Published online Nov 6, 2020. doi: 10.12998/wjcc.v8.i21.5070
Peer-review started: April 12, 2020
First decision: July 25, 2020
Revised: August 6, 2020
Accepted: September 28, 2020
Article in press: September 28, 2020
Published online: November 6, 2020
Processing time: 208 Days and 0.6 Hours
Evaluating patients with chronic venous leg ulcers (CVLUs) is essential to find the underlying etiology. The basic tenets in managing CVLUs are to remove the etiological causes, to address systemic and metabolic conditions, to examine the ulcers and artery pulses, and to control wound infection with debridement and eliminating excessive pressure on the wound. The first-line treatments of CVLUs remain wound care, debridement, bed rest with leg elevation, and compression. Evidence to support the efficacy of silver-based dressings in healing CVLUs is unavailable. Hydrogen peroxide is harmful to the growth of granulation tissue in the wound. Surgery options include a high ligation with or without stripping or ablation of the GSVs depending on venous reflux or insufficiency. Yet, not all CVLUs are candidates for surgical treatment because of comorbidities. When standard care of wound for 4 wk failed to heal CVLUs effectively, use of advanced wound care should be considered based on the available evidence. Negative pressure wound therapy facilitates granulation tissue development, thereby helping closure of CVLUs. Autologous split-thickness skin grafting is still the gold standard approach to close huge CVLUs. Hair punch graft appears to have a better result than traditional hairless punch graft for CVLUs. Application of adipose tissue or placenta-derived mesenchymal stem cells is a promising therapy for wound healing. Autologous platelet-rich plasma provides an alternative strategy for surgery for safe and natural healing of the ulcer. The confirmative efficacy of current advanced ulcer therapies needs more robust evidence.
Core Tip: Wound care, debridement, bed rest with leg elevation, and compression are basic therapies for chronic venous leg ulcers (CVLUs). Ablation of the great saphenous veins help heal some ulcers. Negative pressure wound therapy, autologous split-thickness skin grafting, autologous platelet-rich plasma, and administration of adipose tissue or placenta-derived mesenchymal stem cells are effective approaches for wound healing. Hair punch graft appears to have a better result than traditional hairless punch graft for CVLUs. There are little data to support the efficacy of silver-based dressings in the healing of CVLUs. Hydrogen peroxide is harmful to the growth of granulation tissue in CVLUs.
- Citation: Ren SY, Liu YS, Zhu GJ, Liu M, Shi SH, Ren XD, Hao YG, Gao RD. Strategies and challenges in the treatment of chronic venous leg ulcers. World J Clin Cases 2020; 8(21): 5070-5085
- URL: https://www.wjgnet.com/2307-8960/full/v8/i21/5070.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i21.5070
Leg ulcer refers to areas of epidermal discontinuity in lower limbs with causes of venous, arterial, diabetic, pressure, traumatic, allergic, or inflammation (Figure 1). Chronic venous leg ulcers (CVLUs) are defined as leg ulcers persisting for 4 wk or more due to chronic venous insufficiency[1,2] and account for up to 70% of all chronic leg ulcers. CVLUs have an overall prevalence of up to 2% in the general population of western countries with significant morbidity and a negative socioeconomic impact[3-6].
CVLUs are susceptible to microbial invasion and can cause serious complications, such as delayed healing, cellulitis, increasing ulcer size, debilitating pain, and deeper wound infections causing systemic illness[7]. It is critical to assess and manage patients with effective approaches. Yet, there are some controversies on the treatment of patients with CVLUs; therefore, we review in this article the most recent literature on the management of patients with CVLUs to provide valuable and practical information for clinical workers.
A systematic assessment of patient and CVLUs is essential to find the underlying etiology. The basic principles in managing CVLUs are to remove the etiological causes, to evaluate the wound and artery pulses, and to manage systemic conditions and wound infection with debridement, and to reduce excessive pressure on the wound. The first-line treatments of CVLUs are bed rest with leg elevation, wound care, debridement, and compression. Surgical approaches remain a high ligation with or without stripping or ablation of the great saphenous veins (GSVs) depending on venous reflux or insufficiency; not all CVLUs are suitable for surgery due to comorbidities[1,2,8]. Standard wound care should be used for an initial period of 4 wk and can fail to heal approximately 25% of CVLUs. Even advanced therapies do not heal > 60%. Wound failing to heal 50% ulcer area at 4 wk should be reevaluated and then considered for advanced therapies in the absence of underlying disease[3-6]. When standard care of wound for 4 wk failed to heal CVLUs effectively, advanced wound care should be considered to use based on the available evidence. Unfortunately, many advanced approaches for CVLUs do not have strong evidence or a randomized prospective study to evaluate the efficacy.
Most CVLUs are not an isolated disorder but the manifestation of underlying conditions, such as hypertension, diabetes, and peripheral vascular conditions. History of comorbidities should be carefully consulted to make a confirmed diagnosis[3].
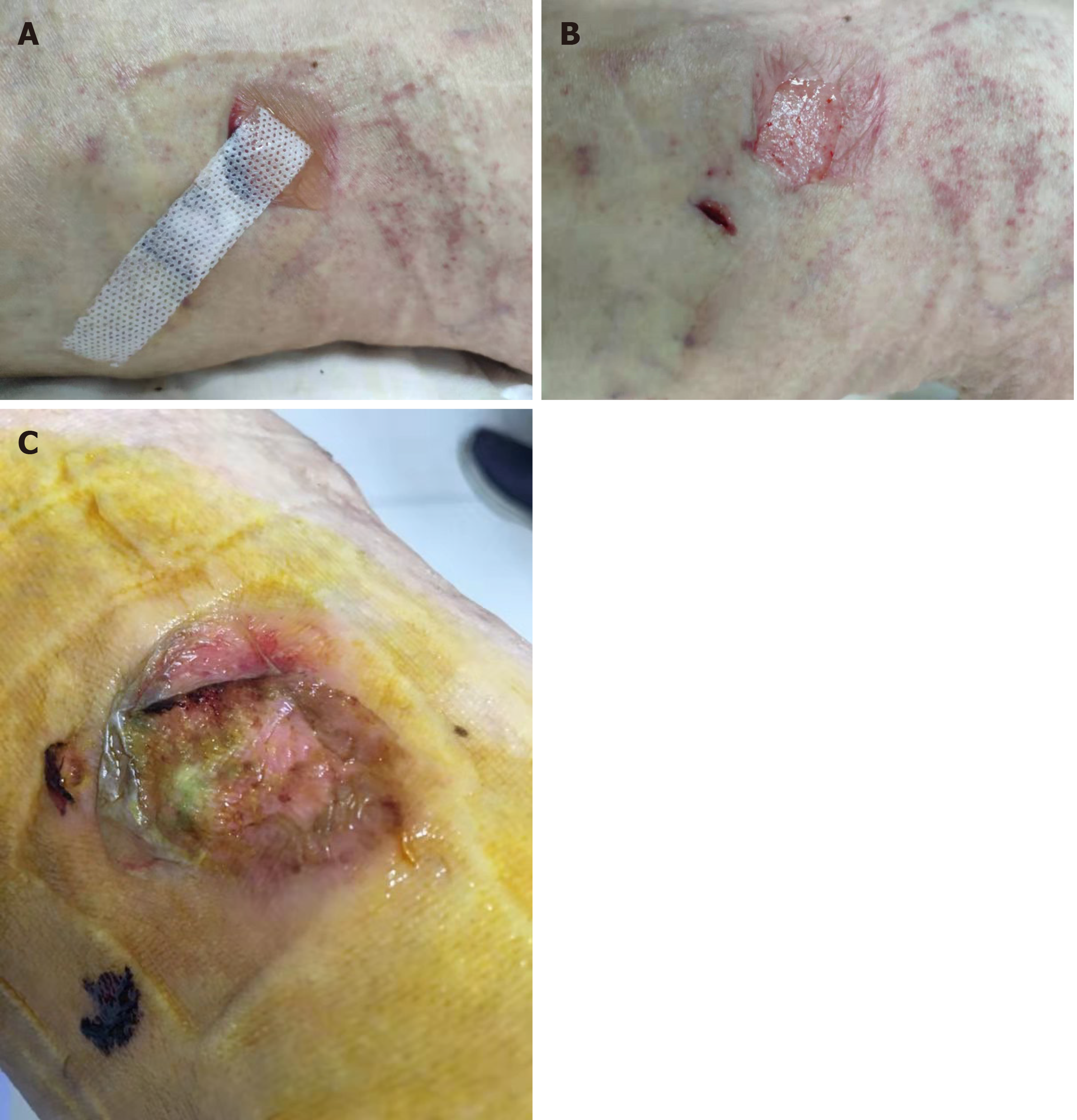
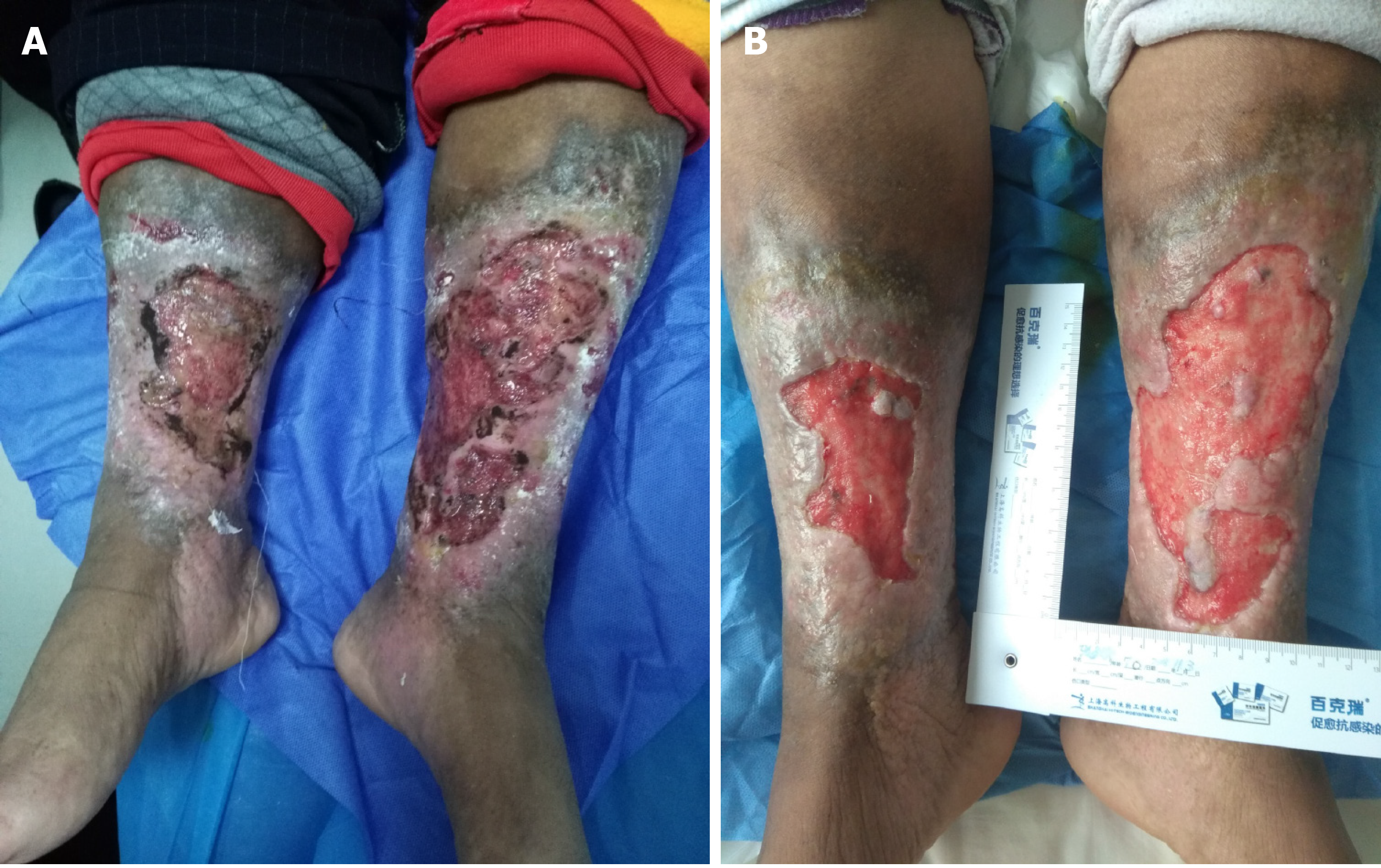
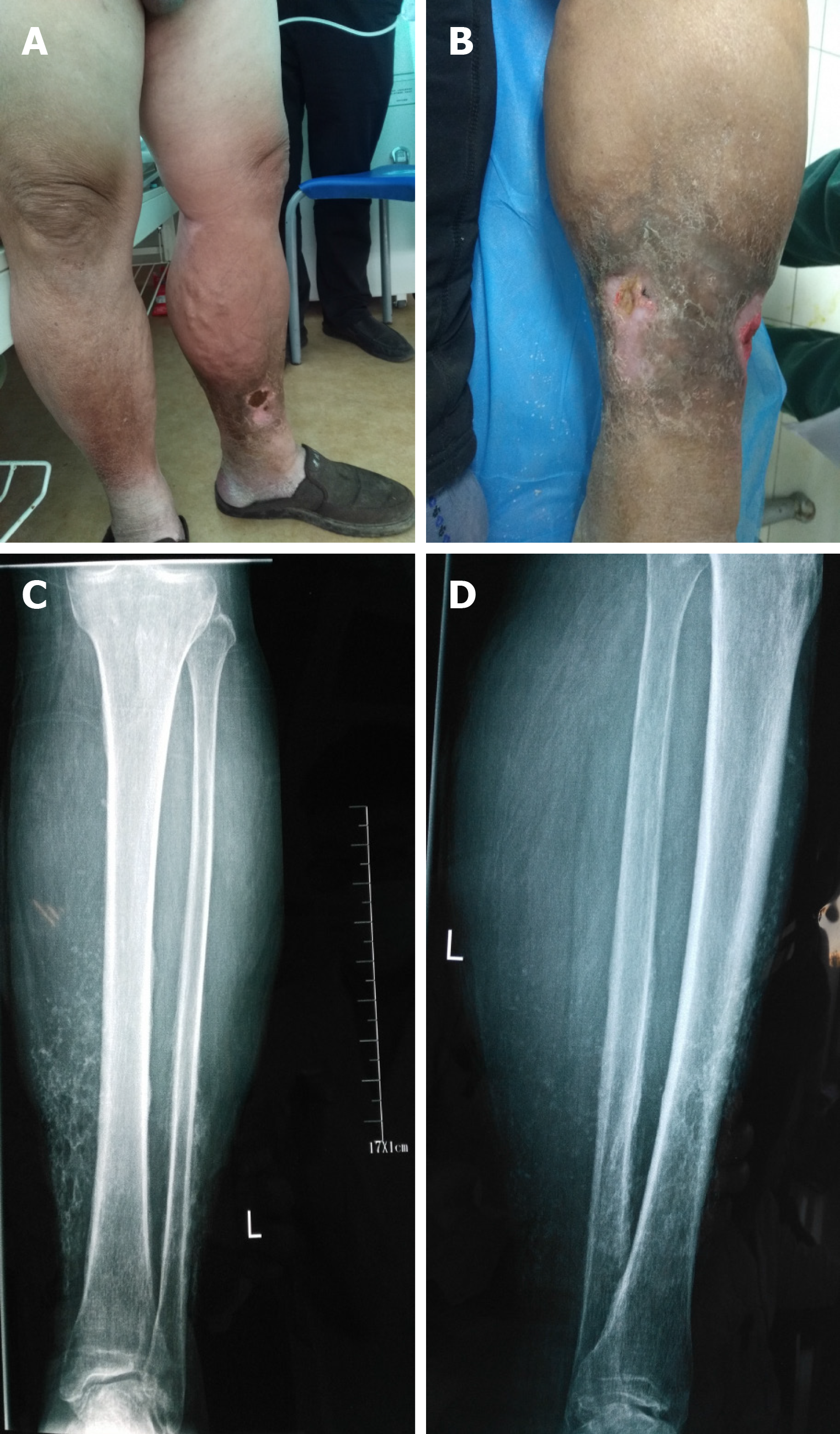
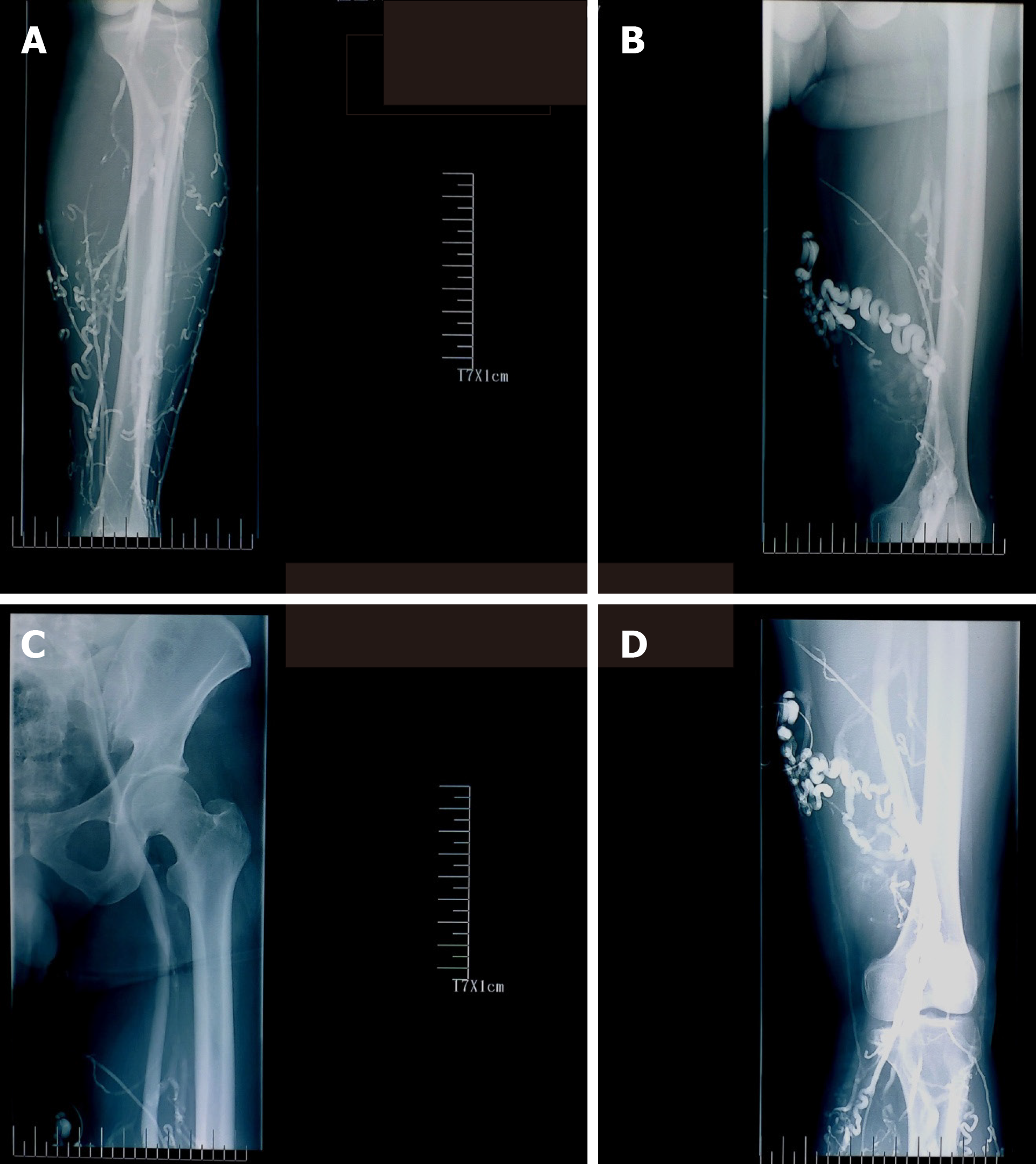
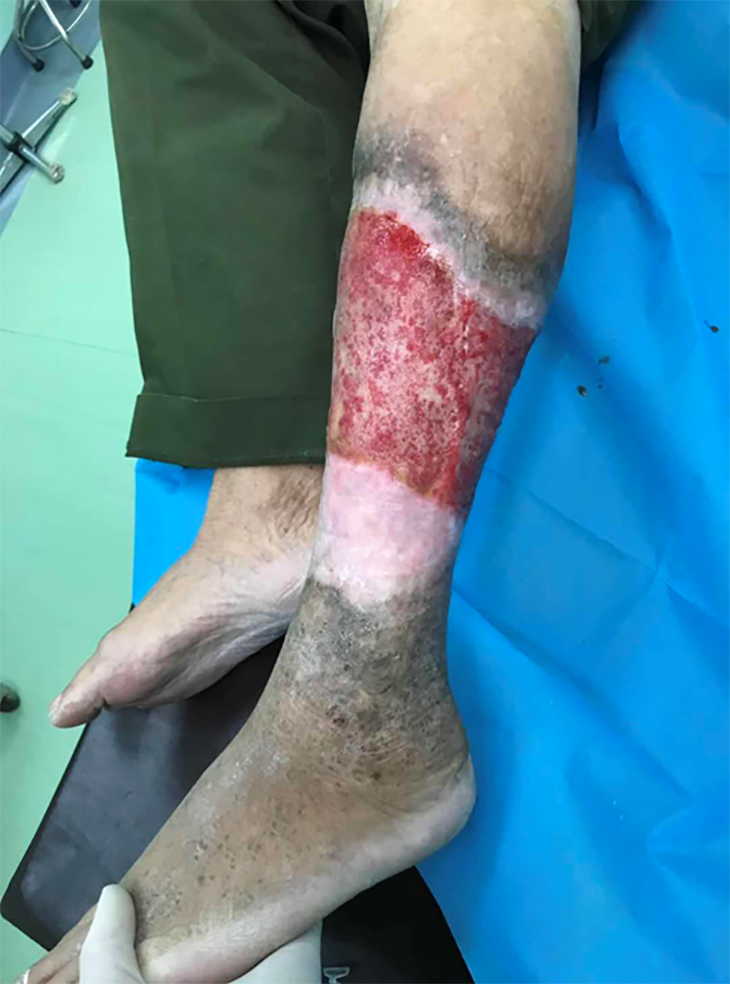
On consulting with a patient, the location, size, and number of ulcers, the color of the exudates, offensive odor from the wound exudates if any, and arterial pulses in the limbs should be examined initially. The survival ability and the extent of skin around the ulcer should be judged, and a photograph should be taken with a ruler adjacent to the ulcers. The offensive odor or increasing pain in a chronic wound usually indicates the presence of ulcer infection (Figure 2). A swab swept from the wound bed is usually cultured to guide the further selection of antibiotics against infection, a negative culture guarantees no bacterial infection in the wound due to the skill of sampling, the process of a sample, or test techniques. A biopsy should be considered for any patients with a history of atypical chronic refractory ulcers to exclude skin cancer (e.g., squamous cell carcinoma)[9] (Figure 3). X-ray film may demonstrate if there is an involvement of the bone or inflammation of tissue underneath or around the ulcers (Figure 4). Patients with leg edema, healed or active ulcers, especially with a history of deep venous thrombosis (DVT), should be examined with duplex ultrasound for iliocaval venous obstruction. Loss of respiratory variation in the common femoral vein or reversed flow in the superficial epigastric vein on duplex ultrasound should be referred for ascending venogram (Figure 5) or computed tomography (CT) to assess the venous insufficiency or valve failure[10-12]. In patients with complicated CLUVs, CT angiography for artery in limbs to confirm the presence and degree of arterial occlusion is a wise choice.
Microbial biofilms in the wound cause non-healing and infection due to their increased resistance to antibiotics. The microbial-host environment and the pathophysiology of venous hypertension in the lower extremities demand a multimodality approach for closing CVLUs. In addition to systemic and topical antibiotics, wound bandages, compression therapy, and wound debridement and restoring blood supply to the wound help sustain the infection and healing the wound[7].
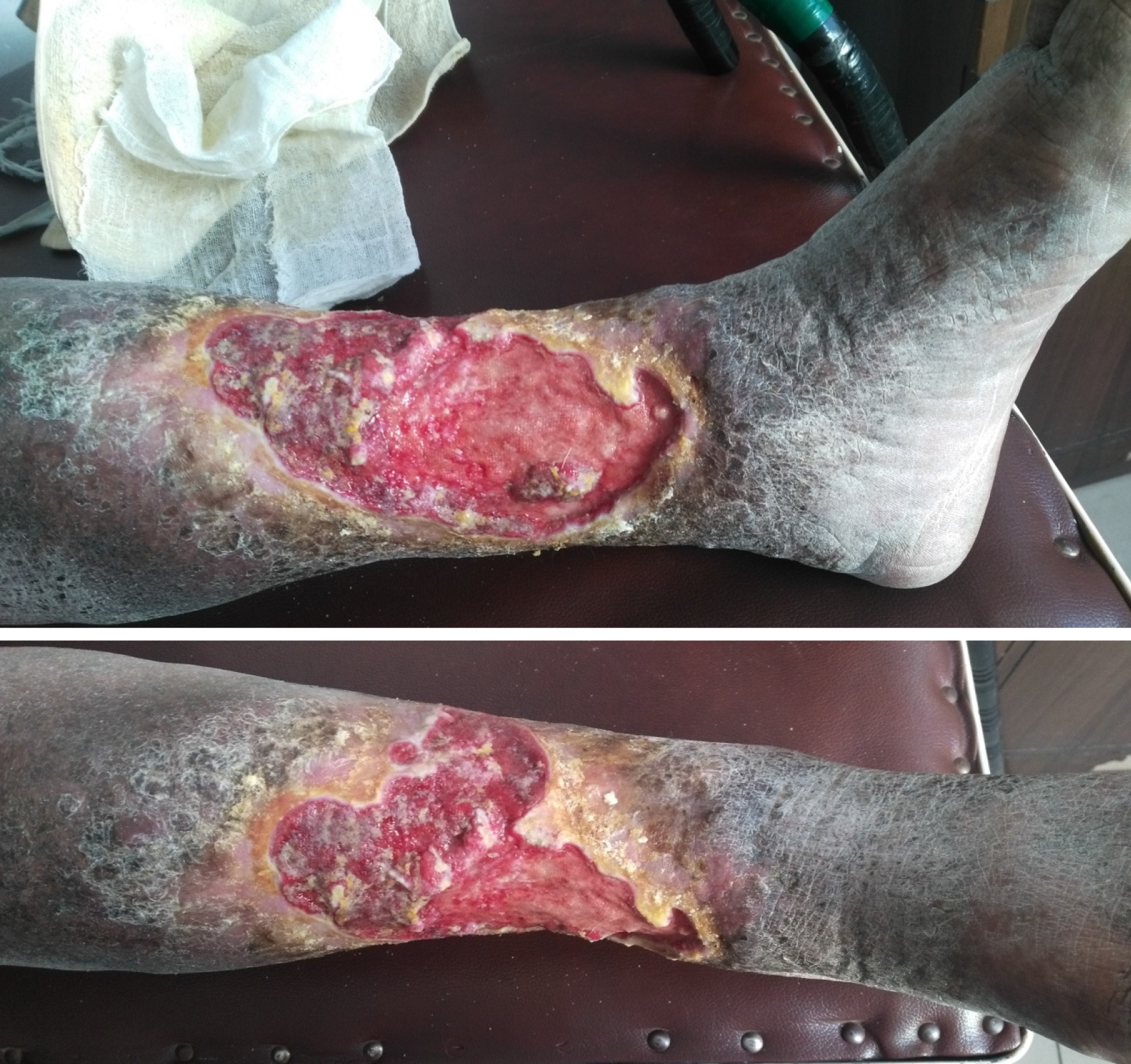
The types of debridement include mechanical debridement, autolytic debridement, and enzymatic debridement[13]. Surgical debridement is performed for extensive devitalized or necrotic tissue, severe infection of skin or cellulitis, infected bone or sepsis that causes the risk of poor wound healing, osteomyelitis, generalized infection, and sepsis. Frequent mechanical debridement of a chronic ulcer is essential to reduce the necrotic burden and achieve healthy granulation[14]. The edge of the granulation tissue looks white and should be preserved instead of being removed (Figure 6), especially in patients with diabetes and peripheral vascular disease.
A variety of dressings for the management of CVLUs include hydrocolloids, hydrogels, alginates, foam, and antimicrobial dressing[15]. Foam dressings containing hydrophilic foam can be used for exuding CVLUs. Alginate dressing is suitable for wound with a cavity or complicated ulcers; when it contacts with exudates, a gel will be formed to facilitate wound rehydration[16]. Hydrogel dressing consists of cross-linked insoluble polymers and is easier to remove than alginate dressing and is suitable for dry wounds[17]. Unfortunately, no robust evidence is available to favor one dressing over another for CVLUs[18,19]. It is worthwhile to remind that selection of wound dressings is sometimes influenced by advertisement instead of evidence[20,21].
A common concern for ulcers is pain on changing dressings or at night that can be relieved with pain killer. Preclinical data support a role for dextrose prolotherapy in promoting tissue repair that is required for healing chronic wounds and ameliorating the associated pain. Five percent dextrose can cause the production of growth factors that have critical roles in repair[4]. Furthermore, numerous clinical trials report pro-reparative effects of dextrose prolotherapy in joint diseases, tendon and ligament damage, and painful musculoskeletal issues[4].
An adequate arterial supply must be ensured initially before compression therapy. A graduated compression bandage (two to four layers) or stocking is the most effective management of CVLUs and should be started as early as possible to relieve the symptoms of venous insufficiency. Stockings are more welcomed than bandages because of their uniform pressure on the skin, comfort, and cosmetic reasons. The patients should be warned to prevent a band or rope-like structure in the edge of the bandage or stockings around the leg to avoid unnecessary injury to the skin. The patients should be educated to walk and elevate the legs if possible. CVLUs heal faster with compression than without[20,21].
The topical use of cadexomer iodine is recommended for CVLUs. The comprehensive use of silver-containing dressings is not substantiated by scientific evidence. The primary use of silver-containing products is to reduce bacterial infection, but no data are available to favor the effect of wound closure[22]. The results of a randomized controlled trial (RCT) demonstrated that a microorganism-binding dressing is more effective than a silver-containing hydrofiber dressing in controlling the bacterial loads of infected CVLUs in terms of bacterial reduction rate (73.1% vs 41.6%, P < 0.00001)[23]. Besides, the role of povidone-iodine, peroxide-containing products for healing CVLUs cannot be concluded until an additional qualified study is performed[22].
Hydrogen peroxide (H2O2) is an endogenous reactive oxygen species that contributes to oxidative stress directly as a molecular oxidant and indirectly through the free radical generation. It is commonly used to irrigate wounds because of its hemostatic and antiseptic properties. However, H2O2 should be used with caution due to its oxidant adverse effects. Low H2O2 concentrations cause only transient symptoms (blanching and blistering), but exposure to 9%-45% H2O2 can cause more severe skin damage, including epidermal necrosis, leading to erythema and bullae, toxicity to keratinocytes and fibroblasts, oxygen embolism demonstrated intracranial air trapping in the right frontal lobe, and multiple cerebral infarction foci in postoperative computed tomography[24,25].
Micronized purified flavonoid fraction (MPFF), pentoxifylline, and sulfoxide are reported to improve CVLU healing and recommended as an effective adjunct to compression therapy for CVLUs[26,27]. MPFF counteracts the pathophysiologic mechanism of venous insufficiency and is the only venoactive drug to control the inflammatory reaction induced by venous hypertension[26-28].
Systemic antibiotics can only be used for patients with evidence of clinical infection rather than bacterial colonization; it is not recommended to use antibiotics locally in ulcers[22]. One study reported that oral administration of low doses of doxycycline 20 mg twice daily for 3 mo could improve extracellular matrix functioning, and its immunomodulating and anti-inflammatory actions through the inhibition of matrix metalloproteinase provided a possible solution to support wound healing[6]. Another study[29] compared 10 patients who received doxycycline 20 mg twice daily with another 10 patients who received doxycycline 100 mg twice daily; the results indicated that higher dosage may improve the healing rate of recalcitrant leg ulcers[29]. A case-control study showed that topical 0.5% timolol maleate improves the healing of chronic leg ulcers[30].
Honey has been used as a traditional medicine for centuries by different cultures for the treatment of wounds. However, not all honey exhibits equal antimicrobial potency, and only a few meet the criteria for clinical usage. A prospective cohort study and a case series study support the use of honey[31,32]; the average wound area of 25 patients with CVLUs decreased significantly following the use of honey. Overall, 72% (18/25) patients experienced a decrease in reported pain levels, and 80% patients were satisfied with honey treatment[32]. One trial (108 participants) showed that 44% (24/54) ulcers were closed with honey vs 33% (18/54) with hydrogel (P = 0.037); another trial supported the use of honey over hydrogel in healing ulcers (P < 0.001)[19]. However, three RCTs that evaluated healing CVLUs with honey vs standard wound therapy arrived at contradictory conclusions. One showed better outcomes, one showed equivalent outcomes but more adverse effects, and the third showed a non-significant reduction in bacterial colonization. Overall, the clinical bottom line is that there is no conclusive evidence that honey improves outcome in patients with CVLUs; its benefit should be considered unproven until more robust trials results are available[31].
It is hypothesized that maltodextrin or ascorbic acid treatment could stimulate tissue repair of chronic wounds by changing the stage of inflammation and modifying collagen turnover directly through fibroblast response. Patients with CVLUs treated with maltodextrin and ascorbic acid were found to have decreased microorganism population and improved wound repair, with almost four-fold CVLUs closure during 12 wk in comparison with those treated with zinc oxide[33]. Nevertheless, the clinical effects of this treatment need to be approved with further clinical trials.
Hyperbaric oxygen therapy has been successfully used for patients with hypoxic diabetic ulcers and CVLUs[34,35] and has demonstrated no significant risk of cell damage caused by a high concentration of oxygen[34]. A retrospective study of 200 patients with chronic wounds treated with hyperbaric oxygen showed that their wounds were healed or reduced (62.0%) compared to those with acute wounds[35,36]. Topical oxygen therapy can stimulate chronic wounds to heal. After oxygen therapy for over 25 d, 83% wound area reduction and 47% wound closure were observed in CVLUs patients[36].
Marston et al[37] reported that patients with CVLUs treated with saphenous stripping had less ulcer recurrence (15%) than those treated with compression therapy (34%); no statistical significance in ulcer healing between compression plus surgery (ligation and stripping) group and compression alone group (82% vs 76%) was observed. Most patients with varicose veins and leg ulcers are treated with endovenous thermal ablation (EVTA), and ulcers recurred in a minority of CVLUs patients after EVTA. Patients with varicose veins who have no deep venous reflux and undertake both phlebectomy and EVTA have experienced less ulcer recurrence. Thus, it is suggested to perform both phlebectomy and EVTA for patients with varicose veins and CVLUs if possible[37].
Treatment of superficial venous reflux has been shown to reduce the rate of ulcer recurrence[1,2,38]. Endovenous laser ablation of superficial venous reflux early resulted in faster closure of CVLUs and more time free from ulcers than deferred endovenous ablation[1,39]. Patients with CEAP 5 venous ulcers healed faster with EVLT and compression therapy than with compression alone[40]. Although the ablation of the GSV appears to help reduce ulcer recurrence, there was no impact of combined superficial and perforator ablations on ulcer recurrence rates. The role of incompetent perforating veins ablation alone or concomitant with GSV treatment needs further RCT study to be performed[39].
Correction of both superficial truncal vein reflux and deep vein stenosis contributes to the healing of CVLUs[1,2,38]. Patients with iliofemoral DVT have a high chance to develop post-thrombotic syndrome. Patients who fail to heal their CVLUs after superficial and perforator ablation should examine the iliocaval system to find hemodynamically significant stenoses or occlusions amenable to stenting[41]. Iliac vein stenting can significantly help improve clinical outcomes in patients with venous stenosis or obstruction. Venous duplex ultrasound in diagnosing iliac vein stenosis with 2.5 s can predict ≥ 50% iliac vein stenosis. Stent placement and pentoxifylline were associated with ulcer healing and reduced risk of venous ulcer recurrence[42]. The endovascular iliocaval and infrainguinal venous stenting help to improve clinical symptoms, improve 1-year patency rate, and heal recalcitrant venous ulcers. Clinical outcomes in patients with IVC filters were improved significantly after removing the filter compared to those in which the filter could not be removed (100% vs 17%; P < 0.01)[11,12].
Skin grafting helps expedite the closure of CVLUs. Currently available grafts are taken from the patient's normal skin, preserved animal’s skin, human cadaveric dermis, porcine dermal, or a sheet of bioengineered skin grown from donor cells. Most grafts have an acellular collagen scaffold, providing room for neovascularization or tissue incorporation[43]. A porcine dermal matrix (Permacol) was reported to be used successfully for a woman with a complex and infected abdominal wall defect wound. One year after discharge, the patient was pain-free with complete wound healing[43].
Currently, autologous split-thickness skin grafting (STSG) is still the first choice to treat huge CVLUs or diabetic ulcers[44] and has greater success rates in CVLUs than in other types of wounds[45,46]. A study assessed the effectiveness of skin autografting at 6 mo after grafting[47], and large CVLUs (> 50 cm2) in 67.5% elderly patients (> 60 years) were closed within 2-3 wk by skin grafting, compared to the conservative group, and maintained closure up to 6 mo[47].
In a retrospective, descriptive study of outpatients with CVLUs, diabetic foot ulcers, surgical, or traumatic wounds were treated with a meshed, partial-thickness, cryopreserved human skin allograft. The results favored the use of allograft skin for chronic wounds[48].
Double layer dermal substitute is composed of a superficial silicon layer and a three-dimensional collagen structure and can be placed within the ulcers to boost tissue regeneration. Nevelia® is a new collagen dermal template substitute to treat chronic venous or arterial ulcers and facilitates tissue formation and angiogenesis[49,50]. Furthermore, 11 trials comparing a variety of grafts with standard care concluded that bilayer artificial skin, used in conjunction with compression bandaging, increases venous ulcer healing compared with a simple dressing plus compression. Further research is needed to assess whether other forms of skin grafts increase ulcer healing[51].
The hair follicle is a large reservoir of progenitor cells; the hair bulge in a hair follicle has epithelial and melanocytic stem cells that can produce the interfollicular epidermis, hair follicle structures, and sebaceous glands and reconstitute in an artificial in vivo system to a new hair follicle[52,53]. The isolated bulge epithelial stem cells can increase the hair density in patients with androgenetic alopecia[54]. Gentile et al[52,55] performed a placebo-controlled, randomized, evaluator-blinded, half-head group study that showed that hair regrowth with micrografts containing hair follicle mesenchymal stem cells may represent a safe and viable treatment alternative against hair loss.
Hair follicle-derived cells play a role as a wound-healing promoter in epidermal wound closure. Hair punch grafting is a minimally invasive surgical procedure for CVLUs[56,57]. An RCT with an intraindividual comparison of hair follicle scalp grafts and hairless skin grafts transplanted in parallel into two halves of the same ulcer was performed. Autologous transplantation of terminal hair follicles by scalp punch grafts led to better healing than hairless punch grafts. The average percentage reduction of ulcer area at 18 wk post-grafting was significantly higher in the hair follicle graft group than in the hairless grafts (75.15 % vs 33.07%, P = 0.002). Hair punch graft appeared to have a better result than traditional hairless punch graft for CVLUs; epithelialization, neovascularization, and dermal reorganization were improved histologically[53,57,58].
The viability of new skin grafting in chronic ulcers is a major concern due to bacterial infection, chronic inflammation, tissue edema, and low oxygen in CVLUs. Negative pressure wound therapy (NPWT) has been used to manage a variety of wounds by granulation tissue formation, wound drainage, and preparation for delayed ulcer closures or grafting. It facilitates the sustaining and incorporation of skin grafts or flaps onto the recipient wound bed[59-62]. A combination of NPWT with irrigation of oxygen loaded fluid can raise the partial pressure of oxygen of the skin around the wounds effectively, enhance the transition of macrophages from type I to type II, and may promote the growth of granulation tissue, leading to a better recipient for skin grafting or epithelization[63].
However, an RCT study of 46 patients comparing NPWT with hydrocolloid dressings over 5 d following autologous grafting on CVLUs failed to show the benefit of NPWT associated with skin graft over traditional dressings. This could be due to the limited number of cases enrolled[64].
NPWT with topical wound solution instillation (NPWTi) helps with wound preparation prior to STSG. NPWTi in a porcine model showed improved tissue oxygenation, granulation tissue, and clearance of wound debris. NPWT after STSG promoted graft catch through reducing shear forces, fluid acquisition, and neovascularization. STSG for massive CVLUs is better than standard compression therapy in terms of less pain, early time to walk, less outpatient visits, and times of dressings required and ulcer healing rate[59-62,65-67]. In a study including 10 patients with CVLUS, the average length of time ulcers was 38 mo (3-120 mo) on admission, and 10 patients received inpatient NPWT with NPWTi for 7d, then STSG with NPWT over the graft for 4 d. Overall, eight patients experienced complete CVLU closure by 6 mo after NPWTi with STSG[62].
Mesenchymal stem cells (MSCs) are multipotent progenitor cells that are capable of directly differentiating into various mesenchymal cell lineages and facilitate wound healing by regulating immune response and inflammation in vivo. The sources of MSC include autogenous, such as bone marrow or adipose tissue-derived stem cells, and allogeneic (e.g., placenta-derived stem cells). MSCs are immune-privileged due to lacking cell surface antigens. MSCs have been used clinically and are reported as a promising and compelling therapy for CVLUs[68].
Placental tissues, including the chorion, the umbilical cord, the amnion, and the amnionic fluid, are full of MSCs, are readily available, and have no ethical concerns for embryonic stem cells[69]. Placental-derived MSCs possess paracrine, metabolic, and immunomodulatory properties while keeping the immune-privileged status[69]. Placental or amniotic membranes (AMs) prepared from fresh cesarean section donors were used for wound healing were used. AMs were initially used for corneal ulcers for many years[69-71].
Placental tissue is beneficial in healing chronic wounds[45]. AMs are available in the cryopreserved state or as dehydrated human amnion/chorion membrane (dHACM) allograft for direct implantation[72-74]. Overcoming the difficulties of fresh transplan-tation, some cryopreserved AM products are commercially available in a micronized formulation to be used topically or hydrated for injection into wounds or other inflamed tissues to shorten wound closure time[75,76]. Further studies should be undertaken to determine if there is a distinction between different amniotic matrices regarding efficacy in wound repair[77].
Adipose-derived MSCs (ADSCs) as well as bone marrow MSCs are the most widely studied cell types[78,79] and display similar results in terms of tissue growth[80]. Adipose-derived stromal vascular fraction (AD-SVFs) and ADSCs warrant careful preparation of the harvested adipose tissue. Gentile et al[80,81] described the procedures to isolate and prepare the ADSCs and platelet-rich plasma as well as the application of ADSCs in practice.
ADSC strategy provides a great opportunity for the treatment of chronic wounds not responding to the standard treatment. ADSCs are easy to obtain due to lower morbidity during the harvesting procedure[78,80], can be implanted at the wound site immediately following debridement, and promote a new tissue formation rich in vascular structures and remodeling collagen[82]. As an additional treatment to primary surgical therapy for CVLUs[83], one study showed that it does not shorten the venous recovery period but decreases the 1-year recurrence rate[83]. However, preclinical and observational RCT reports indicate that centrifuged adipose tissue containing progenitor cells is safe and may accelerate healing time in CVLUs and reduce wound pain[84]. ADSCs enriched, high-density lipoaspirate (HDL) with topical timolol was used for the healing of CVLUs. A 63-year-old patient with CVLUs and venous stasis for 30 years underwent stripping of varicose veins, the standard of care, and anticoagulation. ADSCs-enriched, HDL on wound matrix and compression therapy was used for her medial ulcer, and, as a control, compression therapy following debridement was applied for the lateral ulcer. Daily topical timolol was used for both ulcers for 1 mo. After 3 mo, the medial ulcer was closed completely and maintained healed for over 15 mo. In contrast, healing signs of lateral ulcers in the leg were minimal[85].
The stromal vascular fraction (SVF) of adipose tissue consists of cellular subpopulations with distinct regenerative potential. A prospective clinical pilot study showed that SVF cells could be used safely for the treatment of CVLUs and mixed arterial-venous ulcers even in multimorbid patients, but one-time application of the used amounts of SVF cells was not sufficient in the majority of cases with larger predominantly ischemic mixed arterial-venous ulcers and comorbidities[86,87].
Autologous platelet-rich plasma (PRP) containing fibrin and growth factors can stimulate tissue regeneration with the potential to enhance the healing of chronic wounds. PRP is easily accessible, is relatively inexpensive and safe, and can be injected into or dressed topically over the ulcers. It is an alternative to surgery for a safe and natural healing ulcer[78,80,88,89].
Gentile et al[88] in Italy described the meticulous preparation approach for PRP. Cervelli et al[90] reported that post-traumatic leg ulcers treated with enhanced SVF and PRP combined with fat grafting healed better than those treated with hyaluronic acid (HA). De Angelis et al[91] reported that PRP plus HA treatment showed stronger regenerative potential in terms of epidermal proliferation and dermal renewal than with HA alone. A total of 182 patients with chronic ulcers (diabetic and vascular) treated with PRP plus HA within 80 d showed stronger re-epithelialization than another 182 patients in the control group treated with HA alone (98.4%, 1.3% vs 87.8%, 4.1%, P < 0.05)[91]. Two RCTs results showed PRP injection enhances the healing of CVLUs more than PRP application and compression therapy in terms of healing rate, healing time, and ulcer area reduction[92,93].
The overall quality of evidence of PRP for treating chronic ulcers remains low. PRP may boost the healing of diabetic foot ulcers, but this conclusion is based on low quality evidence from two small RCTs. Well designed and adequately powered clinical trials are needed to show convincingly the efficacy of autologous PRP in the healing of CVLUs and other chronic wounds[94-96].
The efficacy of recombinant granulocyte-macrophage colony-stimulating factor in the treatment of CVLUs remains insignificant clinically[97]. An open-label, prospective, multicenter RCT shows that human fibroblast-derived dermal substitute and compression therapy have no benefit over compression therapy alone in terms of healing time, healing rate, and adverse effects for CVLUs[98].
An RCT compared dHACM allograft with multilayer compression only for CVLUs. Of 47 patients without complete healing during the initial study, wound size of ≥ 40% decreased in 20 (45.4%) patients; < 40% ulcer area decreased in 24 (55%) patients. All 47 patients were treated with dHACM allograft for a mean of 46 d. Eighty percent (16/20) patients in the ≥ 40% group had ulcer closure (P = 0.0027), and 33.3% (8/24) patients in the < 40% group experienced ulcer closure at a mean of 103.6 d (P = 0.0023)[72]. Besides, dHACM allograft can be used as an adjunct to immunosuppressive therapy to reduce pain and heal the pyoderma gangrenosum ulcer in a patient with multiple comorbidities, including venous insufficiency and diabetes mellitus[73].
An RCT studied patients with nonhealing full-thickness CVLUs, and the ulcers treated weekly with dHACM allograft healed more significantly than those with standard wound care and compression (60% vs 35% at 12 wk, P = 0.0128; 71% vs 44% at 16 wk, P = 0.0065)[74]. Another RCT study enrolled 84 participants, and venous leg ulcers (n = 53) treated with allograft for 4 wk had significant healing compared with multilayer compression therapy alone (n = 31) (48.1% vs 19.0%)[99]. A retrospective study showed that the average time to healing leg ulcers with dHACM grafts was less than that with conservative treatment (33 d vs 87 d)[100]. Another two retrospective cohort studies showed all wounds (venous, diabetic foot ulcers) were closed effectively after the use of dHACM allograft[101,102].
Viable cryopreserved human placental membrane (vCHPM) is an effective therapy for CVLUs refractory to standard of care. Adjunctive therapy with human viable wound matrix (hVWM) provides superior healing rates in refractory CVLUs[45]. A retrospective review describes patients with chronic wounds that had failed standard of care treatments for more than 4 wk and were subsequently treated with weekly use of vCHPM grafts. All ulcers reached full closure in 4-5 wk with no complication[103]. After using hVWM of cryopreserved placental tissue, 53% (16/30) of CVLUs refractory to standard therapy healed completely. CVLUs were reduced in size by half more with hVWM than standard therapy (80% vs 25%, P < 0.001). The mean rate of reduction in ulcer area was more significant after hVWM than with standard therapy (1.69% per day vs 0.73% per day, P = 0.01)[104].
Wound care, debridement, bed rest with leg elevation, and compression are basic approaches for CVLUs. Ablation of the GSVs can help to heal some ulcers. Negative pressure wound therapy is very helpful for the preparation of ulcers and promotes the growth of granulation tissue. Split-thickness skin grafting is the first choice for the management of huge CVLUs. Hair punch graft appears to have a better result than that with traditional hairless punch graft for CVLUs. Administration of adipose tissue or placenta-derived MSCs is a promising therapy for wound healing. Autologous platelet-rich plasma provides an alternative strategy for surgery for a safe and natural healing of the ulcer. There are little data to support the efficacy of silver-based dressings in the healing of CVLUs. Hydrogen peroxide is harmful to the growth of granulation tissue in CVLUs. The confirmative efficacy of current advanced ulcer therapies needs more robust evidence.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gentile P S-Editor: Gao CC L-Editor: Filipodia P-Editor: Xing YX
| 1. | Li X, Fan L, Ren S, Li X. Outcomes of Foam Sclerotherapy plus Ligation versus Foam Sclerotherapy Alone for Venous Ulcers in Lower Extremities. Ann Vasc Surg. 2017;45:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Lin F, Zhang S, Sun Y, Ren S, Liu P. The management of varicose veins. Int Surg. 2015;100:185-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 3. | Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, Mills JL, Ricco JB, Suresh KR, Murad MH, Aboyans V, Aksoy M, Alexandrescu VA, Armstrong D, Azuma N, Belch J, Bergoeing M, Bjorck M, Chakfé N, Cheng S, Dawson J, Debus ES, Dueck A, Duval S, Eckstein HH, Ferraresi R, Gambhir R, Gargiulo M, Geraghty P, Goode S, Gray B, Guo W, Gupta PC, Hinchliffe R, Jetty P, Komori K, Lavery L, Liang W, Lookstein R, Menard M, Misra S, Miyata T, Moneta G, Munoa Prado JA, Munoz A, Paolini JE, Patel M, Pomposelli F, Powell R, Robless P, Rogers L, Schanzer A, Schneider P, Taylor S, De Ceniga MV, Veller M, Vermassen F, Wang J, Wang S; GVG Writing Group for the Joint Guidelines of the Society for Vascular Surgery (SVS); European Society for Vascular Surgery (ESVS); and World Federation of Vascular Societies (WFVS). Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. Eur J Vasc Endovasc Surg 2019; 58: S1-S109. e33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 923] [Article Influence: 153.8] [Reference Citation Analysis (0)] |
| 4. | Siadat AH, Isseroff RR. Prolotherapy: Potential for the Treatment of Chronic Wounds? Adv Wound Care (New Rochelle). 2019;8:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 5. | Todd M. Compression therapy for chronic oedema and venous leg ulcers: CoFlex TLC Calamine. Br J Nurs. 2019;28:S32-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Serra R, Gallelli L, Buffone G, Molinari V, Stillitano DM, Palmieri C, de Franciscis S. Doxycycline speeds up healing of chronic venous ulcers. Int Wound J. 2015;12:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Pugliese DJ. Infection in Venous Leg Ulcers: Considerations for Optimal Management in the Elderly. Drugs Aging. 2016;33:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Li X, Yang B, Li X, Ren S. Prospective Comparison of Effect of Ligation and Foam Sclerotherapy with Foam Sclerotherapy Alone for Varicose Veins. Ann Vasc Surg. 2018;49:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Sîrbi AG, Florea M, Pătraşcu V, Rotaru M, Mogoş DG, Georgescu CV, Mărgăritescu ND. Squamous cell carcinoma developed on chronic venous leg ulcer. Rom J Morphol Embryol. 2015;56:309-313. [PubMed] |
| 10. | Sermsathanasawadi N, Pruekprasert K, Pitaksantayothin W, Chinsakchai K, Wongwanit C, Ruangsetakit C, Mutirangura P. Prevalence, risk factors, and evaluation of iliocaval obstruction in advanced chronic venous insufficiency. J Vasc Surg Venous Lymphat Disord. 2019;7:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Rollo JC, Farley SM, Jimenez JC, Woo K, Lawrence PF, DeRubertis BG. Contemporary outcomes of elective iliocaval and infrainguinal venous intervention for post-thrombotic chronic venous occlusive disease. J Vasc Surg Venous Lymphat Disord. 2017;5:789-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | George R, Verma H, Ram B, Tripathi R. The effect of deep venous stenting on healing of lower limb venous ulcers. Eur J Vasc Endovasc Surg. 2014;48:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Davies P. Current thinking on the management of necrotic and sloughy wounds. Prof Nurse. 2004;19:34-36. [PubMed] |
| 14. | Hall L, Adderley U. Active debridement of venous leg ulcers: a literature review to inform clinical practice. Br J Community Nurs. 2019;24:S24-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Medical Advisory Secretariat. Negative pressure wound therapy: an evidence-based analysis. Ont Health Technol Assess Ser. 2006;6:1-38. [PubMed] |
| 16. | Hermans MH. Silver-containing dressings and the need for evidence. Adv Skin Wound Care. 2007;20:166-73; quiz 174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Mosti G. Wound care in venous ulcers. Phlebology. 2013;28 Suppl 1:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | O'Meara S, Martyn-St James M. Foam dressings for venous leg ulcers. Cochrane Database Syst Rev. 2013;(5):CD009907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Gethin G, Cowman S, Kolbach DN. Debridement for venous leg ulcers. Cochrane Database Syst Rev. 2015;(9):CD008599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | O'Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2012;11:CD000265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 21. | Wong IK, Andriessen A, Lee DT, Thompson D, Wong LY, Chao DV, So WK, Abel M. Randomized controlled trial comparing treatment outcome of two compression bandaging systems and standard care without compression in patients with venous leg ulcers. J Vasc Surg. 2012;55:1376-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | O'Meara S, Al-Kurdi D, Ologun Y, Ovington LG, Martyn-St James M, Richardson R. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev. 2014;(1):CD003557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 23. | Mosti G, Magliaro A, Mattaliano V, Picerni P, Angelotti N. Comparative study of two antimicrobial dressings in infected leg ulcers: a pilot study. J Wound Care. 2015;24:121-2; 124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Zou P, Yang JS, Wang XF, Wei JM, Guo H, Zhang B, Zhang F, Chu L, Hao DJ, Zhao YT. Oxygen Embolism and Pneumocephalus After Hydrogen Peroxide Application During Minimally Invasive Transforaminal Lumbar Interbody Fusion Surgery: A Case Report and Literature Review. World Neurosurg. 2020;138:201-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Murphy EC, Friedman AJ. Hydrogen peroxide and cutaneous biology: Translational applications, benefits, and risks. J Am Acad Dermatol. 2019;81:1379-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 26. | Nicolaides AN. The Most Severe Stage of Chronic Venous Disease: An Update on the Management of Patients with Venous Leg Ulcers. Adv Ther. 2020;37:19-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Nicolaides AN. The Benefits of Micronized Purified Flavonoid Fraction (MPFF) Throughout the Progression of Chronic Venous Disease. Adv Ther. 2020;37:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Bush R, Comerota A, Meissner M, Raffetto JD, Hahn SR, Freeman K. Recommendations for the medical management of chronic venous disease: The role of Micronized Purified Flavanoid Fraction (MPFF). Phlebology. 2017;32:3-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Sadler GM, Wallace HJ, Stacey MC. Oral doxycycline for the treatment of chronic leg ulceration. Arch Dermatol Res. 2012;304:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Thomas B, Kurien JS, Jose T, Ulahannan SE, Varghese SA. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg Venous Lymphat Disord. 2017;5:844-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Holland LC, Norris JM. Medical grade honey in the management of chronic venous leg ulcers. Int J Surg. 2015;20:17-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 32. | Mayer A, Slezak V, Takac P, Olejnik J, Majtan J. Treatment of non-healing leg ulcers with honeydew honey. J Tissue Viability. 2014;23:94-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Salgado RM, Cruz-Castañeda O, Elizondo-Vázquez F, Pat L, De la Garza A, Cano-Colín S, Baena-Ocampo L, Krötzsch E. Maltodextrin/ascorbic acid stimulates wound closure by increasing collagen turnover and TGF-β1 expression in vitro and changing the stage of inflammation from chronic to acute in vivo. J Tissue Viability. 2017;26:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Zimanova J, Batora I, Dusinska M, Burghardtova K, Blazicek P, Vojtech I, Bizik A. Short term oxidative DNA damage by hyperbaric oxygenation in patients with chronic leg ulcers. Bratisl Lek Listy. 2011;112:447-452. [PubMed] |
| 35. | Andrade SM, Santos IC. Hyperbaric oxygen therapy for wound care. Rev Gaucha Enferm. 2016;37:e59257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Kaufman H, Gurevich M, Tamir E, Keren E, Alexander L, Hayes P. Topical oxygen therapy stimulates healing in difficult, chronic wounds: a tertiary centre experience. J Wound Care. 2018;27:426-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Marston WA, Crowner J, Kouri A, Kalbaugh CA. Incidence of venous leg ulcer healing and recurrence after treatment with endovenous laser ablation. J Vasc Surg Venous Lymphat Disord. 2017;5:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Sun Y, Li X, Chen Z, Li X, Ren S. Feasibility and safety of foam sclerotherapy followed by a multiple subcutaneously interrupt ligation under local anaesthesia for outpatients with varicose veins. Int J Surg. 2017;42:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Gohel MS, Heatley F, Liu X, Bradbury A, Bulbulia R, Cullum N, Epstein DM, Nyamekye I, Poskitt KR, Renton S, Warwick J, Davies AH; EVRA Trial Investigators. A Randomized Trial of Early Endovenous Ablation in Venous Ulceration. N Engl J Med. 2018;378:2105-2114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 30.3] [Reference Citation Analysis (1)] |
| 40. | Kheirelseid EA, Bashar K, Aherne T, Babiker T, Naughton P, Moneley D, Walsh SR, Leahy AL. Evidence for varicose vein surgery in venous leg ulceration. Surgeon. 2016;14:219-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Lawrence PF, Hager ES, Harlander-Locke MP, Pace N, Jayaraj A, Yohann A, Kalbaugh C, Marston W, Kabnick L, Saqib N, Pouliot S, Piccolo C, Kiguchi M, Peralta S, Motaganahalli R. Treatment of superficial and perforator reflux and deep venous stenosis improves healing of chronic venous leg ulcers. J Vasc Surg Venous Lymphat Disord. 2020;8:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 42. | Harlander-Locke M, Lawrence P, Jimenez JC, Rigberg D, DeRubertis B, Gelabert H. Combined treatment with compression therapy and ablation of incompetent superficial and perforating veins reduces ulcer recurrence in patients with CEAP 5 venous disease. J Vasc Surg. 2012;55:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Gentile P, Colicchia GM, Nicoli F, Cervelli G, Curcio CB, Brinci L, Cervelli V. Complex abdominal wall repair using a porcine dermal matrix. Surg Innov. 2013;20:NP12-NP15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | De Angelis B, Gentile P, Agovino A, Migner A, Orlandi F, Delogu P, Cervelli V. Chronic ulcers: MATRIDERM(®) system in smoker, cardiopathic, and diabetic patients. J Tissue Eng. 2013;4:2041731413502663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Farivar BS, Toursavadkohi S, Monahan TS, Sharma J, Ucuzian AA, Kundi R, Sarkar R, Lal BK. Prospective study of cryopreserved placental tissue wound matrix in the management of chronic venous leg ulcers. J Vasc Surg Venous Lymphat Disord. 2019;7:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Prakash TV, Chaudhary DA, Purushothaman S, K V S, Arvind K V. Epidermal Grafting for Chronic Complex Wounds in India: A Case Series. Cureus. 2016;8:e516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Jankunas V, Bagdonas R, Samsanavicius D, Rimdeika R. An Analysis of the Effectiveness of Skin Grafting to Treat Chronic Venous Leg Ulcers. Wounds. 2007;19:128-137. [PubMed] |
| 48. | Desman E, Bartow W, Anderson LH. Human Skin Allograft for Patients With Diabetic Foot Ulcers, Venous Leg Ulcers, or Surgical/Traumatic Wounds Retrospective, Descriptive Study. Ostomy Wound Manage. 2015;61:16-22. [PubMed] |
| 49. | De Angelis B, Orlandi F, Fernandes Lopes Morais D'Autilio M, Scioli MG, Orlandi A, Cervelli V, Gentile P. Long-term follow-up comparison of two different bi-layer dermal substitutes in tissue regeneration: Clinical outcomes and histological findings. Int Wound J. 2018;15:695-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | De Angelis B, Orlandi F, Morais D'Autilio MFL, Di Segni C, Scioli MG, Orlandi A, Cervelli V, Gentile P. Vasculogenic Chronic Ulcer: Tissue Regeneration with an Innovative Dermal Substitute. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Jones JE, Nelson EA, Al-Hity A. Skin grafting for venous leg ulcers. Cochrane Database Syst Rev. 2013;(1):CD001737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Gentile P. Autologous Cellular Method Using Micrografts of Human Adipose Tissue Derived Follicle Stem Cells in Androgenic Alopecia. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 53. | Jiménez F, Garde C, Poblet E, Jimeno B, Ortiz J, Martínez ML, Gutiérrez-Rivera A, Pérez-López V, Etxaniz U, Naveda C, Higuera JL, Egüés N, Escario E, Izeta A. A pilot clinical study of hair grafting in chronic leg ulcers. Wound Repair Regen. 2012;20:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017;4:58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 55. | Gentile P, Scioli MG, Cervelli V, Orlandi A, Garcovich S. Autologous Micrografts from Scalp Tissue: Trichoscopic and Long-Term Clinical Evaluation in Male and Female Androgenetic Alopecia. Biomed Res Int. 2020;2020:7397162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 56. | Fox JD, Baquerizo-Nole KL, Van Driessche F, Yim E, Nusbaum B, Jimenez F, Kirsner RS. Optimizing Skin Grafting Using Hair-derived Skin Grafts: The Healing Potential of Hair Follicle Pluripotent Stem Cells. Wounds. 2016;28: 109-111. [PubMed] |
| 57. | MartÃnez ML, Escario E, Poblet E, Sánchez D, Buchón FF, Izeta A, Jimenez F. Hair follicle-containing punch grafts accelerate chronic ulcer healing: A randomized controlled trial. J Am Acad Dermatol. 2016;75:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 58. | Martínez Martínez ML, Escario Travesedo E, Jiménez Acosta F. Hair-follicle Transplant Into Chronic Ulcers: A New Graft Concept. Actas Dermosifiliogr. 2017;108:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Ye J, Xie T, Wu M, Ni P, Lu S. Negative pressure wound therapy applied before and after split-thickness skin graft helps healing of Fournier gangrene: a case report (CARE-Compliant). Medicine (Baltimore). 2015;94:e426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Tian Y, Liu T, Zhao CQ, Lei ZY, Fan DL, Mao TC. Negative pressure wound therapy and split thickness skin graft aided in the healing of extensive perineum necrotizing fasciitis without faecal diversion: a case report. BMC Surg. 2018;18:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Pearce FB Jr, Richardson KA. Negative pressure wound therapy, staged excision and definitive closure with split-thickness skin graft for axillary hidradenitis suppurativa: a retrospective study. J Wound Care. 2017;26:S36-S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 62. | Yang CK, Alcantara S, Goss S, Lantis JC 2nd. Cost analysis of negative-pressure wound therapy with instillation for wound bed preparation preceding split-thickness skin grafts for massive (>100 cm(2)) chronic venous leg ulcers. J Vasc Surg. 2015;61:995-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Wen HD, Li ZQ, Zhang MG, Wang JH, Wang GF, Wu Q, Tong S. Effects of vacuum sealing drainage combined with irrigation of oxygen loaded fluid on wounds of pa- tients with chronic venous leg ulcers. Zhonghua Shaoshang Zazhi. 2015;2:86-92. |
| 64. | Leclercq A, Labeille B, Perrot JL, Vercherin P, Cambazard F. Skin graft secured by VAC (vacuum-assisted closure) therapy in chronic leg ulcers: A controlled randomized study. Ann Dermatol Venereol. 2016;143:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 65. | Wu CC, Chew KY, Chen CC, Kuo YR. Antimicrobial-impregnated dressing combined with negative-pressure wound therapy increases split-thickness skin graft engraftment: a simple effective technique. Adv Skin Wound Care. 2015;28:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Suzuki H, Watanabe T, Okazaki T, Notsuda H, Niikawa H, Matsuda Y, Noda M, Sakurada A, Hoshikawa Y, Aizawa T, Miura T, Okada Y. Prolonged Negative Pressure Wound Therapy Followed by Split-Thickness Skin Graft Placement for Wide Dehiscence of Clamshell Incision After Bilateral Lung Transplantation: A Case Report. Transplant Proc. 2016;48:982-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 67. | Leong S, Lo ZJ. Use of disposable negative pressure wound therapy on split-thickness skin graft recipient sites for peripheral arterial disease foot wounds: A case report. Int Wound J. 2020;17:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 68. | Athanerey A, Patra PK, Kumar A. Mesenchymal stem cell in venous leg ulcer: An intoxicating therapy. J Tissue Viability. 2017;26:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 69. | Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012;1:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 565] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 70. | Malek A, Bersinger NA. Human placental stem cells: biomedical potential and clinical relevance. J Stem Cells. 2011;6:75-92. [PubMed] |
| 71. | Lim R. Concise Review: Fetal Membranes in Regenerative Medicine: New Tricks from an Old Dog? Stem Cells Transl Med. 2017;6:1767-1776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 72. | Serena TE, Yaakov R, DiMarco D, Le L, Taffe E, Donaldson M, Miller M. Dehydrated human amnion/chorion membrane treatment of venous leg ulcers: correlation between 4-week and 24-week outcomes. J Wound Care. 2015;24:530-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 73. | Snyder RJ, Ead J, Glick B, Cuffy C. Dehydrated Human Amnion/Chorion Membrane as Adjunctive Therapy in the Multidisciplinary Treatment of Pyoderma Gangrenosum: A Case Report. Ostomy Wound Manage. 2015;61:40-49. [PubMed] |
| 74. | Bianchi C, Cazzell S, Vayser D, Reyzelman AM, Dosluoglu H, Tovmassian G; EpiFix VLU Study Group. A multicentre randomised controlled trial evaluating the efficacy of dehydrated human amnion/chorion membrane (EpiFix® ) allograft for the treatment of venous leg ulcers. Int Wound J. 2018;15:114-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 75. | Kogan S, Sood A, Granick MS. Amniotic Membrane Adjuncts and Clinical Applications in Wound Healing: A Review of the Literature. Wounds. 2018;30:168-173. [PubMed] |
| 76. | Ward DJ, Bennett JP. The long-term results of the use of human amnion in the treatment of leg ulcers. Br J Plast Surg. 1984;37:191-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 77. | Towler MA, Rush EW, Richardson MK, Williams CL. Randomized, Prospective, Blinded-Enrollment, Head-To-Head Venous Leg Ulcer Healing Trial Comparing Living, Bioengineered Skin Graft Substitute (Apligraf) with Living, Cryopreserved, Human Skin Allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 78. | Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Concise Review: The Use of Adipose-Derived Stromal Vascular Fraction Cells and Platelet Rich Plasma in Regenerative Plastic Surgery. Stem Cells. 2017;35:117-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 79. | Scioli MG, Bielli A, Gentile P, Cervelli V, Orlandi A. Combined treatment with platelet-rich plasma and insulin favours chondrogenic and osteogenic differentiation of human adipose-derived stem cells in three-dimensional collagen scaffolds. J Tissue Eng Regen Med. 2017;11:2398-2410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 80. | Gentile P, Cervelli V. Adipose-Derived Stromal Vascular Fraction Cells and Platelet-Rich Plasma: Basic and Clinical Implications for Tissue Engineering Therapies in Regenerative Surgery. Methods Mol Biol. 2018;1773:107-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 81. | Gentile P, Calabrese C, De Angelis B, Pizzicannella J, Kothari A, Garcovich S. Impact of the Different Preparation Methods to Obtain Human Adipose-Derived Stromal Vascular Fraction Cells (AD-SVFs) and Human Adipose-Derived Mesenchymal Stem Cells (AD-MSCs): Enzymatic Digestion Versus Mechanical Centrifugation. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 82. | Zollino I, Zuolo M, Gianesini S, Pedriali M, Sibilla MG, Tessari M, Carinci F, Occhionorelli S, Zamboni P. Autologous adipose-derived stem cells: Basic science, technique, and rationale for application in ulcer and wound healing. Phlebology. 2017;32:160-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 83. | Kavala AA, Turkyilmaz S. Autogenously derived regenerative cell therapy for venous leg ulcers. Arch Med Sci Atheroscler Dis. 2018;3:e156-e163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 84. | Zollino I, Campioni D, Sibilla MG, Tessari M, Malagoni AM, Zamboni P. A phase II randomized clinical trial for the treatment of recalcitrant chronic leg ulcers using centrifuged adipose tissue containing progenitor cells. Cytotherapy. 2019;21:200-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Larsen L, Tchanque-Fossuo CN, Gorouhi F, Boudreault D, Nguyen C, Fuentes JJ, Crawford RW, Dahle SE, Whetzel T, Isseroff RR. Combination therapy of autologous adipose mesenchymal stem cell-enriched, high-density lipoaspirate and topical timolol for healing chronic wounds. J Tissue Eng Regen Med. 2018;12:186-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 86. | Konstantinow A, Arnold A, Djabali K, Kempf W, Gutermuth J, Fischer T, Biedermann T. Therapy of ulcus cruris of venous and mixed venous arterial origin with autologous, adult, native progenitor cells from subcutaneous adipose tissue: a prospective clinical pilot study. J Eur Acad Dermatol Venereol. 2017;31:2104-2118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 87. | Gentile P, Garcovich S. Concise Review: Adipose-Derived Stem Cells (ASCs) and Adipocyte-Secreted Exosomal microRNA (A-SE-miR) Modulate Cancer Growth and proMote Wound Repair. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 88. | Gentile P, Calabrese C, De Angelis B, Dionisi L, Pizzicannella J, Kothari A, De Fazio D, Garcovich S. Impact of the Different Preparation Methods to Obtain Autologous Non-Activated Platelet-Rich Plasma (A-PRP) and Activated Platelet-Rich Plasma (AA-PRP) in Plastic Surgery: Wound Healing and Hair Regrowth Evaluation. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 89. | Cervelli V, Bocchini I, Di Pasquali C, De Angelis B, Cervelli G, Curcio CB, Orlandi A, Scioli MG, Tati E, Delogu P, Gentile P. P.R.L. platelet rich lipotransfert: our experience and current state of art in the combined use of fat and PRP. Biomed Res Int. 2013;2013:434191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 90. | Cervelli V, Gentile P, De Angelis B, Calabrese C, Di Stefani A, Scioli MG, Curcio BC, Felici M, Orlandi A. Application of enhanced stromal vascular fraction and fat grafting mixed with PRP in post-traumatic lower extremity ulcers. Stem Cell Res. 2011;6:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 91. | De Angelis B, D'Autilio MFLM, Orlandi F, Pepe G, Garcovich S, Scioli MG, Orlandi A, Cervelli V, Gentile P. Wound Healing: In Vitro and In Vivo Evaluation of a Bio-Functionalized Scaffold Based on Hyaluronic Acid and Platelet-Rich Plasma in Chronic Ulcers. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 92. | Elbarbary AH, Hassan HA, Elbendak EA. Autologous platelet-rich plasma injection enhances healing of chronic venous leg ulcer: A prospective randomised study. Int Wound J. 2020;17:992-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 93. | Miłek T, Nagraba Ł, Mitek T, Woźniak W, Mlosek K, Olszewski W, Ciostek P, Deszczyński J, Kuchar E, Stolarczyk A. Autologous Platelet-Rich Plasma Reduces Healing Time of Chronic Venous Leg Ulcers: A Prospective Observational Study. Adv Exp Med Biol. 2019;1176:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 94. | Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I, Expósito JA, Bolíbar I, Rodríguez L, Garcia J, Zaror C. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev. 2016;(5):CD006899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 95. | Weller CD, Gardiner EE, Arthur JF, Southey M, Andrews RK. Autologous platelet-rich plasma for healing chronic venous leg ulcers: Clinical efficacy and potential mechanisms. Int Wound J. 2019;16:788-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 96. | Hesseler MJ, Shyam N. Platelet-rich plasma and its utility in medical dermatology: A systematic review. J Am Acad Dermatol. 2019;81:834-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 97. | Bianchi L, Ginebri A, Hagman JH, Francesconi F, Carboni I, Chimenti S. Local treatment of chronic cutaneous leg ulcers with recombinant human granulocyte-macrophage colony-stimulating factor. J Eur Acad Dermatol Venereol. 2002;16:595-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 98. | Harding K, Sumner M, Cardinal M. A prospective, multicentre, randomised controlled study of human fibroblast-derived dermal substitute (Dermagraft) in patients with venous leg ulcers. Int Wound J. 2013;10:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 99. | Serena TE, Carter MJ, Le LT, Sabo MJ, DiMarco DT; EpiFix VLU Study Group. A multicenter, randomized, controlled clinical trial evaluating the use of dehydrated human amnion/chorion membrane allografts and multilayer compression therapy vs. multilayer compression therapy alone in the treatment of venous leg ulcers. Wound Repair Regen. 2014;22:688-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 100. | Miranda EP, Friedman A. Dehydrated Human Amnion/Chorion Grafts May Accelerate the Healing of Ulcers on Free Flaps in Patients With Venous Insufficiency and/or Lymphedema. Eplasty. 2016;16:e26. [PubMed] |
| 101. | Lullove EJ. Use of a Dehydrated Amniotic Membrane Allograft in the Treatment of Lower Extremity Wounds: A Retrospective Cohort Study. Wounds. 2017;29:346-351. [PubMed] |
| 102. | Garoufalis M, Nagesh D, Sanchez PJ, Lenz R, Park SJ, Ruff JG, Tien A, Goldsmith J, Seat A. Use of Dehydrated Human Amnion/Chorion Membrane Allografts in More Than 100 Patients with Six Major Types of Refractory Nonhealing Wounds. J Am Podiatr Med Assoc. 2018;108:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 103. | Anselmo DS, McGuire JB, Love E, Vlahovic T. Application of Viable Cryopreserved Human Placental Membrane Grafts in the Treatment of Wounds of Diverse Etiologies: A Case Series. Wounds. 2018;30:57-61. [PubMed] |
| 104. | Bianchi C, Tettelbach W, Istwan N, Hubbs B, Kot K, Harris S, Fetterolf D. Variations in study outcomes relative to intention-to-treat and per-protocol data analysis techniques in the evaluation of efficacy for treatment of venous leg ulcers with dehydrated human amnion/chorion membrane allograft. Int Wound J. 2019;16:761-767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |