Published online Oct 26, 2020. doi: 10.12998/wjcc.v8.i20.5042
Peer-review started: May 6, 2020
First decision: May 21, 2020
Revised: June 16, 2020
Accepted: September 2, 2020
Article in press: September 2, 2020
Published online: October 26, 2020
Processing time: 172 Days and 22.4 Hours
Primary chondrosarcoma of the liver are extremely rare. Moreover, there are few reports focusing on typical clinical symptoms and imaging characteristics. Therefore, the diagnosis of chondrosarcoma of the liver remains a challenge.
A 59-year-old male was admitted due to a lesion occupying the right liver lobe that was found by physical examination. Magnetic resonance imaging showed a lobular mass with high T2 weighted image and low T1 weighted image with enhanced internal separation and edge in the right liver. He was diagnosed with liver cystadenoma by using magnetic resonance imaging. At 3 mo later, the magnetic resonance scan showed that the mass was enlarged. Laparoscopic liver tumor resection was performed with a pathological diagnosis of liver chondrosarcoma. Then he received a surgical resection for the recurrent lesion. However, intrahepatic and abdominal metastases were found again at 8 mo after the second operation. The patient then received conservative management and is now under follow-up.
Primary liver chondrosarcoma generally is presented as lobulated and heterogeneous density/signal, cystic, solid masses without calcification with enhanced edge, internal septa and solid part. The imaging features are closely related to pathology, which may be helpful for clinical diagnosis.
Core Tip: Primary chondrosarcoma of the liver is extremely rare. Moreover, there are few studies focusing on typical clinical symptoms and imaging characteristics. Therefore, the diagnosis of chondrosarcoma of the liver remains a challenge. This study analyzed the imaging characteristics of a case of primary liver chondrosarcoma to improve the understanding and diagnosis of this disease, which may help to improve the understanding of the disease.
- Citation: Liu ZY, Jin XM, Yan GH, Jin GY. Primary chondrosarcoma of the liver: A case report. World J Clin Cases 2020; 8(20): 5042-5048
- URL: https://www.wjgnet.com/2307-8960/full/v8/i20/5042.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i20.5042
Chondrosarcoma is a malignancy characterized by cartilage matrix production by tumor cells, which mainly occurs in cartilage tissue or connective tissue. It more commonly involves the cartilage tissue or connective tissue of long bones of the extremities and pelvis and rarely occurs in soft tissue or other organs, such as the lung, heart, liver, pancreas and kidney, which are known as extraskeletal chondrosarcomas. Extraskeletal chondrosarcomas were first reported by Stout et al[1] and account for 3% of all soft tissue sarcomas. It has been shown that extraskeletal chondrosarcomas have a histopathological characteristic of chondrocyte-like differentiation but no obvious cartilage areas, no connection with bone structure or punctate calcification. Extraskeletal chondrosarcomas are classified as “uncertain differentiated tumors” in the 2013 World Health Organization Soft Tissue Sarcomas[2]. Extraskeletal chondrosarcomas originating in the liver are rarely observed. Therefore, imaging features of liver chondrosarcoma remain insufficient. In this study, we analyzed the imaging characteristics of a case with primary liver chondrosarcoma to improve the understanding and diagnosis of this disease.
A 59-year-old male was admitted after a physical examination discovered a lesion occupying the right liver lobe.
He had a 40-year history of hepatitis B and received oral “Entecavir” antiviral therapy.
Laboratory tests and tests on tumor markers showed no abnormalities. Ultrasound showed an irregular hypoechoic region in the right posterior lobe of the liver with a poorly smoothed capsule. The uneven echo distribution and a 4.3 cm × 3.8 cm strip-shaped blood flow signal were observed within the hypoechoic region.
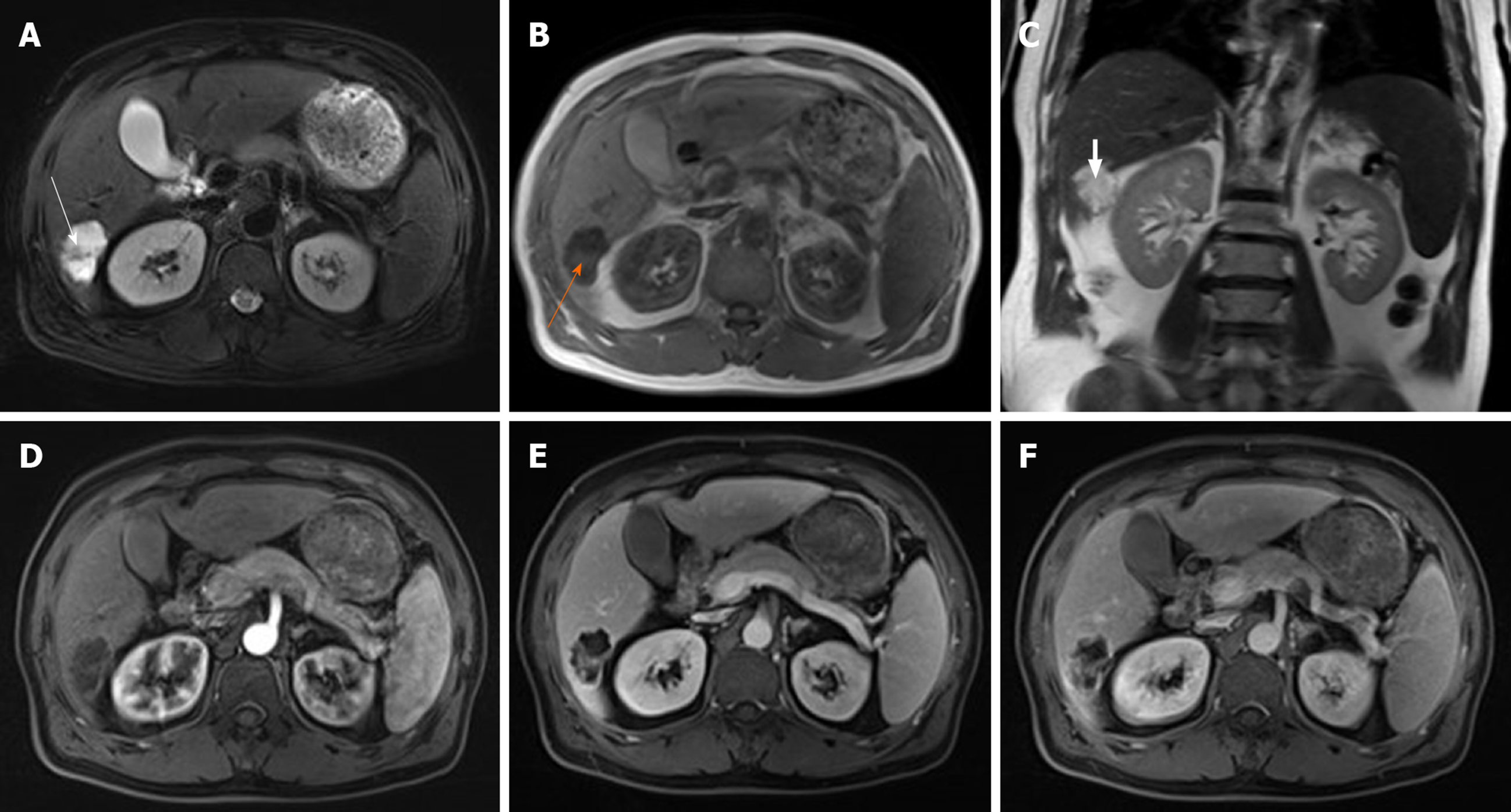
Magnetic resonance imaging (MRI) showed masses in the posterior lower lobe of the right lobe with a high T2 weighted image (T2WI) signal, low signal rings and separated low signals. The lesion had a low signal in the T1WI positive and negative phases with patches and other signals in the center, had a high diffusion weighted imaging signal with an intralesion patchy low signal and a significantly high signal apparent diffusion coefficient with a low intralesion signal. The lesion had clear boundaries and defoliated edges with a size of 3.1 cm × 4.3 cm × 3.7 cm. No dilatation of the internal bile duct was observed (Figure 1A-C). The edge of the lesion and its internal separating arteries were slightly enhanced during the enhanced scan and continuously enhanced during portal vein and the delayed phase. No lymphadenopathy was seen in the abdominal cavity (Figure 1D-F).
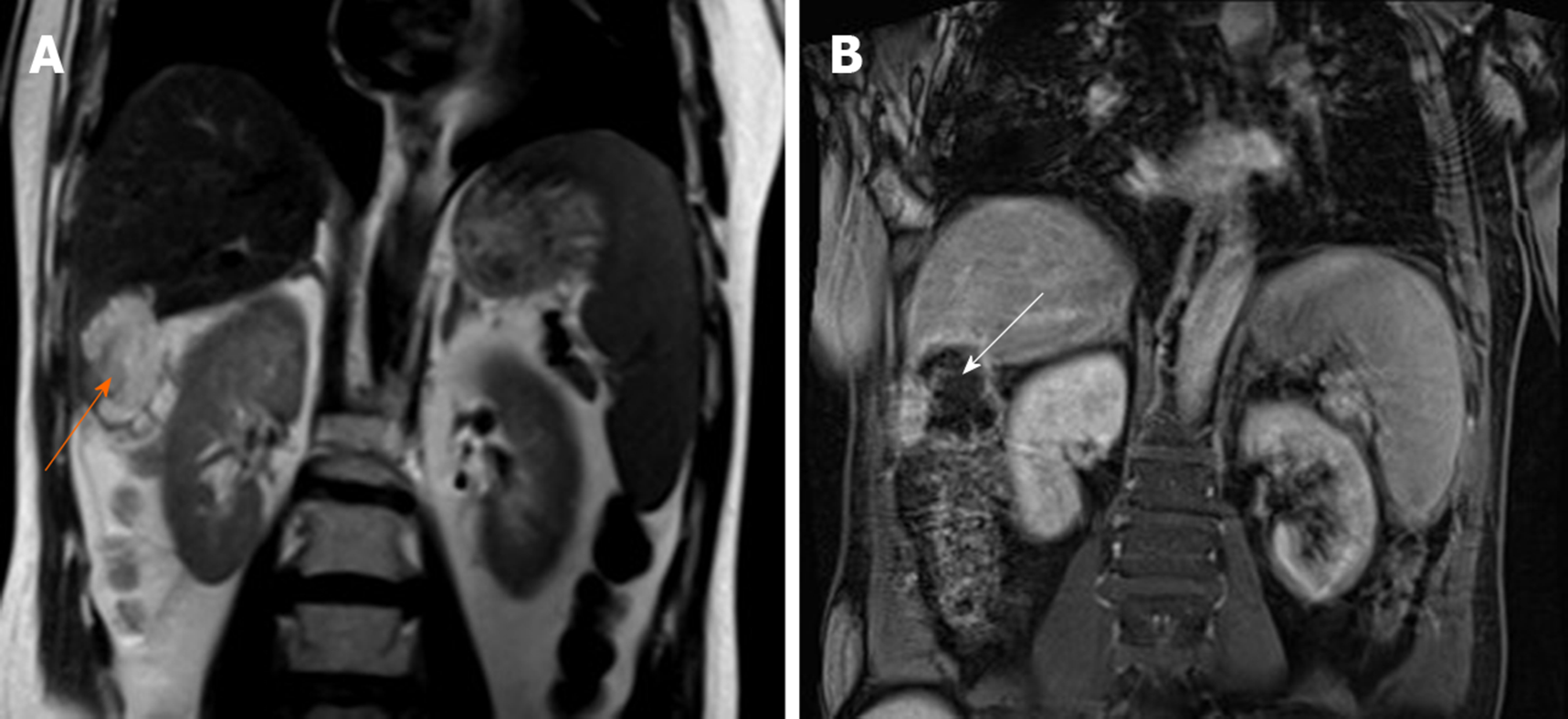
Diagnostic results of MRI: Occupied lesion in the right lobe of the liver, suggesting cystadenomas. He did not receive further treatment. After 3 mo, he received an MRI scan again, which showed a larger mass with obvious lobulated margin in the right lobe with a size of 4.3 cm × 5.3 cm × 4.9 cm (Figures 2A and B). The relationship between lesions and colonic liver curvature were unclear. Therefore, he was admitted for surgical resection. Intraoperatively, a hard, cauliflower-like mass with size of 5 cm × 6 cm × 5 cm in the lower posterior segment of the right lobe of the moderate sclerosis liver was observed, which adhered to the colonic hepatic mesangium and the right renal capsule.
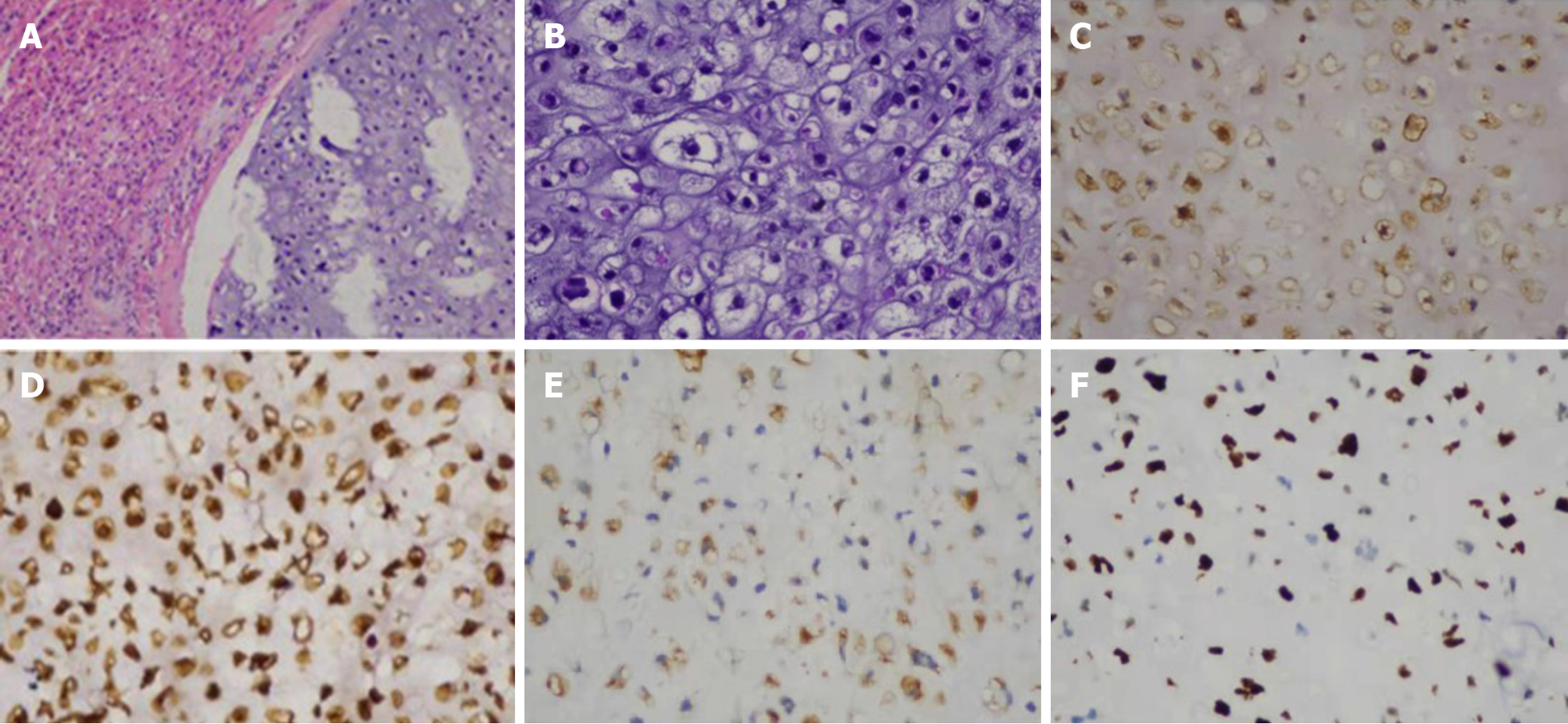
Pathological gross appearance showed that the cut surface of the tumor was gray-blue, nodular, with a sense of mucus, hard and brittle, and the tumor showed swelling to the surrounding liver tissue. Microscopic pathological examination showed a lobulated tumor with sparsely distributed tumor cells and cartilage pit formation (Figures 3A and B). Tumor cell nuclei were heterogeneous. The cells were mostly mononuclear but double nuclei were also observed. Mitotic cells were commonly observed. There was a transparent cartilage matrix among the cells. The tumor was presented as infiltrating growth to the surrounding liver tissue. The surrounding liver tissue showed nodular cirrhosis. Immunohistochemistry showed vimentin (+), S-100 (+) and Ki67 (60% +) (Figures 3C-E). The pathological diagnosis was chondrosarcoma of the liver.
Emission computed tomography (CT) was performed after surgery and found no space-occupying lesions. Therefore, he was diagnosed as primary liver chondrosarcoma. He had an uneventful postoperative course and received no other treatments. A relapse was found at 8 mo following surgery. He received laparoscopic tumor resection. At 8 mo following the second surgery, he was admitted due to melanosis, fatigue and intermittent midupper abdominal pain.
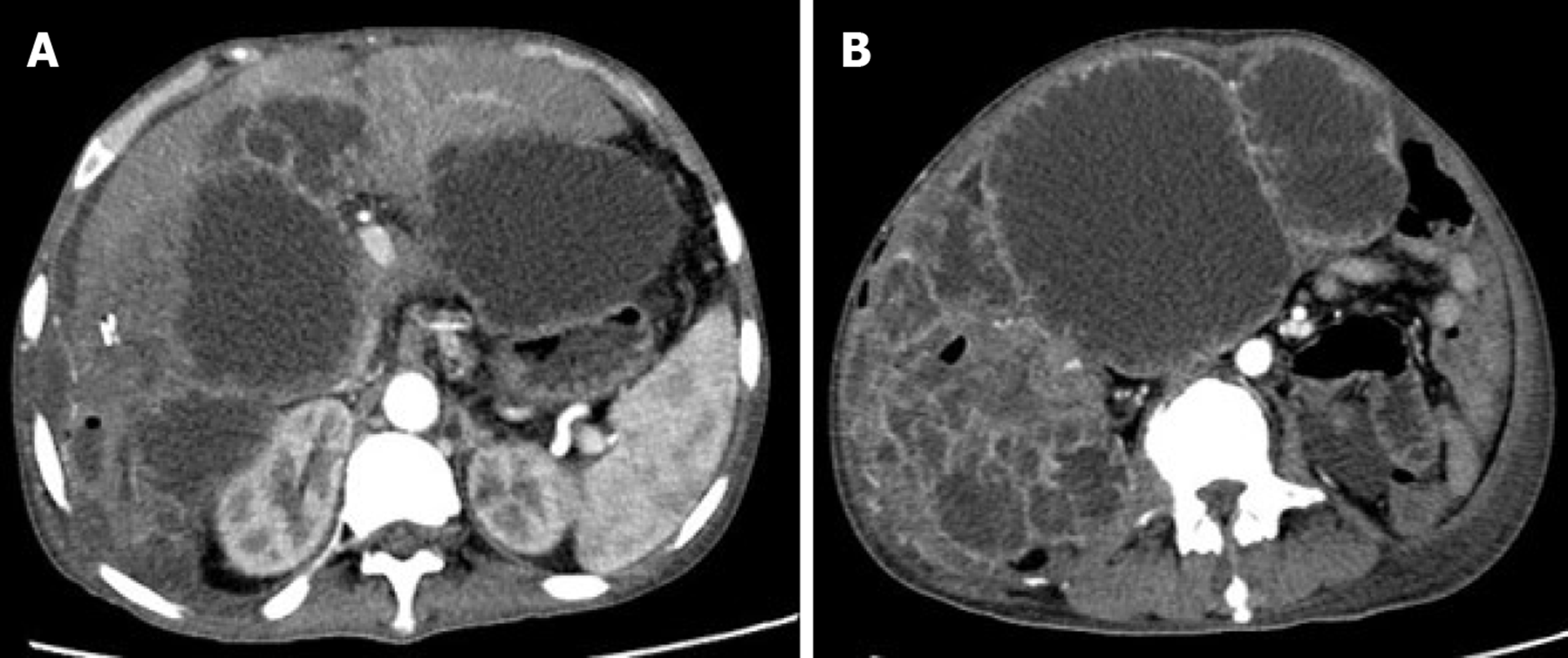
Physical examination showed a 10 cm × 15 cm mass in the right abdomen. CT scan showed multiple metastases within the liver and abdomen, presenting as multiple cystic masses with marginal lobes and enhanced posterior marginal enhancement, which were like the primary imaging findings (Figures 4A and B). Then, the patient received conservative management and is being followed up until now.
Primary liver chondrosarcoma after surgery, multiple metastases within the liver and abdomen.
Abdominal drainage and anti-inflammatory and liver-protecting symptomatic treatment
The patient was discharged after treatment in good and stable condition, and the general condition of the patient was fair after follow-up.
Extraskeletal chondrosarcoma is a rare ectopic tumor. Over 90% of patients have a characteristic translocation that involves the NR4A3 gene on chromosome 9[3]. Extraskeletal chondrosarcoma, which can be divided into myxoid, mesenchymal and differentiated types[4], is commonly observed in the deep soft tissues of the proximal limbs with the highest incidence at the age of 50-60 years and more common in males[5,6].
The incidence of chondrosarcoma in the liver is extremely low. There is no relevant literature abroad. Only two case reports focusing on primary hepatic chondrosarcoma in China were found[7,8]. Both cases were middle-aged/elderly and male. The main clinical symptom was upper abdominal discomfort but without obvious pain, and both lesions occurred in the right lobe of the liver. Their imaging manifestations were very similar to those of liver cystic tumors, and both were misdiagnosed as hepatic cystadenoma or cystadenocarcinoma before surgery. In this study, the MRI manifestations of liver chondrosarcoma were lobular masses with clear boundaries, obvious high T2WI and diffusion weighted imaging signals with uneven intratumor low signal separation. The lesion had a low T1WI signal without bleeding. There was a capsule around the lesion with a low T2WI signal. After enhancement, the edge of the lesion and its internal separation continually enhanced. CT showed a liquid density mass with lobulated margin and no calcification and same enhancement as that of the MRI.
Chondrosarcoma of the liver should be differentiated from hepatic cystadenoma or cystadenocarcinoma, hepatic hydatid cyst, secondary infection of liver cyst, liver abscess and cystic liver metastases. Hepatic cystadenoma or cystadenocarcinoma is a rare liver epithelial tumor that occurs in middle-aged and elderly females without history of hepatitis and cirrhosis[9]. The typical CT image characteristics of hepatic cystadenoma or cystadenocarcinoma are single or multiple circular cystic masses with smooth capsule wall, intracapsule separation and calcification[10]. In the enhanced phrase, the posterior capsule wall, the capsule nodules and intracapsule separation are enhanced lightly and moderately but not the capsule fluid, which is very similar to the imaging of the case in our study.
Most patients with hepatic hydatid cyst have a history of nomadic life. The hepatic hydatid cyst is usually presented as single or multiple cystic lesions of the liver with a thick cyst wall and intracyst separation, cyst wall calcification, floating ascus and scolices in the cyst[11]. For cases with secondary infection of liver cysts, the cyst wall is thickened uniformly and without wall nodules, small air bubbles can appear in the cyst, and a circular edema band is seen around the cyst wall[12]. The cyst wall is evenly enhanced following the enhancement phase. Clinical manifestations of patients with liver abscess are fever, elevated white blood cell levels and pain in the liver area[13]. CT of a liver abscess is manifested as circular or quasicircular low-density shadows in the liver parenchyma with even or uneven density[14]. CT values are higher than water but lower than liver parenchyma. Enhanced scanning shows lesions with a “double ring sign” and a “petal sign,” which is decreased in the delayed scan phase[15].
Primary lesions of cystic liver metastases are found in leiomyosarcoma of the digestive tract and colorectal cancer. Most lesions present as multiple lesions with a round shape, while a few are presented as lobulated shape[16]. When cystic lesions or cystic and parenchymal lesions coexist, the cyst wall can be thin and thick, wall nodules are sometimes seen, and the lesion may have incomplete separation and fluid-fluid level[17]. The capsule wall, wall nodules and separation can be enhanced during the enhanced phase.
Primary liver chondrosarcoma is extremely rare in the clinic, which also has no specified clinical manifestations. The diagnosis of liver chondrosarcoma mainly depends on pathological evaluation. Imaging characteristics can facilitate the early diagnosis of liver chondrosarcoma. The local recurrence rate of extraskeletal chondrosarcomas is 37%-48%[5], with a metastasis rate of 50%[5]. Extraskeletal chondrosarcoma is not sensitive to chemotherapy and radiotherapy. Currently, early surgical resection is considered the most effective treatment method for extraskeletal chondrosarcomas[18,19]. In this study, the patient was misdiagnosed as a hepatic cystadenoma due to lack of understanding of the imaging manifestations of liver chondrosarcoma. The lesion was increasingly enlarged, and surgical resection was performed after 3 mo. Thereafter, he received a surgical resection for recurrent lesion. However, intrahepatic and abdominal metastases were found again after the second operation. Thereafter, conservative treatments and regular follow-up was administrated.
In conclusion, primary liver chondrosarcoma is very rare, and it is difficult to accurately diagnose only by imaging findings. The diagnosis is confirmed mainly based on pathological evaluation and immunohistochemical phenotypes. The imaging characteristics of the patient with primary liver chondrosarcoma in this study may help to improve the understanding of the disease.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ieni A S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Liu JH
| 1. | Stout AP, Verner EW. Chondrosarcoma of the extraskeletal soft tissues. Cancer. 1953;6:581-590. [PubMed] [DOI] [Full Text] |
| 2. | Balanzá R, Arrangoiz R, Cordera F, Muñoz M, Luque-de-León E, Moreno E, Molinar L, Somerville N. Pulmonary extraskeletal myxoid chondrosarcoma: A case report and literature review. Int J Surg Case Rep. 2016;27:96-101. [PubMed] [DOI] [Full Text] |
| 3. | Paoluzzi L, Ghesani M. Extraskeletal myxoid chondrosarcoma with massive pulmonary metastases. Clin Sarcoma Res. 2018;8:20. [PubMed] [DOI] [Full Text] |
| 4. | Kim H, Nakaichi M, Itamoto K, Taura Y. Primary chondrosarcoma in the skull of a dog. J Vet Sci. 2007;8:99-101. [PubMed] [DOI] [Full Text] |
| 5. | Zhang L, Wang R, Xu R, Qin G, Yang L. Extraskeletal Myxoid Chondrosarcoma: A Comparative Study of Imaging and Pathology. Biomed Res Int. 2018;2018:9684268. [PubMed] [DOI] [Full Text] |
| 6. | Drilon AD, Popat S, Bhuchar G, D'Adamo DR, Keohan ML, Fisher C, Antonescu CR, Singer S, Brennan MF, Judson I, Maki RG. Extraskeletal myxoid chondrosarcoma: a retrospective review from 2 referral centers emphasizing long-term outcomes with surgery and chemotherapy. Cancer. 2008;113:3364-3371. [PubMed] [DOI] [Full Text] |
| 7. | Zheng Q, Xiao F, Luo C. Chondrosarcoma of liver: case report. J Clin Exp Pathol. 2011;27:794-795. [DOI] [Full Text] |
| 8. | Zhou W, Guo J. Chondrosarcoma of the liver: 1 case report. Shandong Yiyao. 2011;51:115. [DOI] [Full Text] |
| 9. | Martel G, Alsharif J, Aubin JM, Marginean C, Mimeault R, Fairfull-Smith RJ, Mohammad WM, Balaa FK. The management of hepatobiliary cystadenomas: lessons learned. HPB (Oxford). 2013;15:617-622. [PubMed] [DOI] [Full Text] |
| 10. | Tholomier C, Wang Y, Aleynikova O, Vanounou T, Pelletier JS. Biliary mucinous cystic neoplasm mimicking a hydatid cyst: a case report and literature review. BMC Gastroenterol. 2019;19:103. [PubMed] [DOI] [Full Text] |
| 11. | Mihmanli M, Idiz UO, Kaya C, Demir U, Bostanci O, Omeroglu S, Bozkurt E. Current status of diagnosis and treatment of hepatic echinococcosis. World J Hepatol. 2016;8:1169-1181. [PubMed] [DOI] [Full Text] |
| 12. | Balbo BE, Sapienza MT, Ono CR, Jayanthi SK, Dettoni JB, Castro I, Onuchic LF. Cyst infection in hospital-admitted autosomal dominant polycystic kidney disease patients is predominantly multifocal and associated with kidney and liver volume. Braz J Med Biol Res. 2014;47:584-593. [PubMed] [DOI] [Full Text] |
| 13. | Law ST, Li KK. Is hepatic neoplasm-related pyogenic liver abscess a distinct clinical entity? World J Gastroenterol. 2012;18:1110-1116. [PubMed] [DOI] [Full Text] |
| 14. | Mortelé KJ, Segatto E, Ros PR. The infected liver: radiologic-pathologic correlation. Radiographics. 2004;24:937-955. [PubMed] [DOI] [Full Text] |
| 15. | Kang S, Zhang H, Liu Q. The Correlation of the Early Stage of Pyogenic Liver Abscess Between Dynamic Contrast Enhanced CT Scan and MR Plain Scan. Zhongguo CT and MRI Zazhi. 2013;11:53-57. [DOI] [Full Text] |
| 16. | Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, Alexander DD, Choti MA, Poston G. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283-301. [PubMed] [DOI] [Full Text] |
| 17. | Yu RS, Fu LP, Li RF. CT diagnosis of malignant tumors of the liver cystic. J Clin Radiol. 1999;158. [DOI] [Full Text] |
| 18. | Davis EJ, Wu YM, Robinson D, Schuetze SM, Baker LH, Athanikar J, Cao XH, Kunju LP, Chinnaiyan AM, Chugh R. Next generation sequencing of extraskeletal myxoid chondrosarcoma. Oncotarget. 2017;8:21770-21777. [PubMed] [DOI] [Full Text] |