Published online Jan 26, 2020. doi: 10.12998/wjcc.v8.i2.425
Peer-review started: November 8, 2019
First decision: November 19, 2019
Revised: December 2, 2019
Accepted: December 21, 2019
Article in press: December 21, 2019
Published online: January 26, 2020
Processing time: 70 Days and 2.6 Hours
Cumulative evidence suggests that the aberrant immune responses in acquired aplastic anemia (AA) are sustained by active chronic infections in genetically susceptible individuals. Recently, the constant source to trigger and sustain the pathophysiology has been proposed to come from the altered gut microbiota and chronic intestinal inflammation. In this case, our serendipitous finding provides convincing evidence that the persistently dysregulated autoimmunity may be generated, at least in a significant proposition of AA patients, by the altered gut microbiota and compromised intestinal epithelium.
A 30-year-old Chinese male patient with refractory severe AA experienced a 3-month-long febrile episode, and his fever was refractory to many kinds of injected broad-spectrum antibiotics. When presenting with abdominal cramps, he was prescribed oral mannitol and gentamycin to get rid of the gut infection. This treatment resulted in a quick resolution of the fever. Unanticipatedly, it also produced an excellent hematological response. He had undergone three episodes of recurrence within the one-year treatment, with each recurrence occurring 7-8 wk from the gastrointestinal inflammation eliminating preparations. However, subsequent treatments were able to produce subsequent remissions and consecutive treatments were successful in achieving durative hematological improvements, strongly indicating an etiological association between chronic gut inflammation and the development of AA. Interestingly, comorbid diseases superimposed on this patient (namely, psychiatric disorders, hypertension, insulin resistance, and renal dysfunction) were ameliorated together with the hematological improvements.
Chronic gut inflammation may be responsible for AA pathogenesis. The comorbidities and AA may share a common etiological association.
Core tip: The aberrant immune responses in aplastic anemia had been proposed to be triggered by altered gut microbiota, and our serendipitous finding that a 30-year-old man with refractory severe aplastic anemia had gained an inadvertently excellent hematological response following oral administration of mannitol and gentamycin provides convincing evidence to support the hypothetical but plausible pathogenic association. Several comorbid diseases on this patient were ameliorated together with the hematological improvements, indicating commonly shared etiological associations between the comorbidities and aplastic anemia in a background consisting of altered gut microbiota, chronic intestinal inflammation, increased epithelial permeability, and an autoimmune nature, instead of the adverse events of cyclosporine and iron overload.
- Citation: Zhao XC, Zhao L, Sun XY, Xu ZS, Ju B, Meng FJ, Zhao HG. Excellent response of severe aplastic anemia to treatment of gut inflammation: A case report and review of the literature. World J Clin Cases 2020; 8(2): 425-435
- URL: https://www.wjgnet.com/2307-8960/full/v8/i2/425.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i2.425
Acquired aplastic anemia (AA) is an autoimmune disease resulting from aberrant and antigen-driven immune responses to hematopoietic stem/progenitor cells (HSPCs)[1,2]. In the immune-mediated destruction of bone marrow (BM) hematopoiesis, a growing body of evidence suggests that the dysregulated autoimmunity is sustained by some active chronic infections[3,4]. Multiple infectious agents are involved[5]; however, no putative evidence has confirmed these pathogens to be capable of sustaining the chronic inflammation.
Very recently, the constant source of persistent stimulation to trigger and sustain the immune pathophysiology has been proposed to come from the altered gut microbiota and chronic intestinal inflammation[6,7]. A serendipitous finding reported in this paper explains how a 30-year-old male patient with refractory severe AA (RSAA) showed an inadvertently excellent hematological response to treatment of gut inflammation, which provides direct and convincing evidence to support the hypothetical but plausible pathogenic association.
A 30-year-old male patient was detected as having pancytopenia for 23 years and fever for 11 d.
Twenty-three years ago, at six years of age, a Chinese boy sought medical attention with major complaints of rapidly progressive weakness, fatigue, and pallor. This patient was detected as having pancytopenia and diagnosed with AA by BM aspirate and biopsy at several centers. He was prescribed cyclosporine (CsA) and stanozolol, showing a good hematological response. His symptoms gradually improved, but his peripheral blood cell counts had never reached the normal levels. In 2004, he experienced his first relapse 8 mo after the discontinuation of CsA, and was reinstituted on CsA, again showing a good response. In 2011, he underwent his second relapse 3 mo after the discontinuation of CsA. This time, he was referred to our center. On admission, major complaints included progressive weakness and fatigue for 1 mo and high-grade fever for 11 d. His peak body temperature had risen to 39.7 °C, with the absence of evident localized signs or symptoms.
The patient had no history of diseases of the hematopoietic system or significant infections before the diagnosis of AA.
He had a history of dimethylbenzene exposure due to his house being decorated 8 mo before being sent to the doctor.
No family history of inherited, hematological, or autoimmune diseases was recorded.
His height was 173 cm, and his body weight 63.5 kg. The body temperature was 38.7 °C; breathing rate 23 bp/min; heart rate 98 bp/min; and blood pressure (BP) 124/82 mmHg. Upon physical examination, except for the bruising pallor complexion, no physical abnormalities were recorded. Conspicuous mucocutaneous hemorrhage, jaundice, or exanthemata was not presented. No significant signs of the nervous system, respiratory system, cardiovascular system, gastrointestinal symptom, urogenital system, and skeletal musculature system were found.
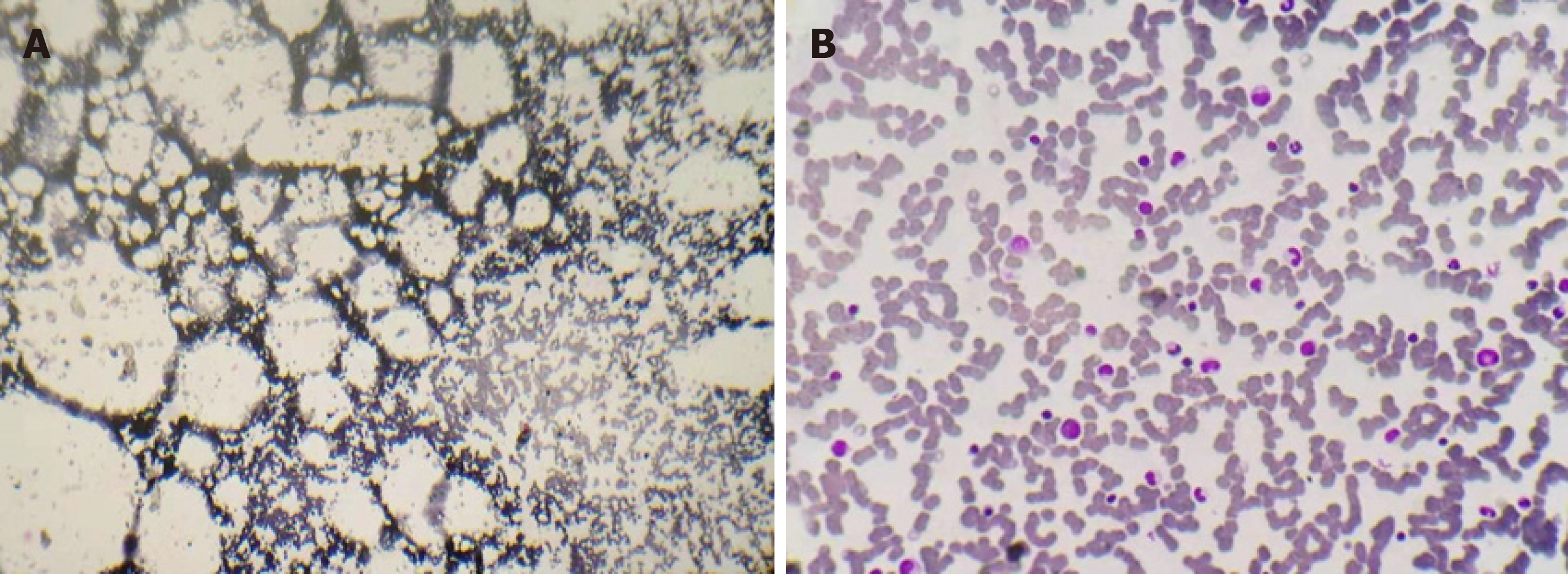
On admission, routine blood test revealed the following results: white blood cells (WBCs), 1730/µL; absolute neutrophil count (ANC), 270/µL; red blood cells (RBCs), 1860000/µL; hemoglobulin level (Hb), 5.4 g/dL; platelets (Plts), 6000 /µL; absolute reticulocyte count (Ret), 7600/µL; and C-reaction protein (CRP), 145.8 mg/L. No abnormalities were detected in his urine examination, coagulation profile, liver and renal function, lactate dehydrogenase, antinuclear antibodies, or serological tests for hepatitis A, B, and C, and anti-HIV antibodies. His blood culture was sterile. Expression of CD55 and CD59 was within the normal limits. Evaluation of BM smears from the posterior superior iliac spine revealed severe hypocellularity with severe fatty replacement, predominantly lymphocytes, with few erythrocyte and myeloid precursors as well as no megalokaryocytes or dysplasia (Figure 1A). Peripheral blood smears confirmed the severe neutropenia without atypical blood cells. No positive cytogenetic findings were identified by culturing the BM sample. A reticulocyte reaction was not observed following a tentative treatment with folinate and mecobalamin.
No signs of pulmonary infection were noted by chest computed tomography. Lymphadenopathy and hepatosplenomegaly were not observed by ultrasonographic examination.
Laboratory tests and tentative treatment fulfilled the diagnostic criteria for severe AA (SAA) according to guidelines for the diagnosis and management of adult aplastic anaemia.
Apart from the treatment with injected antibiotics and blood transfusion, he was retreated with CsA (250 mg/d) and stanozolol (6 mg/d). The fever was resolved successfully by intensive antibiotic treatments, but the BM suppression showed a reduced sensitivity to this immunosuppressive therapy that was once highly effective before the two relapses.
Although this patient had been treated all the while with CsA and stanozolol, significant hematological improvements had never been observed. During the seven years of treatment at our center, this patient had been subject to many fluctuating infectious episodes, with each episode lacking positive microbiological findings and localized presentations. He presented with moderate psychiatric disorders (alternate recurrences of anxiety and depression), escalated BP, and elevated serum creatinine (SCr) levels for the past six years. The distinct complexion and the comorbid diseases were, together with the progressive hematopoietic injury, frequently aggravated by the agnogenic infections. Recombinant human granulocyte colony stimulating factor (rhG-CSF) was initiated at 200 µg/d five years ago due to his severe granulocytopenia, but a significant increase in WBCs had never been noted. Deferasirox was started at 500 mg/d due to markedly elevated serum ferritin levels four years ago. Since then, a rapidly progressive elevation in serum glucose concentration (SGC) was detected, and NovoNorm30R was prescribed. Eltrombopag was supplemented at 100 mg/d three years ago. All these medications showed little improvements on autologous hematopoiesis. This patient had displayed seriously reduced sensitivity to CsA, eltrombopag, and insulin, which is making things worse and accelerating the hematological injury. The frequency of blood transfusion had gradually increased.
From March 2018 on, this patient suffered from a low-grade febrile episode. His fever lasted for 3 mo without obvious localized presentations or positive microbiological evidence, although laboratory tests revealed markedly elevated serum CRP levels. He had been treated consecutively and jointly with many kinds of injected antibiotics including piperacillin-sulbactam, levofloxacin, ceftriaxone, ornidazole, azithromycin, imipenem-cilastatin, vancomycin, and fluconazole. However, these intensive antibiotic treatments failed to relieve the inexplainable fever. Furthermore, along with the aggravated hematopoietic injury, the comorbid diseases superimposed on this patient were gradually or rapidly exacerbated. Only when presenting with abdominal cramps with vogue tenderness in the right lower quadrant of his abdomen, was he prescribed oral administration of mannitol (250 mL:50 mg for 2 d) and gentamycin (2 mL:80 mg, bid for 3 d) [mannitol-gentamycin (MG) regimen] to get rid of the gut infection. Prior used drugs were administered at the same dose as before. This treatment resulted in a quick resolution of the febrile episode. Unanticipatedly, this treatment also produced a good hematological response and the RSAA patient became transfusion-independent.
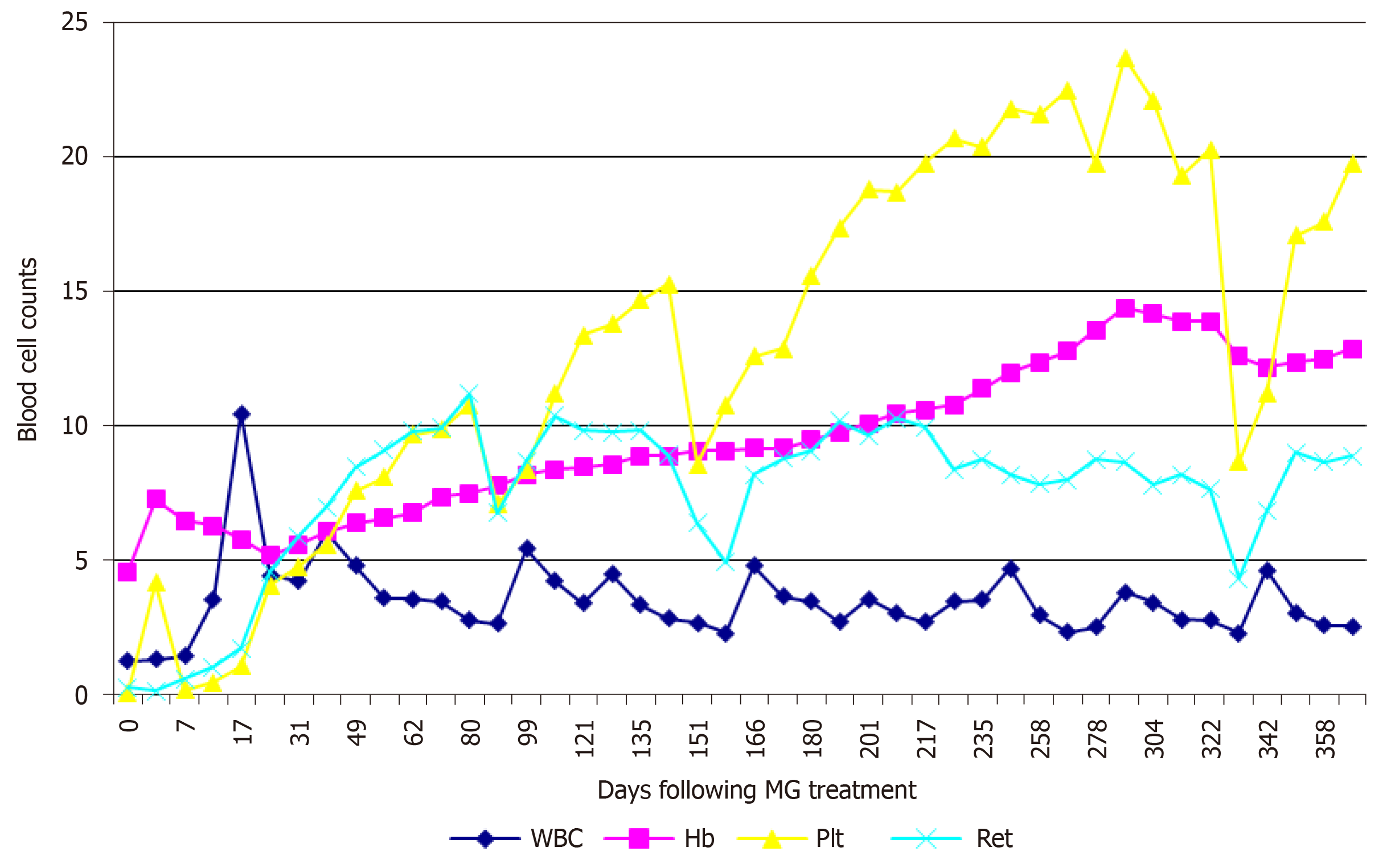
Within 2 d following the treatment, aside from the resolution of the fever, there was a perceptible amelioration of the chronic toxic complexion (mainly the bruising appearance frequently seen on patients with various autoimmune diseases), followed by the alleviation of the fatigue, muscular soreness, and psychiatric disorders, despite a slight decrease in Hb level perhaps due to the relax of the increased angiotasis. A rapid decline in BP and an evident reduction in SGC were also noted. A transiently high responsiveness to rhG-CSF (significant increase in WBCs) was observed around day 17, but it would decrease to considerably low levels before long and no longer increase to the high level, despite continuous administration of rhG-CSF at the same dose as before. SCr decreased to the normal level on day 17. A rapid increase in Plt and Ret levels was recorded. A month later, his second MG was prescribed. Eltrombopag was tapered according to Plt levels, with the drug tapered from 100 mg/d to 75 mg/d on day 25, to 50 mg/d on day 32, to 25 mg/d on day 42, and to 25 mg every other day on day 81.
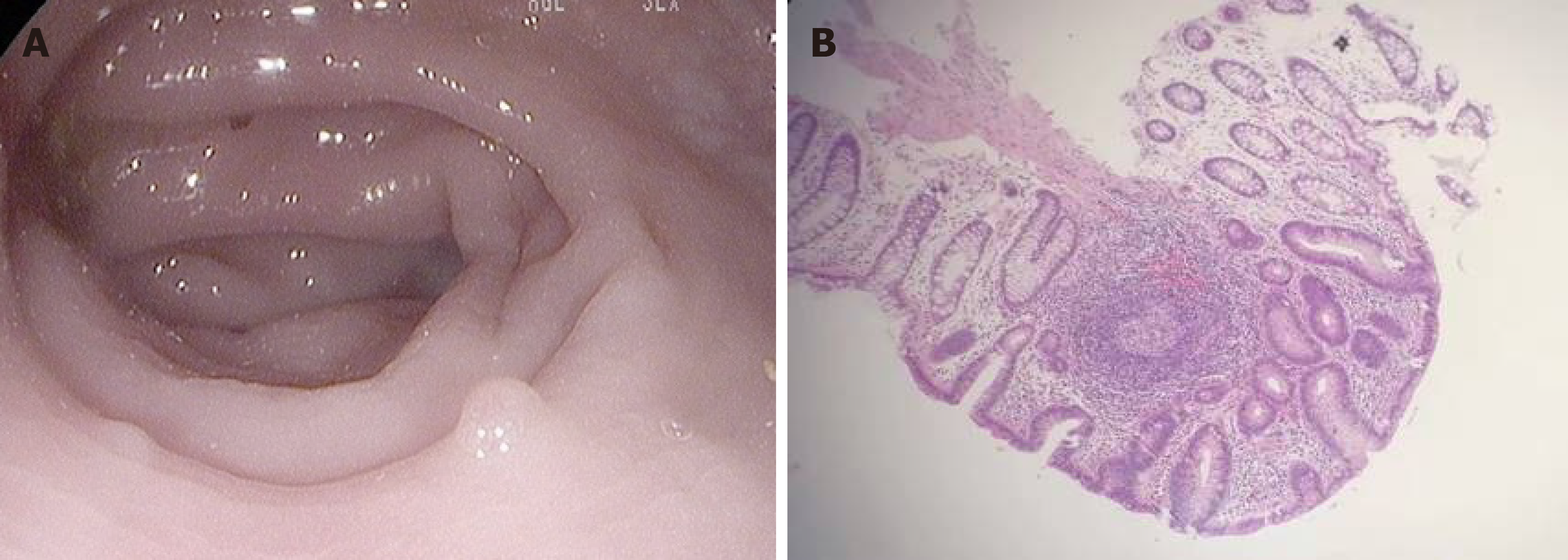
On day 92, this patient underwent the first precipitous decrease in Plt and Ret levels with the recurrence of psychiatric disorders and abdominal cramps. Eltrombopag was prescribed again at 25 mg/d, and his third MG was prescribed. After this treatment, the psychiatric disorders and abdominal pain were quickly resolved, and Plt and Ret levels increased rapidly. On day 151, this patient experienced the second precipitous decrease in Plt and Ret levels after 2 wk of constipation and recurrent abdominal pain, accompanied by elevated SGC and CRP levels. Endoscopic examination of the large intestine was performed and showed that the mucous membranes were congested and swollen, the vascular lakes were absent, and there were several polyps in the sigmoid colon and rectum, confirming the presence of gut inflammation (Figure 2A). Pathological examination of the polyps showed that these polyps were enlarged lymphoid follicles which were structurally integrated, with the infiltration of a large number of lymphocytes and plasma cells around the lymphoid follicles and underneath the mucous membranes, in accordance with inflammatory proliferation (Figure 2B). Followed by treatment with rifaximin (200 mg, qid for 14 d), a rapid increase in Plt and Ret levels was recorded. When insulin sensitivity was restored, NovoNorm30R was tapered, and then discontinued on day 162.
Over the ensuing 4 mo, this patient was monthly prescribed a polyethylene-rifaximin (PR) regimen (1500 mL polyethylene glycol electrolyte solution for 2 d and 200 mg rifaximin, qid for 14 d). These consecutive treatments resulted in steady hematological improvements. The last PR preparation was on day 278. Routine blood test on day 291 revealed a Plt count of 237000/µL and an Hb level of 14.4 g/dL. BM aspirate was performed again, and the smears revealed a normal cellularity (Figure 1B). On day 337, this patient experienced the third precipitous decrease in Plt and Ret levels. He was given another PR, and a steady increase in Plt and Hb level was observed.
Before MG treatment, the patient’s BP was 146/94 mmHg and SCr level was 1.78 mg/dL. NovoNorm30R was injected at 28 U before breakfast and 26 U before dinner. Routine blood test recorded the following results: WBCs, 1280/µL; ANC, 87/µL; RBCs, 1500000/µL; Hb, 4.6g/dL; PLT, 1000/µL; and Ret, 2900/µL. He was transfused with 4 U packed RBC and 10 U Plt. Hematological examinations of WBCs, Hb, Plt, and Ret levels during the one year of treatment are shown in Figure 3.
The MG regimen was originally designed to eliminate gut infections in patients with hematopoietic malignancies after intensive chemotherapies. Infectious episodes in these patients were sometimes refractory to various injected antibiotics and complicated by gastrointestinal (GI) signs and symptoms. Since it is difficult for using injected antibiotics to get rid of pathogens in the gut, gentamycin, a poorly absorbable and broad-spectrum antibiotic, prescribed orally can efficiently eliminate most bacilli and partial cocci without killing anaerobic bacteria[8]. Mannitol can produce an osmotic diarrhea and bowel cleansing[9], thus substantially reducing the amount of gut bacteria and minimizing the endotoxin absorption. MG has displayed excellent and efficacious therapeutic effects for eliminating gut infections. In an attempt to treat the 3-month-long febrile episode probably generated by the gut infection, MG was prescribed to this patient. The treatment gave rise to a quick resolution of the febrile episode. Unexpectedly, it also produced a good hematological response.
When comparing the durative resistance before MG treatment with the high responsiveness to the same drugs and the same doses after the treatment, it is reasonably inferred that the active chronic gut inflammation was responsible not only for the development and perpetuation of AA but also for the seriously decreased sensitivity to CsA and the progressive hematological injury. This patient had undergone 7-year serious suppression of autologous hematopoiesis and durative resistance to various drugs that are considered to be effective in the treatment of AA and that were demonstrated to be effective in the previous treatment in this patient. It is well-known that the host immune system would normally return to the immune homeostasis rapidly after the resolution of offending pathogens in acute infections; the exhaustion of HSPCs in AA patients strongly indicates the chronic activation of inflammatory signals[4]. The permanently progressive hematopoietic suppression and drug resistance, together with the resolution of the 3-month-long febrile episode, had been relieved rapidly after MG treatment, meaning that the gut inflammation had existed at least for 7 years and had a direct relationship with the BM suppression; the 3-month-long febrile episode and the accelerated hematopoietic deterioration are nothing more than the result of aggravated chronic gut inflammation. The episodes of recurrence uniformly occurred 7-8 wk from the gastrointestinal inflammation eliminating preparations (GIIEPs), meaning that the gut inflammation is a continuous pathophysiology instead of reinfections; the duration of the maintenance of autologous hematopoiesis by these treatments lasted for about 7-8 wk and the gut inflammation gradually return to the original state. The subsequent treatments able to produce subsequent remissions and consecutive treatments successful in achieving durative hematological improvements further confirmed the direct role of chronic gut inflammation in the suppression of autologous hematopoiesis. This patient presented with moderate GI symptoms rather than recurrently acute onset of serious GI symptoms, suggesting the gut inflammation to be an actively chronic disease with irregular episodes of aggravation. Recurrence and subsequent therapeutic responses suggest that the immunocompromised state in this patient may be the result of the active chronic gut inflammation and the serious suppression of BM hematopoiesis. The immunocompromised state induced by BM suppression would be corrected after the recovery of autologous hematopoiesis, and this patient was unable to get rid of the gut infection, indicating that the gut inflammation was not the result of neutropenia caused by BM suppression but rather the result of altered gut microbiota and compromised intestinal epithelium. On the basis of this finding, defects in HSPCs[1-4], whether in active disease or in remission, are sustained by the persistent stimulation originally generated by the active chronic gut inflammation. From this point of view, administration of drugs that were previously reported to induce AA may be somewhat coincidental events or indirect consequences. Except for genotoxic drugs that may induce malignant transformations, most of them do not exert direct toxic effects on HSPCs[10], but rather disturb the gut microbiota or damage the intestinal epithelial integrity[10,11], which induces inappropriate immune responses and then primes the autoimmune responses to HSPCs.
In the human body, the GI tract provides the largest and most vulnerable interface linking the host psycho-neuro-endocrino-immune system to external environmental factors. It also harbors the most enriched lymphatic tissue and the most complex microbial community[12], which are crucial for the education and maturation of the host immune system[13,14]. Given the fact that genetically driven mouse models under germ-free (GF) conditions have gained the ability to protect themselves from developing autoimmune diseases, this onset of autoimmunity indicates the contribution of commensal microbiota to the initiation and maintenance of the immune pathophysiology[15-17]. Dysbiotic gut microbiota and chronic gut inflammation are closely associated with almost all known autoimmune diseases, with the GI tract becoming the common spot for chronic inflammation to trigger and sustain the autoimmunity[18,19]. Although the precise underlying mechanism in initiating and perpetuating the autoimmunity remains unclear, the role of gut inflammation may be that it provides gut microbes with the opportunity to interact with the host immune system via increased intestinal permeability[20], sustains the inflammatory environment through pattern recognition receptors (PPRs) on the intestinal epithelium and innate immune cells sensing pathogen associated molecular patterns and microbe associated molecular patterns on commensal microbes[21], and links immunogenetics and environmental exposures to amplify the autoimmune reactions[22].
Until now, there are only a handful of available publications documenting the association of complicating gastrointestinal inflammatory conditions (GIICs) with the development of AA. Reported AA-associated GIICs include inflammatory bowel disease (IBD), celiac disease, and neutropenic enterocolitis[23-49]. The majority of these reported cases were afflicted with more severe gut inflammation and presented as an acute hematological episode. They presented with both more severe systemic and GI symptoms such as hyperthermia, abdominal cramps, and watery and/or blood diarrhea, followed by rapidly progressive pancytopenia and hypocellular marrow. In most of these reported cases with IBD, the authors had directly attributed the onset of AA to the adverse events of antibiotics, non-steroid anti-inflammatory drugs (NSAIDs), and immunosuppressants[23-34], which have been widely used to treat gut inflammation. However, successful treatment with intensive immunosuppressive therapy strongly indicates the autoimmune nature in NSAIDs-associated AA[26,27]. In azathioprine and 6-mercaptopurine-induced AA, the reversible autologous hematopoiesis suggests the direct BM suppression, which is attributed to the defects in thiopurine methyltransferase[31-34]. In a small fraction of patients with IBD, the onset of AA preceded the drug use or the drugs had not ever been prescribed[35-37]. Epstein-Barr virus and cytomegalovirus have been reported to be closely associated with the development of AA[5]. Matsumoto et al[38] reported an SAA patient with severe colitis with both Epstein-Barr virus and cytomegalovirus reactivation, suggesting that the gut may become the constant supply of these viruses or the colitis may share the common etiological association with AA. A total of 12 cases in six publications have described the association between AA and celiac disease, out of which celiac disease was diagnosed concomitantly with AA in eight patients, whereas in the remaining cases, the diagnosis of celiac disease preceded that of AA. Among these cases, hematological improvements were recorded in three patients after gluten-free diet and micronutrient supplementation[39-44], clearly indicating the pathogenic association between gut inflammation and AA. In publications of AA-associated neutropenic colitis, the authors had attributed the onset of colitis to severe neutropenia in the treatment of AA, and these patients commonly presented with rapidly progressive BM failure (BMF)[45-49], which may represent the association of AA with acute gut inflammation. However, the presence of bleeding polypoid lesions in the colon indicates an chronic inflammation preceding the onset of severe leukocytopenia and necrotic colitis[48].
These cases had been reported probably because of the obvious GI symptoms and signs when presenting with serious pancytopenia. Nevertheless, a large proportion of AA patients in clinical practice lack noticeable systemic and GI symptoms. The patient we are reporting here had undergone a persistently moderate fever with the absence of noticeable GI signs and symptoms until mild abdominal cramps occurred. The presence of gut inflammation was later confirmed by endoscopic and pathological examinations. On investigation of the medical history of AA patients treated in our center, nearly all patients have experienced multiple episodes of mild to moderate symptoms of IBD or IBS such as agnogenic fever, cramping abdominal pain, flatulence, diarrhea or constipation, and altered quality and quantity of stools, but their GI symptoms have never been thought to be associated with the pathogenesis of BMF ever before. We have observed that, in AA patients, the blood cell counts commonly fluctuate in correlation with alterations of physical and mental states, which are frequently catalyzed by agnogeneic infectious episodes, however, the GI symptoms were so subtle that they were always overshadowed by the serious hematologic manifestations. Thus, gut inflammation may exist in most, if not all, patients with AA. On the other hand, AA may share the common immune mechanism with these GIICs[7].
Recurrence is the most noteworthy issue. This patient had experienced three episodes of recurrence during the one-year treatment, with each recurrence occurring 7-8 wk after MG or PR treatment, although subsequent treatments were able to produce subsequent remissions and consecutive treatments were successful in achieving durative hematological improvements. Possible interpretation for these efficacious therapeutic responses and the high inclination to recurrence is that these treatments may merely result in a short-term specific pathogen-free or low abundant state, in which the significantly reduced gut inflammation loses its ability to overcome the threshold to sustain the aberrant immune responses. The dysbiotic gut microbiota would gradually return to the original state within approximately 7-8 wk. Another question is the memory cytotoxic T cells[50,51]. They could not be eradicated by these GIIEPs, which may be the rootstock for rapid recurrence.
Enlightened by our reporting case, a preliminary clinical investigation has been conducted on a group of five patients with RSAA and twenty-seven patients with refractory non-severe AA, in an attempt to testify whether or not treatment with these GIIEPs (use PR regimen in this clinical trial) was reproducible and efficacious as that observed in this patient. In fact, while the transiently high responsiveness to rhG-CSF was noted in all RSAA patients, a significant increase in Plt and Ret levels was recorded in all of these 32 patients. However, as have been described in this reporting case, nearly all patients had undergone recurrence within 6-10 wk from PR treatment, suggesting that this stable hematologic response could be sustained for only 6-10 wk if subsequent GIIEP treatment was not performed. This preliminary clinical investigation has been limited by the lack of strict prospective clinical studies.
Treatment of active chronic gut inflammation to manage autoimmune diseases may become a practicable pathway in the near future. As far, studies on the manipulation of gut microbiota to treat autoimmune diseases have been investigated extensively and have shown that it is highly effective to ameliorate the disease severity and to improve survival[52]. Rifamycin may be a much promising drug to treat AA patients for its eubiotic properties[53]. Apart from the eubiotic effect, rifampicin partially absorbed by the gastrointestines could serve as a mammalian target of rapamycin (mTOR) inhibitor to break down the vicious circle induced by the activation of mTOR signaling pathway and has been shown to be highly effective to ameliorate the immune-mediated hematopoietic insult in AA[54-56]; the therapeutic responses to mTOR inhibitors in the treatment of AA have been confirmed by the use of sirolimus to successfully treat relapsed and refractory AA patients[57].
Before popularizing the treatment option, several open questions must be raised and warrant extensive investigations: (1) How to evaluate the possible protective role of BM suppression in limiting the gut inflammation caused by overwhelming pathogenic infections, chronic gut inflammation, or dysbiotic gut microbiota as evidenced by the observation that a long-term remission of IBD could be achieved by the pancytopenia state in the course of azathioprine treatment[33]? (2) How to evaluate the possible protective role of BM suppression in preventing the over-proliferation of various pathogens that are able to infect and proliferate in hematopoietic progenitor cells and immune cells[5]? (3) How to evaluate the possible protective role of BM suppression in the repression of malignant clonal hematopoiesis as evidenced by the fact that approximately 10% of AA patients develop malignant clonal hematopoiesis after the recovery of autologous hematopoiesis following standard first-line immunosuppressive therapies[1,2]? (4) How to evaluate the presence of low-dose lipopolysaccharide in blood circulation and BM microenvironment[20], as well as the expression of HLA-DR and PPRs on hematopoietic progenitor cells in presenting exogenous and endogenous antigens? (5) How to evaluate the long-term effects of the indiscriminate deletion of gut microbiota on the host immune system and metabolism as evidenced by the studies showing that gut microbiota is indispensable and crucial for the education and sculpting of the host immune system and for the normal physical metabolism[13,14]? (6) How to evaluate and deal with the genetic predisposition which determines an individual whether or not to be able to develop an autoimmune disease? (7) How to deal with the effector memory CTL cells to prevent the rapid relapse[50,51]? (8) Which microbes and which mechanisms induce the actively chronic pathophysiology in AA? And (9) How to select the optimal gut-modifying regimen and dietary? Supply of sufficient indigestible polysaccharides and immunoregulatory vitamins seems to be beneficial for all AA patients.
Interestingly, along with the hematological improvements, the comorbidities superimposed on this patient were rapidly or gradually ameliorated, making it reasonable to believe that they share a common etiological association with AA rather than the adverse effects of CsA and iron overload. Although numerous investigations have reported the pathogenic association of altered gut microbiota and compromised intestinal epithelium with the diseases of the central nervous system, cardiovascular system, kidneys, and insulin sensitivity[16,58-60], no publications have documented these conditions as the comorbidities of AA based on the same etiology.
At least in a significant fraction of AA patients, gut inflammation plays a critical role in initiating and maintaining the autoimmune pathophysiology. The complex and elaborate interplay between gut microbiota and BM hematopoiesis may be the convergence of intricate interaction of host immunogenetics with various environmental exposures. In the treatment of AA with GIIEPs, recurrence was the most noteworthy issue, although subsequent treatments were able to produce subsequent remissions and consecutive treatments were successful in achieving durative hematological improvements. Anyway, this relatively nontoxic, highly efficacious, and readily acceptable treatment may open a novel avenue in etiological research and treatment option, particularly for RSAA patients.
Manuscript source: Unsolicited Manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta R S-Editor: Wang YQ L-Editor: Wang TQ E-Editor: Qi LL
| 1. | Young NS. Aplastic Anemia. N Engl J Med. 2018;379:1643-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 478] [Article Influence: 68.3] [Reference Citation Analysis (60)] |
| 2. | Zeng Y, Katsanis E. The complex pathophysiology of acquired aplastic anaemia. Clin Exp Immunol. 2015;180:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 3. | Maciejewski JP, Kim S, Sloand E, Selleri C, Young NS. Sustained long-term hematologic recovery despite a marked quantitative defect in the stem cell compartment of patients with aplastic anemia after immunosuppressive therapy. Am J Hematol. 2000;65:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Binder D, van den Broek MF, Kägi D, Bluethmann H, Fehr J, Hengartner H, Zinkernagel RM. Aplastic anemia rescued by exhaustion of cytokine-secreting CD8+ T cells in persistent infection with lymphocytic choriomeningitis virus. J Exp Med. 1998;187:1903-1920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Espinoza JL, Kotecha R, Nakao S. Microbe-Induced Inflammatory Signals Triggering Acquired Bone Marrow Failure Syndromes. Front Immunol. 2017;8:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Espinoza JL, Elbadry MI, Nakao S. An altered gut microbiota may trigger autoimmune-mediated acquired bone marrow failure syndromes. Clin Immunol. 2016;171:62-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Nakagawa N, Maruyama H, Zaimoku Y, Maruyama K, Saito C, Katagiri T, Hosokawa K, Ishiyama K, Yamazaki H, Nakao S. Evidence for a Common Immune Pathophysiology in Acquired Aplastic Anemia and Ulcerative Colitis. Blood. 2015;2418. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Chen C, Chen Y, Wu P, Chen B. Update on new medicinal applications of gentamicin: evidence-based review. J Formos Med Assoc. 2014;113:72-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Paulo GA, Martins FP, Macedo EP, Gonçalves ME, Ferrari AP. SAFETY OF MANNITOL USE IN BOWEL PREPARATION: a prospective assessment of intestinal methane (CH4) levels during colonoscopy after mannitol and sodium phosphate (NaP) bowel cleansing. Arq Gastroenterol. 2016;53:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Josefsdottir KS, Baldridge MT, Kadmon CS, King KY. Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood. 2017;129:729-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 236] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 11. | Bjarnason I, Scarpignato C, Holmgren E, Olszewski M, Rainsford KD, Lanas A. Mechanisms of Damage to the Gastrointestinal Tract From Nonsteroidal Anti-Inflammatory Drugs. Gastroenterology. 2018;154:500-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 321] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 12. | Shen L. Functional morphology of the gastrointestinal tract. Curr Top Microbiol Immunol. 2009;337:1-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Nash MJ, Frank DN, Friedman JE. Early Microbes Modify Immune System Development and Metabolic Homeostasis-The "Restaurant" Hypothesis Revisited. Front Endocrinol (Lausanne). 2017;8:349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Takiishi T, Fenero CIM, Câmara NOS. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers. 2017;5:e1373208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 629] [Article Influence: 78.6] [Reference Citation Analysis (1)] |
| 15. | Maeda Y, Kurakawa T, Umemoto E, Motooka D, Ito Y, Gotoh K, Hirota K, Matsushita M, Furuta Y, Narazaki M, Sakaguchi N, Kayama H, Nakamura S, Iida T, Saeki Y, Kumanogoh A, Sakaguchi S, Takeda K. Dysbiosis Contributes to Arthritis Development via Activation of Autoreactive T Cells in the Intestine. Arthritis Rheumatol. 2016;68:2646-2661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 488] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 16. | Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 867] [Cited by in RCA: 936] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 17. | Luce S, Guinoiseau S, Gadault A, Letourneur F, Blondeau B, Nitschke P, Pasmant E, Vidaud M, Lemonnier F, Boitard C. Humanized Mouse Model to Study Type 1 Diabetes. Diabetes. 2018;67:1816-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016;375:2369-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1826] [Cited by in RCA: 2325] [Article Influence: 258.3] [Reference Citation Analysis (0)] |
| 19. | Gianchecchi E, Fierabracci A. Recent Advances on Microbiota Involvement in the Pathogenesis of Autoimmunity. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | Mu Q, Kirby J, Reilly CM, Luo XM. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front Immunol. 2017;8:598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 381] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 21. | Toubi E, Vadasz Z. Innate immune-responses and their role in driving autoimmunity. Autoimmun Rev. 2019;18:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 22. | Vogelzang A, Guerrini MM, Minato N, Fagarasan S. Microbiota - an amplifier of autoimmunity. Curr Opin Immunol. 2018;55:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | NUGUID TP, BACON HE. Aplastic anemia following chloramphenicol therapy in chronic ulcerative colitis: report of a case. Dis Colon Rectum. 1961;4:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 24. | Wiesen A, Wiesen J, Limaye S, Kaushik N. Mesalazine-induced aplastic anemia. Am J Gastroenterol. 2009;104:1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Abboudi ZH, Marsh JC, Smith-Laing G, Gordon-Smith EC. Fatal aplastic anaemia after mesalazine. Lancet. 1994;343:542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Laidlaw ST, Reilly JT. Antilymphocyte globulin for mesalazine-associated aplastic anaemia. Lancet. 1994;343:981-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Otsubo H, Kaito K, Sekita T, Shimada T, Kobayashi M, Hosoya T. Mesalazine-associated severe aplastic anemia successfully treated with antithymocyte globulin, cyclosporine and granulocyte colony-stimulating factor. Int J Hematol. 1998;68:445-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Daneshmend TK. Mesalazine-associated thrombocytopenia. Lancet. 1991;337:1297-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Davies JM, Dunn HG. Interstitial Lung Disease Developing during Treatment with Cyclosporine in a patient with Diffuse Scleroderma, Aplastic Anemia and Crohn's Disease: Implications for Pathogenesis and Treatment. J Clin Rheumatol. 1995;1:287-291. [PubMed] |
| 30. | Fox RM, Firkin FC. Sequential pure red cell and megakaryocyte aplasia associated with chronic liver disease and ulcerative colitis. Am J Hematol. 1978;4:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Tajiri H, Tomomasa T, Yoden A, Konno M, Sasaki M, Maisawa S, Sumazaki R, Shimizu T, Toyoda S, Etani Y, Nakacho M, Ushijima K, Kobayashi A; Japanese Society for Pediatric Inflammatory Bowel Disease. Efficacy and safety of azathioprine and 6-mercaptopurine in Japanese pediatric patients with ulcerative colitis: a survey of the Japanese Society for Pediatric Inflammatory Bowel Disease. Digestion. 2008;77:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Choi YS, Suh JP, Song KH, Lee JB, Lee DS, Lee IT, Kim DS, Lee DH. A case of Crohn's disease with improvement after azathioprine-induced pancytopenia. Case Rep Gastroenterol. 2011;5:344-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Burke DA, Dixon MF, Axon AT. Ulcerative colitis: prolonged remission following azathioprine-induced pancytopenia. J Clin Gastroenterol. 1989;11:327-330. [PubMed] |
| 34. | Al Riyami AZ, Jacobson K, Ford J, Dalal BI. Transient appearance of GPI-deficient population in a patient with azathioprine-associated bone marrow aplasia. Ann Hematol. 2012;91:1659-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Sharma BC, Yachha SK, Mishra RN, Gupta D. Hypoplastic anemia associated with ulcerative colitis in a child. J Pediatr Gastroenterol Nutr. 1996;23:326-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Kishikawa H, Nishida J, Nakano M, Hirano E, Morishita T, Ishii H. Ulcerative colitis associated with aplastic anemia. Dig Dis Sci. 2003;48:1376-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Naithani R, Mahapatra M, Kumar R, Rai S. Aplastic anemia and Crohn's disease - coincidence or association? Indian J Gastroenterol. 2005;24:183. [PubMed] |
| 38. | Matsumoto H, Kimura Y, Murao T, Osawa M, Akiyama T, Mannoji K, Koresawa R, Tokunaga H, Wada H, Sugihara T, Haruma K. Severe Colitis Associated with both Epstein-Barr Virus and Cytomegalovirus Reactivation in a Patient with Severe Aplastic Anemia. Case Rep Gastroenterol. 2014;8:240-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Grey-Davies E, Hows JM, Marsh JC. Aplastic anaemia in association with coeliac disease: a series of three cases. Br J Haematol. 2008;143:258-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 40. | Irfan O, Mahmood S, Nand H, Billoo G. Celiac disease associated with aplastic anemia in a 6-year-old girl: a case report and review of the literature. J Med Case Rep. 2018;12:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Badyal RK, Sachdeva MU, Varma N, Thapa BR. A rare association of celiac disease and aplastic anemia: case report of a child and review of literature. Pediatr Dev Pathol. 2014;17:470-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Maheshwari A, Nirupam N, Aneja S, Meena R, Chandra J, Kumar P. Association of celiac disease with aplastic anemia. Indian J Pediatr. 2012;79:1372-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Basu A, Ray Y, Bowmik P, Rahman M, Dikshit N, Goswami RP. Rare association of coeliac disease with aplastic anaemia: report of a case from India. Indian J Hematol Blood Transfus. 2014;30:208-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Salmeron G, Patey N, de Latour RP, Raffoux E, Gluckman E, Brousse N, Socié G, Robin M. Coeliac disease and aplastic anaemia: a specific entity? Br J Haematol. 2009;146:122-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 45. | Mulholland MW, Delaney JP. Neutropenic colitis and aplastic anemia: a new association. Ann Surg. 1983;197:84-90. [PubMed] |
| 46. | Frick MP, Maile CW, Crass JR, Goldberg ME, Delaney JP. Computed tomography of neutropenic colitis. AJR Am J Roentgenol. 1984;143:763-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Weinberger M, Hollingsworth H, Feuerstein IM, Young NS, Pizzo PA. Successful surgical management of neutropenic enterocolitis in two patients with severe aplastic anemia. Case reports and review of the literature. Arch Intern Med. 1993;153:107-113. [PubMed] |
| 48. | Koh MB, Cheung DY, Noh CH, Lee SM, Park YB, Kim JI, Park SH, Kim JK, Lee JW. Bleeding polypoid lesions in the colon as a presentation of neutropenic colitis in aplastic anemia. Gastrointest Endosc. 2009;69:953; discussion 954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 49. | Alioglu B, Avci Z, Ozcay F, Arda S, Ozbek N. Neutropenic enterocolitis in children with acute leukemia or aplastic anemia. Int J Hematol. 2007;86:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Hosokawa K, Muranski P, Feng X, Townsley DM, Liu B, Knickelbein J, Keyvanfar K, Dumitriu B, Ito S, Kajigaya S, Taylor JG 6th, Kaplan MJ, Nussenblatt RB, Barrett AJ, O'Shea J, Young NS. Memory Stem T Cells in Autoimmune Disease: High Frequency of Circulating CD8+ Memory Stem Cells in Acquired Aplastic Anemia. J Immunol. 2016;196:1568-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 51. | Giudice V, Feng X, Lin Z, Hu W, Zhang F, Qiao W, Ibanez MDPF, Rios O, Young NS. Deep sequencing and flow cytometric characterization of expanded effector memory CD8+CD57+ T cells frequently reveals T-cell receptor Vβ oligoclonality and CDR3 homology in acquired aplastic anemia. Haematologica. 2018;103:759-769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 52. | Balakrishnan B, Taneja V. Microbial modulation of the gut microbiome for treating autoimmune diseases. Expert Rev Gastroenterol Hepatol. 2018;12:985-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 53. | Ponziani FR, Zocco MA, D'Aversa F, Pompili M, Gasbarrini A. Eubiotic properties of rifaximin: Disruption of the traditional concepts in gut microbiota modulation. World J Gastroenterol. 2017;23:4491-4499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 122] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 54. | Zheng P, Chang X, Lu Q, Liu Y. Cytopenia and autoimmune diseases: a vicious cycle fueled by mTOR dysregulation in hematopoietic stem cells. J Autoimmun. 2013;41:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Liu SL, Zhou YM, Tang DB, Zhou N, Zheng WW, Tang ZH, Duan CW, Chen J. Rapamycin ameliorates immune-mediated aplastic anemia by inhibiting the proliferation and metabolism of T cells. Biochem Biophys Res Commun. 2019;518:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Feng X, Lin Z, Sun W, Hollinger MK, Desierto MJ, Keyvanfar K, Malide D, Muranski P, Chen J, Young NS. Rapamycin is highly effective in murine models of immune-mediated bone marrow failure. Haematologica. 2017;102:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 57. | He G, Zhang X, Wu D, Sun A, Wang X. Relapse of aplastic anemia responsive to sirolimus combined with cyclosporine. Pediatr Blood Cancer. 2011;56:1133-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 58. | Yang Q, Vijayakumar A, Kahn BB. Metabolites as regulators of insulin sensitivity and metabolism. Nat Rev Mol Cell Biol. 2018;19:654-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 437] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 59. | Kanbay M, Onal EM, Afsar B, Dagel T, Yerlikaya A, Covic A, Vaziri ND. The crosstalk of gut microbiota and chronic kidney disease: role of inflammation, proteinuria, hypertension, and diabetes mellitus. Int Urol Nephrol. 2018;50:1453-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 60. | Yang T, Zubcevic J. Gut-Brain Axis in Regulation of Blood Pressure. Front Physiol. 2017;8:845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |