Published online Oct 26, 2020. doi: 10.12998/wjcc.v8.i20.4844
Peer-review started: July 16, 2020
First decision: August 21, 2020
Revised: August 23, 2020
Accepted: September 11, 2020
Article in press: September 11, 2020
Published online: October 26, 2020
Processing time: 100 Days and 21.7 Hours
Solitary fibrous tumor (SFT) is a rare fibroblastic mesenchymal neoplasm that affects spindle cell soft tissues with broad-spectrum biological behavior; it is predominantly benign, and rarely metastasizes. SFT occurs mainly in the tissue structure of the serosa in the pleura and the thorax, and can be found throughout the body, though extra-thoracic localization, including the cephalic region, is un-common. We reported the first case of intracranial malignant SFT metastasized to the chest wall.
An 81-year-old Japanese man was referred to our hospital due to progressive gait disturbance and appetite loss. His medical history included partial resection due to brain tumor, four times, and 50-Gray radiation therapy at another hospital, starting when he was 74 years old. An unenhanced head computed tomography (CT) scan revealed an 8 cm × 5.1 cm × 6.5 cm mixed-density mass at the left frontal lobe, accompanying a midline shift, and an unenhanced chest-abdomen CT scan revealed a 6 cm × 4.1 cm × 6.5 cm low-density mass in the left chest wall. A CT-guided percutaneous lung biopsy was performed, and the pathological findings were SFT corresponding to brain tumor. Finally, the correct diagnosis of his brain tumor in history of past illness revealed to be SFT, and the unremovable tumor, namely present brain lesions enlarged and metastasized to the chest wall. We established a definitive diagnosis of intracranial malignant SFT metastasized to the chest wall. We notified him and his family of the disease, and offered palliative care. He passed away on the 29th hospital day.
This case suggests the need for careful, detailed examination, and careful follow-up when encountering patients presenting with a mass.
Core Tip: Solitary fibrous tumor (SFT) is a rare fibroblastic mesenchymal neoplasm that affects spindle cell soft tissues, demonstrating broad-spectrum biological behavior; it is ordinarily benign, and rarely known to metastasize. SFT occurs primarily in the serosa tissue structure in the pleura and the thorax, and can be found throughout the body, though extra-thoracic localization, including the cephalic region, is uncommon. We have reported the first known case of intracranial malignant SFT metastasized to the chest wall; this case suggests the need for careful, detailed examination, and careful follow-up when encountering patients presenting with a mass.
- Citation: Usuda D, Yamada S, Izumida T, Sangen R, Higashikawa T, Nakagawa K, Iguchi M, Kasamaki Y. Intracranial malignant solitary fibrous tumor metastasized to the chest wall: A case report and review of literature. World J Clin Cases 2020; 8(20): 4844-4852
- URL: https://www.wjgnet.com/2307-8960/full/v8/i20/4844.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i20.4844
Solitary fibrous tumor (SFT) is a rare type of fibroblastic mesenchymal neoplasm that affects spindle cell soft tissues, demonstrating broad-spectrum biological behavior; it is ordinarily benign, and rarely known to metastasize[1-7]. SFT occurs primarily in the serosa tissue structure in the pleura and the thorax, and can be found throughout the body, though extra-thoracic localization, including the cephalic region, is uncommon[1-3,8-12].
SFT of the central nervous system (CNS) was first reported by Carneiro et al[13] in 1996, with only limited subsequent reports: Of the roughly 200 described cases, there have been only five reports to date of extracranial hematogenous metastases that include lung, liver, bone, soft tissue, and a combination of two or more regions[14]. Malignant intracranial SFT is exceedingly rare, and in fact the exact origination of CNS SFT remains a controversial topic[9]. Intracranial SFT seemingly tends to demonstrate dural attachment, and is frequently misinterpreted as meningioma when detected either radiologically or surgically[9]. The pleura may be affected by primary tumors, or by the metastasizing and spreading of intrathoracic or extrathoracic neoplasms[12]. A broad variety of other neoplasms also involve the pleura, the most common of which, by a significant margin, is metastatic carcinoma, generally of pulmonary origin[15]. It is less common, but still possible, for other metastatic tumors of various histogenesis to occur, as well[15]. Though SFT of the pleura is rarely encountered as a benign neoplasm, it may have malignant characteristics[16]. To date, there have not been any reports of intracranial malignant SFT metastasizing to the chest wall; therefore, we have reported the first such case, together with a brief review of literature.
An 81-year-old Japanese man was referred to our hospital due to progressive gait disturbance and appetite loss.
His medical history included partial resection due to brain tumor, four times, starting when he was 74 years old, and 50-Gray radiation therapy when he was 77 years old, at another hospital. In addition, he was phlebotomized three times due to polycythemia vera when he was 81 years old, and was diagnosed with Alzheimer’s disease. He was given 90 mg of phenobarbital, 500 mg of hydroxycarbamide, and 5 mg of donepezil on a regular basis. He had no history of smoking or drinking alcohol, and no history of exposure to asbestos. He did not undergo regular medical exams. The patient was unemployed, and had no food or drug allergies. However, he needed full assistance for everyday life activities. He presented no family history of malignant disease.
The patient was 158 cm tall and weighed 52 kg. His vital signs were abnormal, with blood pressure of 94/77 mmHg, heart rate of 80 regular beats/min, body temperature of 36 °C, oxygen saturation of 97% in ambient air, and respiratory rate of 16/min; his glasgow coma scale score was 15 (E4V5M6) points. He complained of right hemiparesis, and manual muscle testing for the right half of the body had a grade of 2. On the other hand, nothing else abnormal was detected upon physical examination, including skin or neurological findings.
Routine laboratory examination revealed increased values of white blood cells, red blood cells, hemoglobin, hematocrit, aspartate transaminase, alanine aminotransferase, lactate dehydrogenase, alkaline phosphatase, gamma-glutamyl transpeptidase, C-reactive protein, and plasma glucose, and decreased values of creatine phosphokinase, creatinine, and sodium. However, other values were normal, including complete blood count, biochemistry, and tumor marker for diagnosing lung cancer (Table 1).
| Parameter (units) | Measured value | Normal value |
| White blood cell (103/µL) | 18 | 3.8-8.5 |
| Neutrophil (%) | 84.4 | 48-61 |
| Lymphocyte (%) | 8.4 | 25-45 |
| Monocyte (%) | 3.2 | 4-7 |
| Eosinophil (%) | 3.6 | 1-5 |
| Basophil (%) | 0.4 | 0-1 |
| Red blood cell (106/µL) | 7.04 | 3.78-4.97 |
| Hemoglobin | 17.8 | 13.7-17.4 |
| Hematocrit | 56.9 | 40.2-51.5 |
| Platelet (103/µL) | 214 | 131-365 |
| Aspartate transaminase (IU/L) | 65 | 12-31 |
| Alanine aminotransferase (IU/L) | 43 | 8-40 |
| Lactic acid dehydrogenase (IU/L) | 592 | 110-210 |
| Alkaline phosphatase (IU/L) | 1597 | 100-330 |
| Gamma-glutamyl transpeptidase (IU/L) | 473 | 9-49 |
| Total bilirubin (mg/dL) | 0.56 | 0.3-1.2 |
| Total protein (g/dL) | 6.9 | 6.7-8.3 |
| Albumin (g/dL) | 4.2 | 3.9-4.9 |
| Creatine phosphokinase (IU/L) | 25 | 65-275 |
| Blood urea nitrogen (mg/dL) | 15.4 | 8-22 |
| Creatinine (mg/dL) | 0.48 | 0.4-0.8 |
| Amylase (IU/L) | 77 | 39-134 |
| Sodium (mEq/L) | 137 | 138-146 |
| Potassium (mEq/L) | 4.8 | 3.6-4.9 |
| Chloride (mEq/L) | 103 | 99-109 |
| C-reactive protein (mg/dL) | 2.01 | 0-0.4 |
| Plasma glucose (mg/dL) | 128 | 70-109 |
| Carcinoembryonic antigen (ng/mL) | 1 | 0-5 |
| Carbohydrate antigen 19-9 (U/mL) | 11.4 | 0-37 |
| Squamous cell carcinoma related antigen antigen (ng/mL) | 1.3 | 0-1.5 |
| Pro-gastrin-releasing peptide (pg/mL) | 45 | 0-69 |
| Beta-D-glucan (pg/mL) | 7.6 | 0-20 |
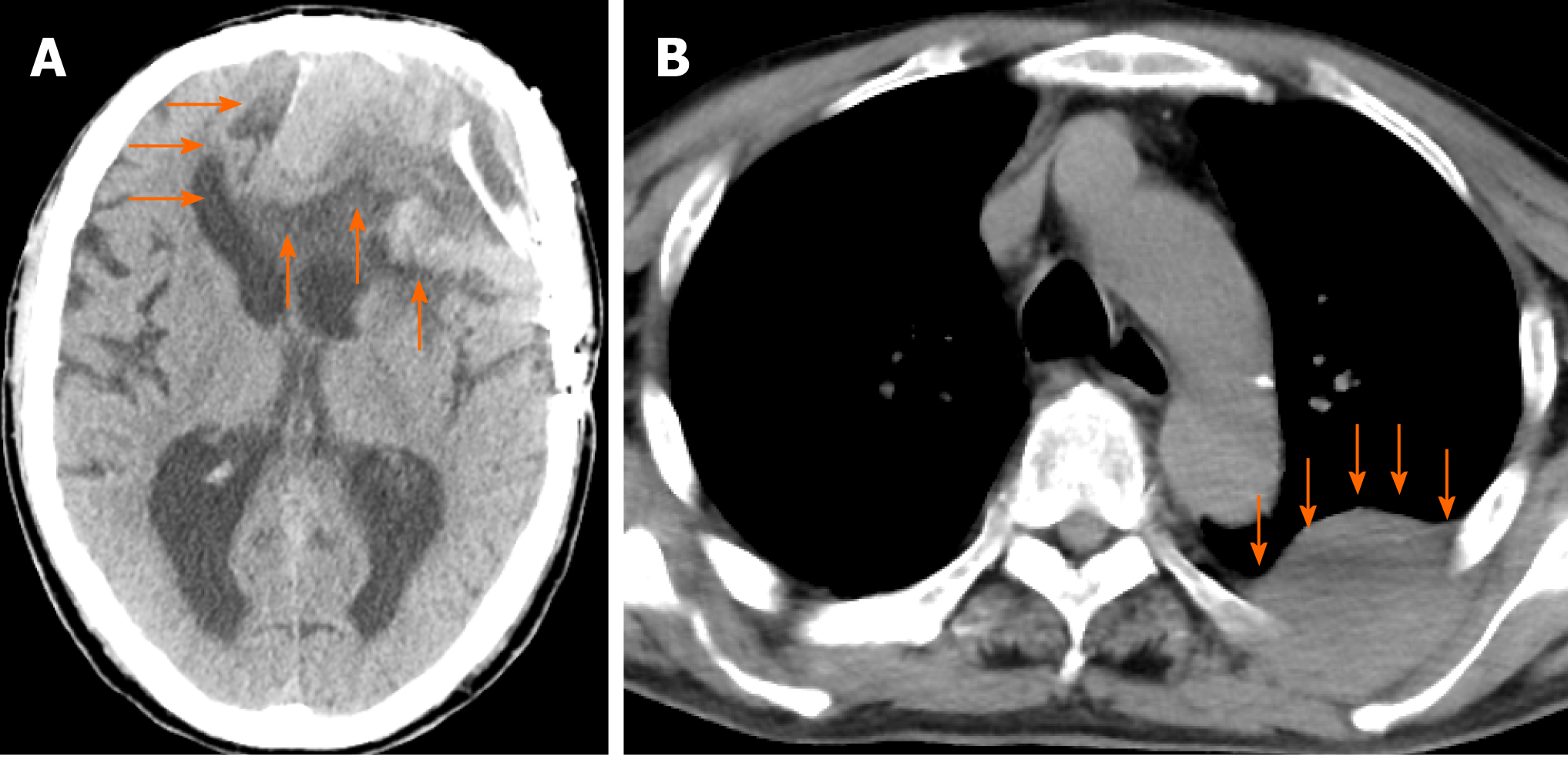
An unenhanced head computed tomography (CT) scan revealed an 8 cm × 5.1 cm × 6.5 cm mixed-density mass at the left frontal lobe, accompanying a midline shift (Figure 1A). An unenhanced chest-abdomen CT scan revealed a 6 cm × 4.1 cm × 6.5 cm low-density mass in the left chest wall at the superior segment, namely S6 (Figure 1B).
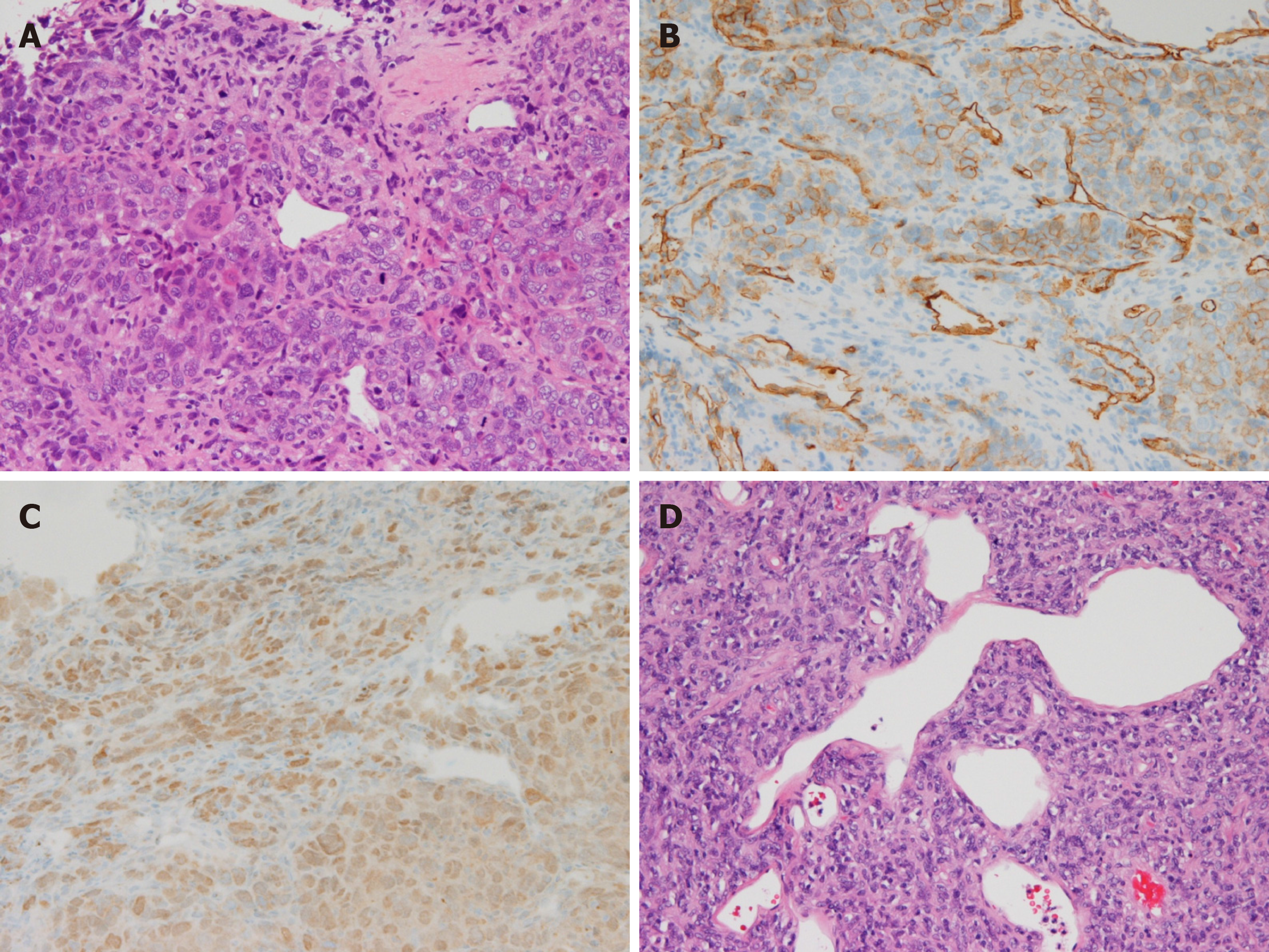
Malignancy was not detected through upper esophagogastroduodenoscopy and colonoscopy. At this point, we suspected a diagnosis of brain tumor plus metastatic chest wall tumor. The patient was hospitalized for further examination and treatment, and we performed a CT-guided percutaneous lung biopsy. The hematoxylin and eosin stain pathological findings revealed the following (Figure 2A): A solid proliferation of atypical polymorphic cells, including spindle-shaped cells, multinucleated giant cells, and blood vessels with a wall irregularity were dominant, with mitosis and pericytomatous patterns in part. Immunohistochemical staining revealed positive results for Vimentin and cluster of differentiation (CD) 34-positive cells, and confirmed signal transducer and activator of transcription (STAT) 6-positive cells (Figure 2B and C, respectively). We obtained the pathological specimen to the hospital where his intracranial surgery had been performed, and it was found to be the same as that of the chest wall mass (Figure 2D). Finally, the correct diagnosis of his brain tumor in history of past illness revealed to be SFT, and the unremovable tumor, namely present brain lesions enlarged and metastasized to the chest wall.
Here, we established a definitive diagnosis of intracranial malignant SFT metastasized to the chest wall.
We notified the patient and his family of the disease, and offered palliative care.
The patient passed away on the 29th hospital day.
We present the first known case of intracranial malignant SFT metastasized to the chest wall. In a study of clinical characteristics and outcomes for SFT, 57% of the patients were women and 89% were of Caucasian descent[17]. Among these patients, the median age was 62 years (with a range from 20 to 89), and 39% of the patients ultimately died of the disease; for 34% of these patients, the primary tumor site was the lung/pleura, the abdomen/pelvis for 28%, an extremity for 16%, and in the head or neck for 11% of patients[17]. On the other hand, the exact incidence of SFT in the CNS is unclear, though reportedly low[9]. CNS SFT occurs with roughly equal gender distribution, in patients of a wide range of ages, with incidence rates peaking in the fifth and sixth decades of life, though a small number of cases have been described as occurring in children[9]. Also, it is well established that the vast majority of SFT involving CNS (over 94%) is benign, and that it rarely recurs or metastasizes[9].
According to Fargen’s review, most patients of SFT in the CNS presented with headache (50%), followed by imbalanced gait (22%), weakness (19%), loss of visual acuity (19%), dysfunction of the cranial nerve (17%), nausea and vomiting (13%), and confusion or other altered mental state (12%)[9].
CT scans remain the norm for evaluating thoracic SFT[18]. Magnetic resonance imaging (MRI) scans are useful for delineating tumors and evaluating their invasion into adjacent organs, such as the diaphragm, the chest wall, or nerves; consequently, MRI scans serve a complementary role to these other imaging modalities for both disease staging and patient evaluation[12,15,18]. SFT imaging findings can vary, and resemble meningioma in imaging; the appearance on T2-weighted MRI scans of nonspecific yet well-circumscribed ovoid or rounded masses with characteristic hypo-intensity, and avid enhancement on CT and MRI scans, may imply an SFT diagnosis[18,19]. In addition, CT scans and/or fluorodeoxyglucose positron emission tomography/CT scans can be used for initial evaluations of pleural neoplasms[12].
The World Health Organization (WHO) classification for soft tissue tumors regards hemangiopericytoma (HPC) and SFT as a continuous spectrum of neoplasia, and many lesions that were considered HPC before 1990 would now be regarded as SFT[9]. Morphologically, SFT is generally characterized by hypercellularity alternating with hypocellularity, with spindle cells between irregular hyalinized collagen bundles, with a pattern-less architecture and a focal HPC-like perivascular pattern rich in staghorn vasculature; however, there is a broad spectrum of both SFT morphology and biological behavior[1,4,9,20]. Over half of all SFT cases test positive for CD34, CD99, ALDH1, and bcl-2, and strong Vimentin staining is also common[1,4,9,20]. In the CNS, SFT is ordinarily a dura-based, meningioma-like mass, known to develop out of the commonly found CD34-positive dendritic interstitial cells of meningeal coverings, with no meningothelial differentiation[9]. SFT ordinarily tests negative for the S-100, GFAP, EMA, cytokeratin, and vascular antigens[9].
Recent research has validated the diagnostic utility of the nuclear expression of STAT6, a novel marker, as a way to distinguish SFT from potential histologic mimics[9,21]. Moreover, identifying the NGFI-A-binding protein 2 (NAB2)-STAT6 fusion gene can provide valuable diagnostic information, including in tissue that is formalin-fixed or paraffin-embedded, or when there is only limited biopsy material[9,21-23]. There are several NAB2-STAT6 fusion subtypes, but their clinical significances are not fully understood[24]. Moreover, the malignant progression mechanisms remain unclear[24]. On the other hand, the telomerase reverse transcriptase (TERT) gene promoter mutation activation is associated with aggressive histopathological features, indicating that it plays a role in the progression of tumors[22].
A conclusive diagnosis depends primarily on close examination of the histological and immunohistochemical features[9,16]. SFT has cytopathologic features that are found whether benign or malignant, increasing the difficulty of distinguishing between them[21]. The 2013 WHO classification of soft tissue tumors defines malignant forms as being hypercellular, mitotically active [> 4 mitosis/10 high-power fields (HPF)], with cytological atypia, tumor necrosis, and/or infiltrative margins[17]. While SFT with classical morphologic features is ordinarily indolent, and while SFT with overtly malignant histologic features tends to be an aggressive neoplasm behaving similarly to a high-grade sarcoma, SFT behavior is ultimately difficult to predict, underscoring the importance of awareness of SFT’s propensity for aggressive behavior in a minority of cases of histologically classical SFT, and the importance of ensuring satisfactory clinical follow-up[4]. At present, there are no criteria used to determine malignancy based on tumor size in cerebral SFT[9]. Furthermore, approximately 8% of cases have demonstrated what have been deemed “atypical features” based upon brain or bone invasion, marked pleomorphism, or high mitotic indices[9].
Surgical excision remains the gold standard for treating localized SFT, and remains the only curative option available; however, a complete resection of intracranial metastatic SFT can prove challenging[4,9,17,18,25]. In recent cases, combining surgery with radiotherapy has led to excellent local control in the treatment of soft tissue SFT[26]. In the event that surgical resection is unfeasible or has proven unsuccessful, alternative therapies have been described as having heterogeneous efficacy[27]. While only limited efficacy has been demonstrated by radiotherapy and conventional chemotherapeutic agents, novel targeted therapies that use antiangiogenic action have been found to show therapeutic efficacy in patients who have advanced or metastatic SFT[4,7,16,18,28]. Pazopanib has been found to be an effective option for the treatment of recurrent or metastatic SFT in first-, second-, and third-line settings[25,29,30]. However, this can lead to liver injury as a major adverse event, and when necessary, it is also important to take adequate treatment withdrawal and dose reduction into consideration[30]. Some scholars have suggested an intraoperative pathologic examination of CNS SFT to exclude the residual of the tumor at the incisal margin[9]. In cases such as these, adjuvant chemotherapy or radiotherapy may help to prevent recurrence[9]. However, to date, no studies have demonstrated radiotherapy’s role in improving long-term prognosis, and there is still too small a number of patients who have undergone adjuvant chemotherapy to be able to evaluate any possible benefit[9]. On the other hand, a study performed recently suggests that gamma knife radiosurgery may serve as a feasible adjunct for the treatment of SFT[9]. Finally, palliative systemic therapy is used in cases that are inoperable or metastatic, but with only low response rates[25].
Limited knowledge is available regarding SFT, about 15% of cases of which are malignant, with a 10%-30% likelihood of metastasizing[18,23,31,32]. Furthermore, meta-stases can occur in any age group, both early and late, in varying locations, and are typically diagnosed only after presentation with symptoms[19]. A minority of cases demonstrate more aggressive behavior, and even years after treatment, relapses, local recurrence, and distant metastasis can sometimes occur[11,19]. The metastatic disease sites vary by primary SFT site and the most common sites of SFT metastasis are, in order from most to least common, the lungs (61%), followed by the pleura (49%), the liver (41%), the bones (41%), and the peritoneum (41%)[33]. In another study, there was a 13% risk of metastasis in the independent cohort after five years, and a 16% risk of metastasis after ten years[31]. There is also a significant association between a mitotic count of ≥ 4 per 10 HPF, or malignant pathology, with metastatic SFT disease[33]. Therefore, long-term monitoring is essential, including regular clinical reviews, and early imaging is useful for recurrence and metastasis symptoms, with imaging modality selected based on clinical concern[19,34,35].
Malignant SFT in particular showed a rapid local recurrence and distant metastasis, with a poor prognosis[9]. In some SFT study, the tumor recurred in 22.5% of cases and metastasized in 11.8% of cases, while tumor death was seen in in 9.6% of cases[20]. The incidence of local recurrence and metastatic recurrence are both strongly associated with disease-specific death, and these events may occur as much as 10-20 years after the initial presentation, including in patients whose tumors were initially considered to be benign[19,34,35].
The key indicators of clinical outcome are still the dimensional pattern, the histopathological features, and curative surgery[1]. Regarding histopathological features, dedifferentiation serves as a key prognostic factor for SFT, and specific locations like cerebro-meningeal and intra-abdominal sites, and hypoglycemia, also led to a high risk of unfavorable prognosis[20]. Regarding curative surgery, it is most likely that the prognosis depends on the extent of complete resection, rather than on histologic grading[9]. Recurrence was commonly noted in cases that involved incomplete excision[9]. While gross total resection is demonstrably superior to subtotal/partial resection in treatment of CNS SFT, with a nearly 16-fold improvement of the odds of recurrence over patients who only undergo subtotal/partial resection, careful follow-up is still necessary, especially when gross total resection is unsuccessful or not an option[9]. According to the soft tissue and bone tumor classification published by the WHO in 2013, necessary criteria suggesting SFT malignant potential include hypercellularity, > 4 mitoses/10 HPFs, variable cytological atypia, tumor necrosis, and/or infiltrative margins; among these, mitoses seem to be the most effectively prognostic[9].
This case study has a limitation: This article only reviews a single case report and case series of SFT. Therefore, the actual situation and nature of SFT may differ from the results of the literature review as a result of reporting bias.
In conclusion, we have presented the first case of intracranial malignant SFT metastasizing to the chest wall. This case suggests the need for careful, detailed examinations when encountering patients presenting with a mass: When a neoplastic lesion is confirmed through image inspection, we should investigate thoroughly, including further image investigations and pathologic examination. In addition, careful follow-up is also necessary.
The author would like to thank Dr. Takeshima K, from Department of General Medicine, Kanazawa Medical University Himi Municipal Hospital, for her invaluable help with review of the literature.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huo Q, Park SB S-Editor: Yan JP L-Editor: A P-Editor: Xing YX
| 1. | Testa DC, Selvaggi F, Andreano T, Mazzola L, Cortellese R. Solitary fibrous tumor of the anterior abdominal wall. A case report and review of the literature. Ann Ital Chir. 2019;8:S2239253X19030068. [PubMed] |
| 2. | Zhang N, Zhou D, Chen K, Zhang H, Huang B. Malignant solitary fibrous tumor of the kidney with liver metastasis: A case report and literature review. J Cancer Res Ther. 2018;14:S1217-S1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Ronchi A, La Mantia E, Gigantino V, Perdonà S, De Sio M, Facchini G, Franco R, De Chiara A. A rare case of malignant solitary fibrous tumor in prostate with review of the literature. Diagn Pathol. 2017;12:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Thway K, Ng W, Noujaim J, Jones RL, Fisher C. The Current Status of Solitary Fibrous Tumor: Diagnostic Features, Variants, and Genetics. Int J Surg Pathol. 2016;24:281-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 5. | Ng HK, Choi PC, Wong CW, To KF, Poon WS. Metastatic solitary fibrous tumor of the meninges. Case report. J Neurosurg. 2000;93:490-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Bianchi G, Sambri A, Pedrini E, Pazzaglia L, Sangiorgi L, Ruengwanichayakun P, Donati D, Benassi MS, Righi A. Histological and molecular features of solitary fibrous tumor of the extremities: clinical correlation. Virchows Arch. 2020;476:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Wang Y, Zhang J, Liu Q, Liu F, Zhu X, Zhang J. Solitary fibrous tumor of the pineal region with delayed ectopic intracranial metastasis: A case report and review of the literature. Medicine (Baltimore). 2019;98:e15737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Chen N, Slater K. Solitary fibrous tumour of the liver-report on metastasis and local recurrence of a malignant case and review of literature. World J Surg Oncol. 2017;15:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Wu Z, Yang H, Weng D, Ding Y. Rapid recurrence and bilateral lungs, multiple bone metastasis of malignant solitary fibrous tumor of the right occipital lobe: report of a case and review. Diagn Pathol. 2015;10:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Baněčková M, Martínek P, Skálová A, Mezencev R, Hadravský L, Michal M, Švajdler M. Solitary fibrous tumors of the head and neck region revisited: a single-institution study of 20 cases and review of the literature. Hum Pathol. 2020;99:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Friis RB, Safwat A, Baad-Hansen T, Aggerholm-Pedersen N. Solitary Fibrous Tumour: A Single Institution Retrospective Study and Further Validation of a Prognostic Risk Assessment System. Clin Oncol (R Coll Radiol). 2018;30:798-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Carter BW, Betancourt SL, Shroff GS, Lichtenberger JP 3rd. MR Imaging of Pleural Neoplasms. Top Magn Reson Imaging. 2018;27:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Carneiro SS, Scheithauer BW, Nascimento AG, Hirose T, Davis DH. Solitary fibrous tumor of the meninges: a lesion distinct from fibrous meningioma. A clinicopathologic and immunohistochemical study. Am J Clin Pathol. 1996;106:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 277] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Osuga T, Hayashi T, Ishiwatari H, Ono M, Yoshida M, Kimura Y, Hasegawa T, Sato Y, Sato T, Miyanishi K, Takimoto R, Kobune M, Kato J. Pancreatic metastasis from a solitary fibrous tumor of the central nervous system. JOP. 2014;15:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 15. | Attanoos RL, Pugh MR. The Diagnosis of Pleural Tumors Other Than Mesothelioma. Arch Pathol Lab Med. 2018;142:902-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Yanik F, Karamustafaoglu YA, Yoruk Y. Surgical outcomes and clinical courses of solitary fibrous tumors of pleura. Niger J Clin Pract. 2019;22:1412-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | DeVito N, Henderson E, Han G, Reed D, Bui MM, Lavey R, Robinson L, Zager JS, Gonzalez RJ, Sondak VK, Letson GD, Conley A. Clinical Characteristics and Outcomes for Solitary Fibrous Tumor (SFT): A Single Center Experience. PLoS One. 2015;10:e0140362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Keraliya AR, Tirumani SH, Shinagare AB, Zaheer A, Ramaiya NH. Solitary Fibrous Tumors: 2016 Imaging Update. Radiol Clin North Am. 2016;54:565-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Ratneswaren T, Hogg FRA, Gallagher MJ, Ashkan K. Surveillance for metastatic hemangiopericytoma-solitary fibrous tumors-systematic literature review on incidence, predictors and diagnosis of extra-cranial disease. J Neurooncol. 2018;138:447-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Yamada Y, Kohashi K, Kinoshita I, Yamamoto H, Iwasaki T, Yoshimoto M, Ishihara S, Toda Y, Itou Y, Koga Y, Hashisako M, Nozaki Y, Kiyozawa D, Kitahara D, Inoue T, Mukai M, Honda Y, Toyokawa G, Tsuchihashi K, Matsushita Y, Fushimi F, Taguchi K, Tamiya S, Oshiro Y, Furue M, Nakashima Y, Suzuki S, Iwaki T, Oda Y. Clinicopathological review of solitary fibrous tumors: dedifferentiation is a major cause of patient death. Virchows Arch. 2019;475:467-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Tani E, Wejde J, Åström K, Wingmo IL, Larsson O, Haglund F. FNA cytology of solitary fibrous tumors and the diagnostic value of STAT6 immunocytochemistry. Cancer Cytopathol. 2018;126:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Lin Y, Seger N, Tsagkozis P, Hesla AC, Ghaderi M, Chen Y, Ehnman M, Warsito D, Wejde J, Larsson O, Haglund F. Telomerase promoter mutations and copy number alterations in solitary fibrous tumours. J Clin Pathol. 2018;71:832-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Demicco EG, Wagner MJ, Maki RG, Gupta V, Iofin I, Lazar AJ, Wang WL. Risk assessment in solitary fibrous tumors: validation and refinement of a risk stratification model. Mod Pathol. 2017;30:1433-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 266] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 24. | Park HK, Yu DB, Sung M, Oh E, Kim M, Song JY, Lee MS, Jung K, Noh KW, An S, Song K, Nam DH, Kim YJ, Choi YL. Molecular changes in solitary fibrous tumor progression. J Mol Med (Berl). 2019;97:1413-1425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Schöffski P, Timmermans I, Hompes D, Stas M, Sinnaeve F, De Leyn P, Coosemans W, Van Raemdonck D, Hauben E, Sciot R, Clement P, Bechter O, Beuselinck B, Woei-A-Jin FJSH, Dumez H, Nafteux P, Wessels T. Clinical Presentation, Natural History, and Therapeutic Approach in Patients with Solitary Fibrous Tumor: A Retrospective Analysis. Sarcoma. 2020;2020:1385978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Bishop AJ, Zagars GK, Demicco EG, Wang WL, Feig BW, Guadagnolo BA. Soft Tissue Solitary Fibrous Tumor: Combined Surgery and Radiation Therapy Results in Excellent Local Control. Am J Clin Oncol. 2018;41:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Bailly C, Bichali Alroumani M, Douane F, Ansquer C, Drui D. Metastatic Solitary Fibrous Tumor With Doege-Potter Syndrome: Hypoglycemia Treated by 90Y Radioembolization. Clin Nucl Med. 2018;43:e93-e95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Haas RL, Walraven I, Lecointe-Artzner E, Scholten AN, van Houdt WJ, Griffin AM, Ferguson PC, Miah AB, Zaidi S, DeLaney TF, Chen YL, Spalek M, Krol SDG, Moeri-Schimmel RG, van de Sande MAJ, Sangalli C, Stacchiotti S. Radiation Therapy as Sole Management for Solitary Fibrous Tumors (SFT): A Retrospective Study From the Global SFT Initiative in Collaboration With the Sarcoma Patients EuroNet. Int J Radiat Oncol Biol Phys. 2018;101:1226-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Martin-Broto J, Stacchiotti S, Lopez-Pousa A, Redondo A, Bernabeu D, de Alava E, Casali PG, Italiano A, Gutierrez A, Moura DS, Peña-Chilet M, Diaz-Martin J, Biscuola M, Taron M, Collini P, Ranchere-Vince D, Garcia Del Muro X, Grignani G, Dumont S, Martinez-Trufero J, Palmerini E, Hindi N, Sebio A, Dopazo J, Dei Tos AP, LeCesne A, Blay JY, Cruz J. Pazopanib for treatment of advanced malignant and dedifferentiated solitary fibrous tumour: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2019;20:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 30. | Ebata T, Shimoi T, Bun S, Miyake M, Yoshida A, Shimomura A, Noguchi E, Yonemori K, Shimizu C, Fujiwara Y, Narita Y, Tamura K. Efficacy and Safety of Pazopanib for Recurrent or Metastatic Solitary Fibrous Tumor. Oncology. 2018;94:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Demicco EG, Griffin AM, Gladdy RA, Dickson BC, Ferguson PC, Swallow CJ, Wunder JS, Wang WL. Comparison of published risk models for prediction of outcome in patients with extrameningeal solitary fibrous tumour. Histopathology. 2019;75:723-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Lv G, Wang K, Li M, Li Z, Zheng A, Pang Q. Recurrence of multiple metastases after surgical removal of a primary malignant solitary fibrous tumor from the main bronchus: A case report. Medicine (Baltimore). 2018;97:e13560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | O'Neill AC, Tirumani SH, Do WS, Keraliya AR, Hornick JL, Shinagare AB, Ramaiya NH. Metastatic Patterns of Solitary Fibrous Tumors: A Single-Institution Experience. AJR Am J Roentgenol. 2017;208:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Gholami S, Cassidy MR, Kirane A, Kuk D, Zanchelli B, Antonescu CR, Singer S, Brennan M. Size and Location are the Most Important Risk Factors for Malignant Behavior in Resected Solitary Fibrous Tumors. Ann Surg Oncol. 2017;24:3865-3871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 35. | Salas S, Resseguier N, Blay JY, Le Cesne A, Italiano A, Chevreau C, Rosset P, Isambert N, Soulie P, Cupissol D, Delcambre C, Bay JO, Dubray-Longeras P, Krengli M, De Bari B, Villa S, Kaanders JHAM, Torrente S, Pasquier D, Thariat JO, Myroslav L, Sole CV, Dincbas HF, Habboush JY, Zilli T, Dragan T, Khan R K, Ugurluer G, Cena T, Duffaud F, Penel N, Bertucci F, Ranchere-Vince D, Terrier P, Bonvalot S, Macagno N, Lemoine C, Lae M, Coindre JM, Bouvier C. Prediction of local and metastatic recurrence in solitary fibrous tumor: construction of a risk calculator in a multicenter cohort from the French Sarcoma Group (FSG) database. Ann Oncol. 2017;28:1979-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |