0.105 | 0.746 |
| Total effective rate of treatment | 16 (34.04) | 14 (29.79) | 0.416 | 0.519 |
There was no significant difference in quality-of-life scores in multiple dimensions between the two groups (P = 0.518, 0.094, 0.338, 0.418) (Table 3 and Figure 1).
| GQOLI-74 score (point) | S-1 group (n = 47) | Gemcitabine group (n = 47) | t | P value |
| Material | 60.53 (4.31) | 59.97 (4.06) | 0.648 | 0.518 |
| Society | 63.84 (3.54) | 62.59 (3.61) | 1.695 | 0.094 |
| Psychology | 67.14 (3.68) | 66.42 (3.56) | 0.964 | 0.338 |
| Body | 63.12 (3.97) | 62.47 (3.78) | 0.813 | 0.418 |
There was no significant difference in progression-free survival and survival between the two groups (P = 0.064, 0.050) (Table 4).
| Follow-up results | S-1 group (n = 47) | Gemcitabine group (n = 47) | t | P value |
| Progress-free survival (mo) | 6.63 (1.02) | 6.25 (0.94) | 1.878 | 0.064 |
| Survival (mo) | 13.63 (1.52) | 13.02 (1.45) | 1.991 | 0.050 |
The incidence of granulocytopenia, nausea, and vomiting, and diarrhea in the S-1 group was lower than that in the gemcitabine group (P = 0.001, 0.001, 0.001), and there was no significant difference in the incidence of thrombocytopenia between the two groups (P = 0.366) (Table 5).
| Toxic and side effects | S-1 group (n = 47) | Gemcitabine group (n = 47) | χ2 | P value |
| Granulocytopenia | 26 (55.32) | 38 (80.85) | 14.998 | 0.001 |
| Nausea and vomiting | 20 (42.55) | 33 (70.21) | 15.555 | 0.001 |
| Diarrhea | 8 (17.02) | 23 (48.94) | 23.048 | 0.001 |
| Thrombocytopenia | 23 (48.94) | 26 (55.32) | 0.816 | 0.366 |
Lung cancer is one of the most fatal and malignant tumors worldwide. With the development of modern industrial society, the air quality continues to deteriorate, and the incidence of lung cancer is increasing year by year. Lung cancers can be divided into the peripheral type and the central type according to the anatomical site, squamous cell carcinoma and adenocarcinoma according to histological classification, and small cell lung cancer (SCLC) and NSCLC according to clinical characteristics[6]. The last classifying method is common in clinical diagnosis and treatment. NSCLC is the main type of lung cancer, with a high mortality rate, and its diagnosis and treatment have been the research focus[7].
Clinical treatment of NSCLC mainly includes surgery, chemotherapy, and radiotherapy. The surgical treatment is to control the progress of the disease which is suitable for patients with stage I to II lung cancer. However, as NSCLC cannot be diagnosed due to absence of symptoms or specific symptoms observed in the early stage, the disease generally progresses to the middle and late stages, and radical surgery can hardly achieve satisfactory outcome[8].
Chemotherapy is the most common treatment for advanced NSCLC. Since the 1990s, the combination of two chemotherapeutic drugs containing platinum has been adopted as the first-line standard chemotherapy regimen to control the rapid growth of tumors and prolong the survival time of patients[9-13]. According to a clinical survey, the current mainstream chemotherapy regimen resulted in a clinical remission rate of about 15 to 36%, the survival time of patients who have received chemotherapy is only 8 to 10 mo, and less than 40% of patients have a survival period of more than one year. Maintenance therapy aims to strengthen the effect of chemotherapy, prolong life, and improve the quality of life of patients after chemotherapy[14].
Currently, drugs for maintenance treatment in patients with NSCLC include gemcitabine, docetaxel and gefitinib[15,16]. Gemcitabine belongs to a cytosine nucleoside derivative, which has the same action mechanism with arabinoside. Once injected into the body, gemcitabine is converted into arabinoside triphosphate and diphosphate under the activating effect of deoxidized cytosine kinase. Arabinoside triphosphate can significantly inhibit the synthesis of DNA polymerase and thereby prevent the synthesis of cancer cell DNA. In contrast, arabinoside diphosphate can hinder the formation of deoxycytidine diphosphate, thus avoiding the polymerization and synthesis of tumor cell DNA and retarding tumor growth[17]. Clinical studies confirmed that patients having received gemcitabine maintenance had progression-free survival of about 5 to 7 mo and survival of 12 to 15 mo, which further prolonged the survival of patients after chemotherapy[18]. However, gemcitabine also has many toxic and side effects, such as granulocytopenia, and thrombocytopenia.
S-1 was developed by Japanese scholars in 1991 and belongs to oral fluorouracil, with the main components including tegafur, interacial, and gimeracil. Its clinical medicinal mechanism includes three aspects: (1) Tegafur, a prodrug of 5-fluorouracil (5-FU), is converted into 5-FU after intake by the body; and (2) Gimeracil, a dihydropyrimidine dehydrogenation inhibitor, can directly inhibit the catabolism of 5-FU, and prolong the effective concentration of 5-FU in the body after the intake of tegafur, thus having an effect similar to that of continuous intravenous drip[19,20].
In the present study, the clinical efficacy, quality of life, survival and progression-free survival of the gemcitabine group were similar to those of the S-1 group, indicating that S-1 had the same effect as gemcitabine in treating advanced NSCLC. However, the S-1 group had significantly lower incidence of granulocytopenia, nausea and vomiting and diarrhea than the gemcitabine group, suggesting that S-1 was superior to gemcitabine in terms of toxic and side effects and safety. Li Ting compared the clinical efficacy of S-1 with that of gemcitabine in the treatment of advanced pancreatic cancer, and found that the clinical efficacy of the two drugs tended to be consistent, but the incidence of adverse reactions in patients receiving S-1 was lower than that in patients receiving gemcitabine. There was no significant difference in the clinical efficacy, quality of life and progression-free survival between the S-1 group and gemcitabine group, and the S-1 group had lower incidence of adverse reactions such as vomiting and diarrhea than the experimental group.
S-1 has the same curative effect with gemcitabine in the maintenance treatment of patients with advanced NSCLC, but S-1 is less toxic and its application should be preferentially promoted.
Chemotherapy is the predominant treatment for advanced non-small-cell lung cancer (NSCLC) patients. Cisplatin, paclitaxel, and other commonly applied drugs can control the progress of the disease to a certain extent. After the chemotherapy, drugs like gemcitabine and docetaxel are usually used to treat advanced NSCLC patients, so as to improve their quality of life and extend their survival time.
S-1 has remarkable effects in the maintenance treatment of advanced NSCLC with less toxic and side effects than conventional drugs.
To investigate the efficacy and safety of S-1 maintenance therapy in patients with advanced NSCLC.
Ninety-four patients with NSCLC admitted to our hospital from September 2015 to April 2018 were included in the study and divided into the S-1 group (47 cases) and the gemcitabine group (47 cases). S-1 group was treated with S-1, while the gemcitabine group was treated with gemcitabine. The clinical efficacy and quality of life of the patients after treatment in the two groups were evaluated.
There was no significant difference in the total effective rate between the two groups (P = 0.519). The quality-of-life scores indicated that there was no significant difference between the two groups. The incidence of nausea and vomiting, granulocytopenia and diarrhea in the S-1 group was significantly lower than that in the gemcitabine group. There was no significant difference in the incidence of thrombocytopenia (P = 0.366), the progression-free survival (P = 0.064), and the survival between the two groups (P = 0.050).
S-1 maintenance therapy shows a significant therapeutic effect in patients with advanced NSCLC. It has the same clinical efficacy as gemcitabine, but with less toxic and side effects, and is more effective and safer than conventional drugs.
Randomized control trails are required to further validate the effect of S-1 maintenance therapy in patients with advanced NSCLC.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Melo FF, Glyn-Owen K, Hellard M, Willock R S-Editor: MedE-Ma JY, Gao CC L-Editor: A P-Editor: Wang LL
| 1. | Eberhardt WE, De Ruysscher D, Weder W, Le Péchoux C, De Leyn P, Hoffmann H, Westeel V, Stahel R, Felip E, Peters S; Panel Members. 2nd ESMO Consensus Conference in Lung Cancer: locally advanced stage III non-small-cell lung cancer. Ann Oncol. 2015;26:1573-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 291] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 2. | Yu T, Liu L, Li J, Yan M, Lin H, Liu Y, Chu D, Tu H, Gu A, Yao M. MiRNA-10a is upregulated in NSCLC and may promote cancer by targeting PTEN. Oncotarget. 2015;6:30239-30250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 3. | Lv X, Gou F, Shen Y, Lu H, Chen J, Liu J, Chen H, Zhang X, Yu D. Long-term clinical response of advanced lung adenocarcinoma to maintenance treatment of gemcitabine: A case report. Medicine (Baltimore). 2018;97:e13464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Liu D, Xu W, Ding X, Yang Y, Su B, Fei K. Polymorphisms of CCNB1 Associated With the Clinical Outcomes of Platinum-Based Chemotherapy in Chinese NSCLC Patients. J Cancer. 2017;8:3785-3794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Miyanaga A, Kubota K, Hosomi Y, Okuma Y, Minato K, Fujimoto S, Okamoto H, Satouchi M, Isobe H, Aono H, Takiguchi Y, Gemma A; Tokyo Cooperative Oncology Group. Phase II trial of S-1 plus cisplatin combined with bevacizumab for advanced non-squamous non-small cell lung cancer (TCOG LC-1202). Jpn J Clin Oncol. 2019;49:749-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Buergy D, Wenz F. [Consolidative local therapy in oligometastatic NSCLC without progression after first-line chemotherapy]. Strahlenther Onkol. 2017;193:341-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Murakami S. Durvalumab for the treatment of non-small cell lung cancer. Expert Rev Anticancer Ther. 2019;19:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Hou L, Zhou C, Wu Y, Yu Y, Hu Y. Transcutaneous electrical acupoint stimulation (TEAS) relieved cancer-related fatigue in non-small cell lung cancer (NSCLC) patients after chemotherapy. J Thorac Dis. 2017;9:1959-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Luo H, Yu X, Liang N, Xie J, Deng G, Liu Q, Zhang J, Zhang J, Ge H. The effect of induction chemotherapy in patients with locally advanced nonsmall cell lung cancer who received chemoradiotherapy: A systematic review and meta-analysis. Medicine (Baltimore). 2017;96:e6165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Murakami S, Saito H, Kondo T, Oshita F, Yamada K. Phase II study of bevacizumab, cisplatin, and pemetrexed in advanced non-squamous non-small cell lung cancer (NS-NSCLC) with EGFR wild-type. J Exp Ther Oncol. 2019;13:131-138. [PubMed] |
| 11. | You R, Liu J, Wu DB, Qian X, Lyu B, Zhang Y, Luo N. Cost-Effectiveness Analysis Of EGFR Mutation Testing And Afatinib Versus Gemcitabine-Cisplatin As First-Line Therapy For Advanced Non-Small-Cell Lung Cancer In China. Cancer Manag Res. 2019;11:10239-10248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Qi F, Hu X, Liu Y, Wang Z, Duan J, Wang J, Dong M. First-line pemetrexed-platinum doublet chemotherapy with or without bevacizumab in non-squamous non-small cell lung cancer: A real-world propensity score-matched study in China. Chin J Cancer Res. 2019;31:749-758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Fujita T, Kuroki T, Hayama N, Shiraishi Y, Amano H, Nakamura M, Hirano S, Tabeta H, Nakamura S. Pemetrexed Plus Platinum for Patients With Advanced Non-small Cell Lung Cancer and Interstitial Lung Disease. In Vivo. 2019;33:2059-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Seto T, Azuma K, Yamanaka T, Sugawara S, Yoshioka H, Wakuda K, Atagi S, Iwamoto Y, Hayashi H, Okamoto I, Saka H, Mitsuoka S, Fujimoto D, Nishino K, Horiike A, Daga H, Sone T, Yamamoto N, Nakagawa K, Nakanishi Y. Randomized Phase III Study of Continuation Maintenance Bevacizumab With or Without Pemetrexed in Advanced Nonsquamous Non-Small-Cell Lung Cancer: COMPASS (WJOG5610L). J Clin Oncol. 2020;38:793-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Jian H, Li W, Ma Z, Huang J, Feng J, Song Y, Gao B, Zhu H, Tao M, Bai C, Ma S, Pan H, Qin S, Hua D, Yu Y, Lu S. Intercalating and maintenance gefitinib plus chemotherapy vs chemotherapy alone in selected advanced non-small cell lung cancer with unknown EGFR status. Sci Rep. 2017;7:8483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Hu X, Pu K, Feng X, Wen S, Fu X, Guo C, He W. Role of Gemcitabine and Pemetrexed as Maintenance Therapy in Advanced NSCLC: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS One. 2016;11:e0149247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Kim HR, Jang JS, Sun JM, Ahn MJ, Kim DW, Jung I, Lee KH, Kim JH, Lee DH, Kim SW, Cho BC. A randomized, phase II study of gefitinib alone vs nimotuzumab plus gefitinib after platinum-based chemotherapy in advanced non-small cell lung cancer (KCSG LU12-01). Oncotarget. 2017;8:15943-15951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Vesel M, Rapp J, Feller D, Kiss E, Jaromi L, Meggyes M, Miskei G, Duga B, Smuk G, Laszlo T, Karner I, Pongracz JE. ABCB1 and ABCG2 drug transporters are differentially expressed in non-small cell lung cancers (NSCLC) and expression is modified by cisplatin treatment via altered Wnt signaling. Respir Res. 2017;18:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Cheng Z, Xin H, Han T. BECN1 promotes the migration of NSCLC cells through regulating the ubiquitination of Vimentin. Cell Adh Migr. 2019;13:249-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Hu H, Zhou Y, Zhang M, Ding R. Prognostic value of RASSF1A methylation status in non-small cell lung cancer (NSCLC) patients: A meta-analysis of prospective studies. Biomarkers. 2019;24:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |