Published online Jan 26, 2020. doi: 10.12998/wjcc.v8.i2.390
Peer-review started: October 24, 2019
First decision: December 5, 2019
Revised: December 23, 2019
Accepted: January 2, 2020
Article in press: January 2, 2020
Published online: January 26, 2020
Processing time: 85 Days and 1.2 Hours
Pancreatic cancer has a poor prognosis; 40%–50% of patients have liver metastases at the time of initial diagnosis and only 15%–20% undergo surgical resection. Irreversible electroporation (IRE) is a new, non-thermal local ablation method for solid tumors, which can induce cell membrane permeabilization, resulting in unrecoverable nanoscale perforation and apoptotic cell death without damaging the structural components of tissues.
We report the case of a 66-year-old female patient with liver metastasis from pancreatic cancer with a pathological diagnosis of poorly differentiated adenocarcinoma. Carbohydrate antigen 19-9 was elevated to 420.3 U/mL. Computed tomography showed a pancreas mass of 2.7 cm × 2.5 cm and single liver metastasis of 1.4 cm × 1.1 cm in the S6 area. The patient underwent IRE and arterial infusion chemotherapy and received tegafur. The therapeutic effect of the combination treatment has been evaluated as complete response. To date, the patient has survived for > 12 mo and is receiving tegafur as maintenance therapy (at the time this case report was written).
IRE plus arterial infusion chemotherapy and tegafur may be synergistic, providing a reference for treating liver metastasis from pancreatic cancer.
Core tip: In this study, we report the case of a 66-year-old female patient with liver metastasis from pancreatic cancer who underwent irreversible electroporation and chemotherapy and showed a complete response after the combination therapy. Our findings suggest that irreversible electroporation plus arterial infusion chemotherapy and tegafur may be synergistic, providing a reference for treating liver metastasis from pancreatic cancer.
- Citation: Ma YY, Shi JJ, Chen JB, Xu KC, Niu LZ. Irreversible electroporation for liver metastasis from pancreatic cancer: A case report. World J Clin Cases 2020; 8(2): 390-397
- URL: https://www.wjgnet.com/2307-8960/full/v8/i2/390.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i2.390
Pancreatic cancer is one of the most aggressive cancers and has a poor prognosis. The 5-year survival rate is < 8%, and the prognosis is extremely poor. Pancreatic cancer is known as the “king of cancers”. At the initial diagnosis, only 15%–20% of patients are resectable[1]. Up to 80%–90% of patients lose their opportunity for surgery due to local invasion or distant metastasis[2,3]. Up to 50% of patients have distant metastasis at the initial diagnosis, where liver metastasis is the most common (40%–50%)[4]. The median survival time of patients with untreated, unresectable locally advanced or distant metastatic pancreatic cancer is only 2–6 mo[5]. A study by Johns Hopkins University found that even by strict screening, the median survival time of patients with periampullary carcinoma with simultaneous liver metastases was only 5.9 mo, compared with 14.2 mo in patients without metastases[6].
As only a few patients with pancreatic cancer are eligible for surgery, and most patients have limited response to combination chemotherapy and radiation therapy, some ablation therapies have been examined as adjuvant or independent treatments. Radiofrequency ablation and microwave thermal ablation are the most commonly used ablation methods. Irreversible electroporation (IRE) is a non-thermal effect–based physical ablation method. Its principle is mainly causing irreversible electroporation of the tumor cell membrane by transient, high-frequency, and repeated high-voltage pulse to the tumor cells to achieve the purpose of ablation[7-9]. Recently, a prospective study showed that the median overall survival (OS) from diagnosis in patients with pancreatic cancer treated with IRE combined with chemotherapy was extended to 20.3 mo (stage III) and 13.56 mo (stage IV); the results suggested that IRE combined with chemotherapy can prolong survival time[10].
Tegafur is an oral fluoropyrimidine derivative designed to improve the anti-tumor activity of 5-fluorouracil while reducing its gastrointestinal toxicity. Recently, studies investigating tegafur have indicated that it has a better clinical curative effect for advanced pancreatic cancer. Many high-quality clinical trials have investigated the therapeutic efficacy and safety of tegafur combined with multiple chemotherapeutic agents[11-13].
Here, we report the case of a 66-year-old female patient with liver metastasis from pancreatic cancer who underwent IRE and chemotherapy and showed a complete response (CR) after the combination therapy.
A 66-year-old female patient had pain under the xiphoid. Abdominal B ultrasound revealed a pancreatic space–occupying lesion; further abdominal computed tomography (CT) and magnetic resonance imaging examination were performed to determine the position. Liver metastasis was disclosed, and the tumor markers carbohydrate antigen (CA) 12-5 and CA19-9 were significantly increased; pancreatic cancer was considered. There was no indication for surgery at the local hospital. At our hospital, she was diagnosed with a pancreatic space-occupying lesion for investigation. She had a body mass index of 24.61 kg/m2 (body weight, 63.5 kg; height, 160 cm) and was a nonsmoker.
Nothing to declare.
Twenty years ago, she underwent cholecystectomy for gallstones, and underwent appendectomy 10 years ago for acute appendicitis.
Her mother had died of gallbladder cancer.
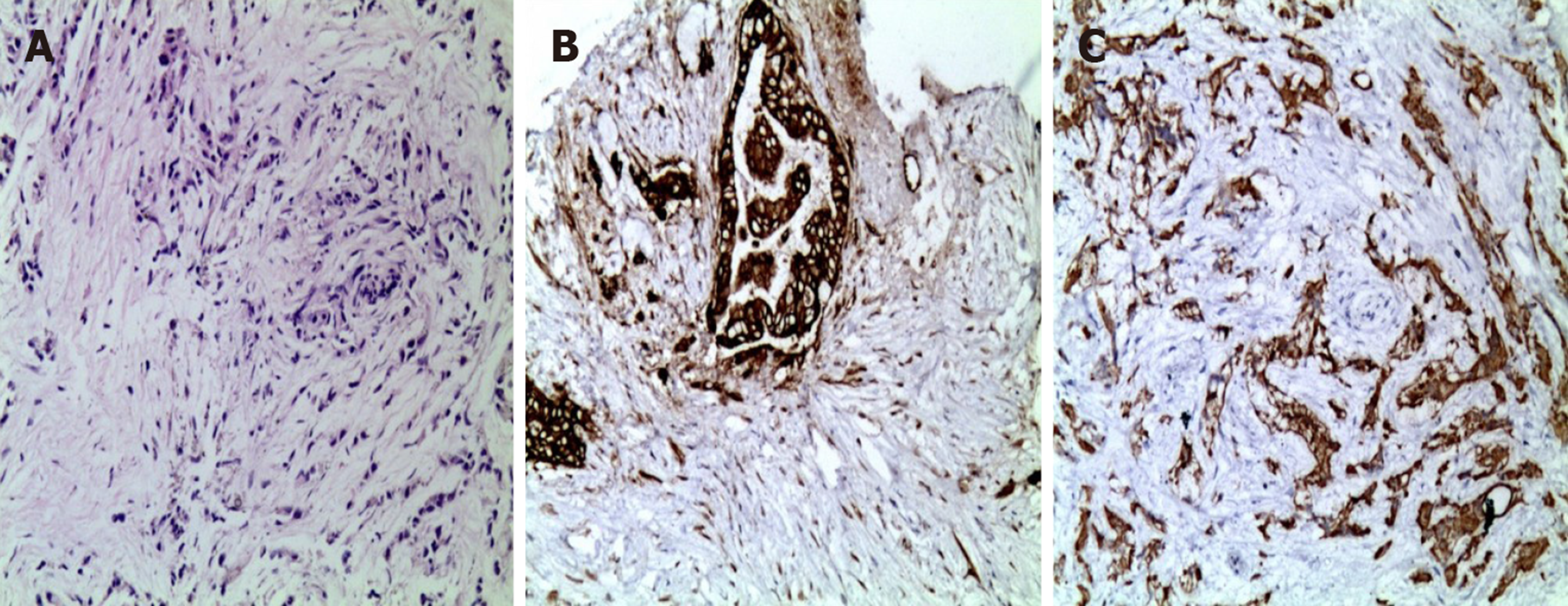
Tumor marker screening showed elevated levels of CA12-5 (15.85 U/mL) and CA19-9 (420.3 U/mL). Immunohistochemistry demonstrated cytokeratin 7(+) and cytokeratin 19(+). Based on the clinical and immunohistochemical results, the lesions were consistent with poorly differentiated adenocarcinoma of the pancreas (Figure 1).
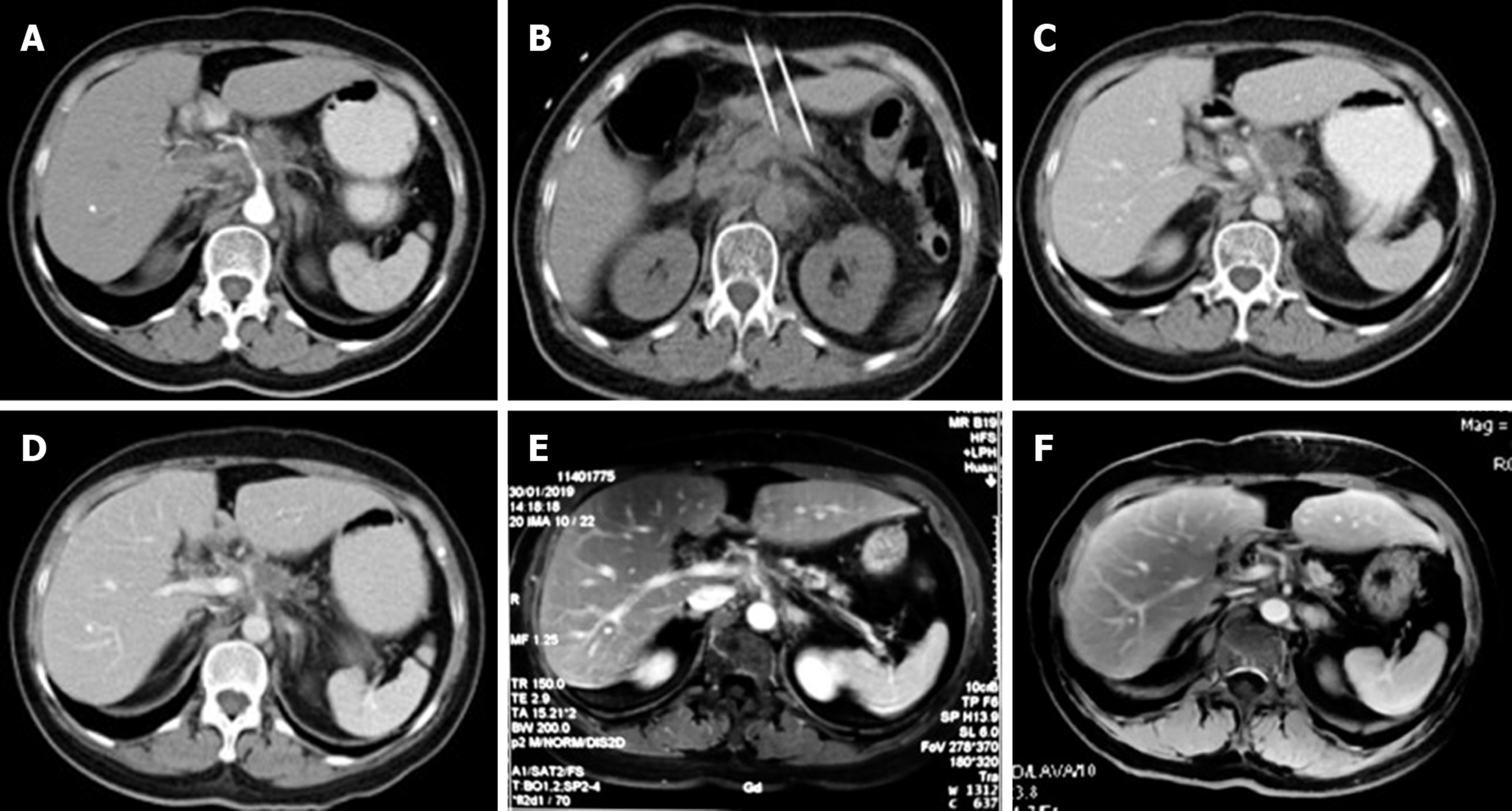
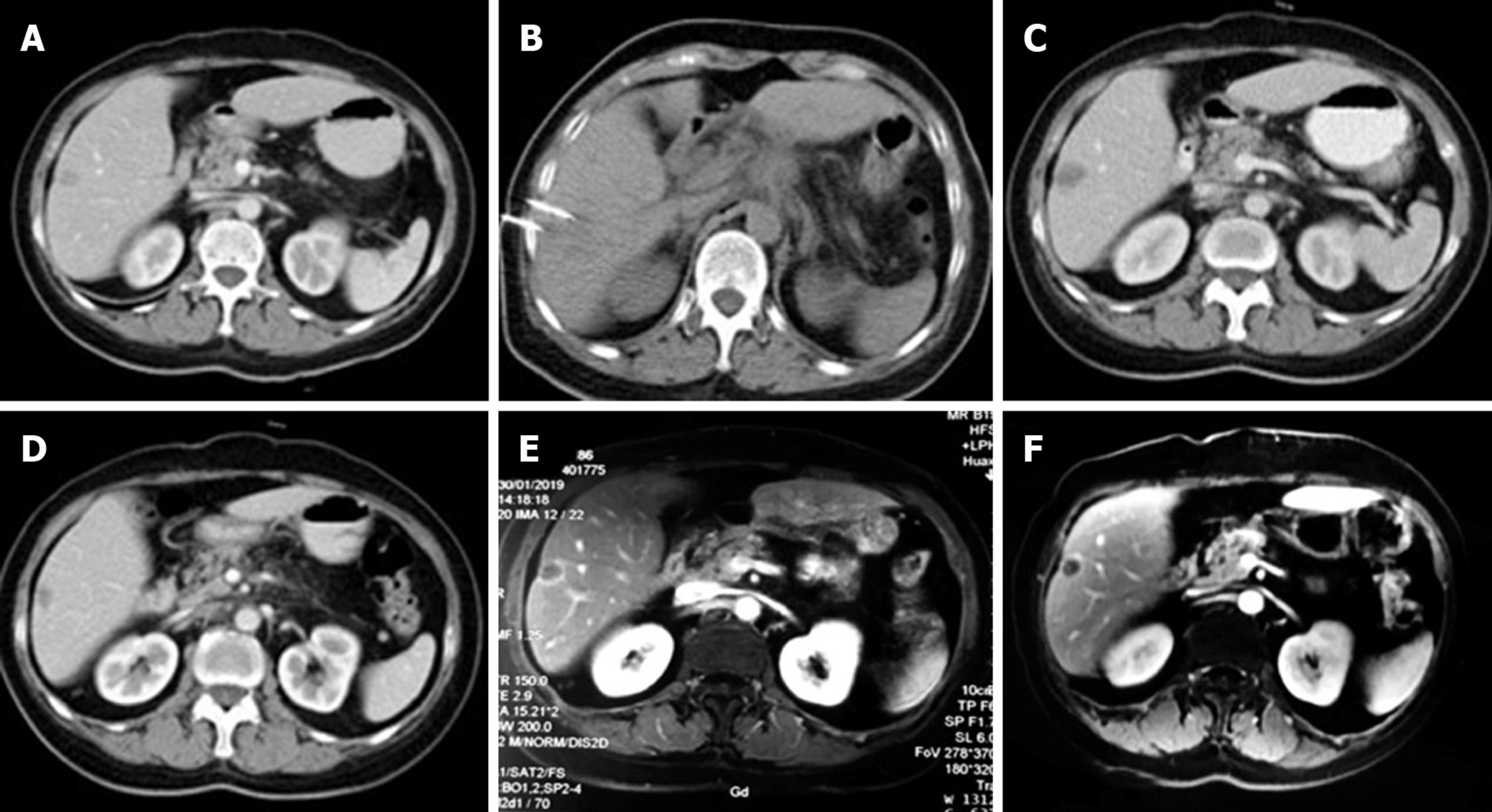
During this hospitalization, a CT scan revealed a nodular mass in the pancreas measuring about 2.7 cm × 2.5 cm (Figure 2A) and a small, nodular low-density lesion in the liver S6 measuring about 1.4 cm × 1.1 cm (Figure 3A). The lesion appeared non-homogeneous after enhancement, with mild reinforcement. Adjacent to the splenic artery, the proper hepatic artery and abdominal cavity were locally compressed. The tail of the pancreas was atrophied. No obvious effusion was seen in the abdominal cavity.
Liver metastasis from pancreatic cancer.
Percutaneous IRE ablation was performed under general anesthesia, two electrodes were used during the operation, the needle tip distance was 1.0 to 2.0 cm, and the effective exposure distance of the electrodes was 1.5 to 2.0 cm. CT combined with ultrasound was used to confirm tumor size, and the parameters for IRE ablation were electric field intensity 1500 V/cm, pulse length 70 μs, and pulse number 90. During the operation, the success of the ablation was determined based on the hyperechoic coverage and real-time impedance changes in the ablation lesion. Gemcitabine intravenous chemotherapy was administered, followed by gemcitabine arterial infusion and simultaneous tegafur for a total of four cycles. Table 1 shows the method of administration.
| Chemotherapy regimen | Dosage | Delivery route | Time | Cycle |
| Gemcitabine | Day 1 (1.2 g); Day 8 (0.8 g) | Intravenous | Days 1 and 8 | Every 21 d |
| Gemcitabine + Tegafur | Days 1 and 8 (1.2 g) + 80 mg | Arterial infusion + oral | Days 1 and 8; days 1–14 | For four cycles |
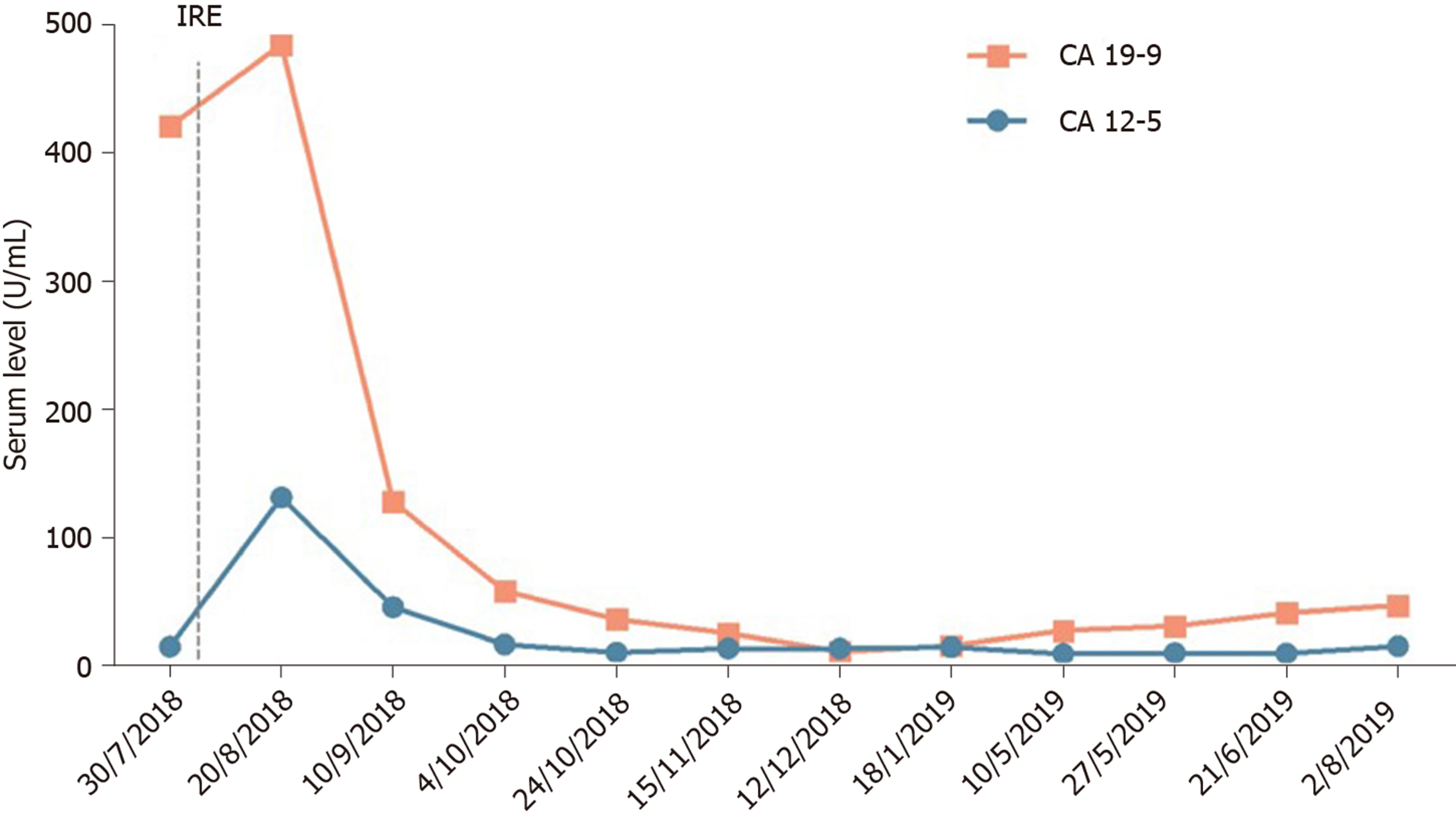
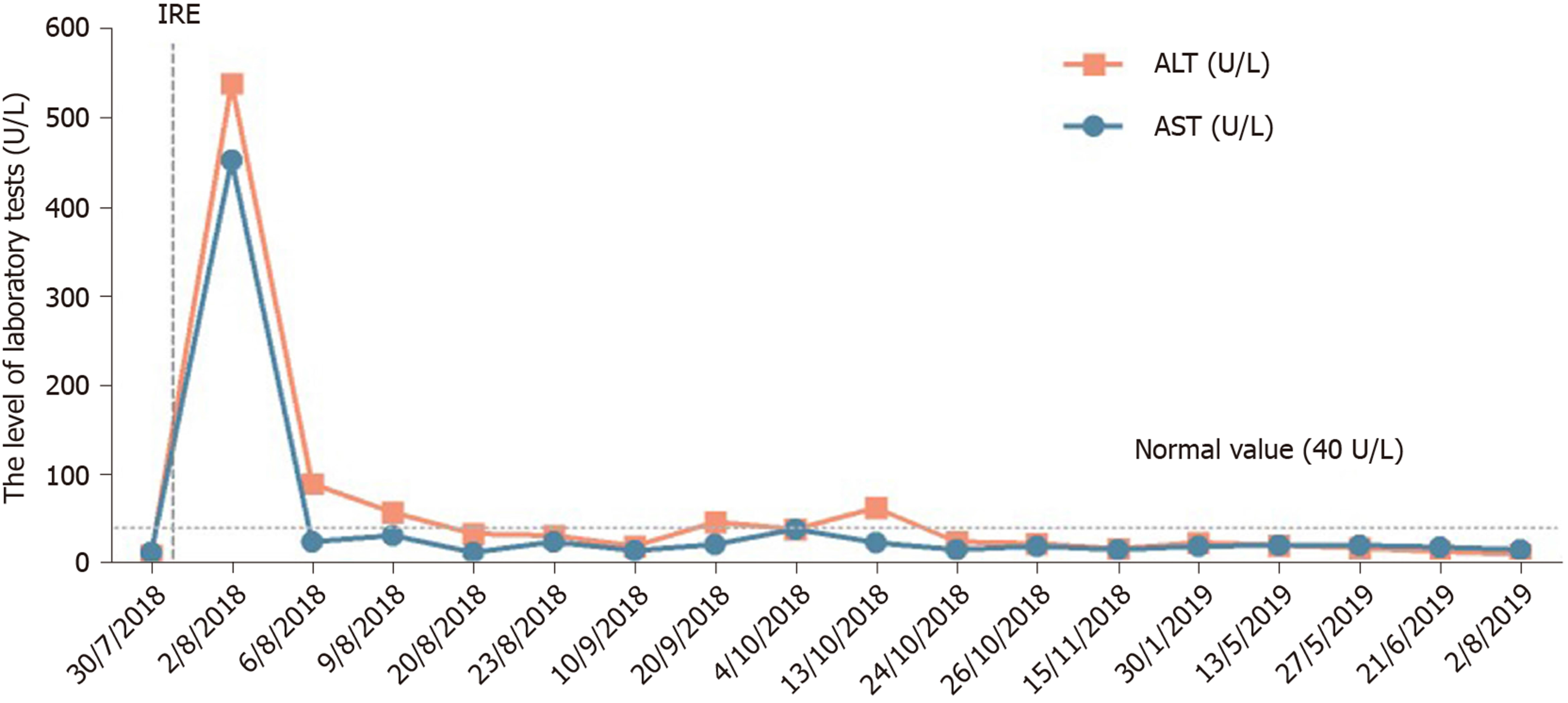
After 12 mo of IRE ablation combined with gemcitabine and tegafur, the patient’s serum tumor markers were measured: CA19-9 was 48.48 U/mL and CA12-5 was 16.09 U/mL; during treatment, her serum CA19-9 level decreased to within normal levels (Figure 4). Her liver enzymes were measured: Alanine aminotransferase (ALT) was 538 U/mL and aspartate aminotransferase (AST) was 452 U/mL, demonstrating elevation at the time of blood collection on postoperative day 1, and peaked within 24 h (Figure 5). The ALT and AST levels returned to baseline on day 9 and day 20. After 12 mo of follow-up, a CT scan showed complete disappearance of the pancreatic mass (Figure 2), and significantly marked shrinkage of the liver mass (Figure 3). The patient had a CR according to the Response Evaluation Criteria in Solid Tumors 1.1, and partial response according to the Solid tumor diagnostic criteria 1.1[14]. In addition, there were no severe adverse reactions during treatment.
We report the case of a 66-year-old female patient with liver metastasis from pancreatic cancer who underwent synchronous IRE ablation of liver and pancreatic lesions combined with gemcitabine arterial infusion chemotherapy and oral tegafur. The treatment relieved the abdominal pain effectively, shrank the pancreatic mass entirely, and reduced the size of the liver mass, with markedly reduced serum CA19-9 (48.18 U/mL) and CA125 (16.09 U/mL). In addition, her serum ALT and AST levels were significantly increased on post-intervention day 1; however, the elevated levels decreased rapidly. She showed a prolonged survival and good health 12 mo after IRE. This indicates that the treatment did not affect liver function and that both of the combined therapies were safe for liver metastasis from pancreatic cancer.
Liver metastasis from pancreatic cancer is a systemic disease and patients have a poor prognosis and short lifespan. The overall 5-year relative survival rate for pancreatic cancer is 7.6%, and is 29.3% for localized disease but only 2.6% for distant metastasis[15]. The liver is the most common location for metastatic lesions of pancreatic cancer, as cancer cells can cross the portal artery or abdominal cavity endolymphatic pathway and metastasize to the liver[16]. Therefore, systemic treatment is needed. Unfortunately, most patients would have lost the opportunity for surgical resection at the time of diagnosis. Therefore, managing the symptoms and improving the quality of life and survival are critical for these patients[16]. IRE ablation has been widely used for treating pancreatic cancer and liver cancer because it is minimally invasive and has a good curative effect[17,18]. Hong et al[19] reported seven patients with pancreatic cancer metastasis after IRE treatment, three of whom had single liver metastasis with a lifespan of up to 16 mo. IRE treatment of Child-Pugh B (7/8) hepatocellular carcinoma resulted in equivalent ablation success, with improved liver tolerance compared with microwave ablation and other ablative modalities[20].
IRE is an emerging physical ablation technique. IRE does not produce a heat sink effect and retains the functions of blood vessels, bile ducts, and nerves located in the ablation area. Unfortunately, these patients with pancreatic cancer have lost their chance of surgical resection. In patients with metastatic pancreatic cancer, gemcitabine-based chemotherapy is the main treatment. Therefore, for this patient, managing symptoms and improving quality of life and survival are critical.
In recent years, with the deepening of research on transcatheter arteriovenous infusion chemotherapy drugs, the effective concentration of local drugs in tumor lesions has increased, and drug concentrations in the systemic blood system have decreased because of adverse reactions. Percutaneous transarterial infusion chemotherapy can inhibit tumor growth through local high-concentration chemotherapy drugs. In patients with metastatic pancreatic cancer, gemcitabine-based chemotherapy is the main treatment; in the present study, we used IRE to treat the patient with stage VI pancreatic cancer. Martin et al[21] recommend using induction gemcitabine- or FOLFIRINOX (oxaliplatin, irinotecan, 5-fluorouracil, and leucovorin)-based chemotherapy, depending on the patient’s age and performance status, for at least 3–4 mo (three cycles of gemcitabine or 4–6 cycles of FOLFIRINOX). Although the required duration of adjuvant chemotherapy remains unclear, given the physical condition of our patient, we decided to administer four chemotherapy cycles after local IRE treatment.
A recent analysis based on randomized trials in Asia showed that gemcitabine in combination with tegafur significantly prolonged OS in patients with advanced or metastatic pancreatic cancer[22]. The results suggest that tegafur can be the first-line treatment for metastatic pancreatic cancer in Asian populations. The Chinese Society of Clinical Oncology Pancreatic Cancer Expert Committee has reached a consensus to include tegafur monotherapy and tegafur combined with gemcitabine regimen in the selection of first-line treatment options for advanced pancreatic cancer. However, the program may be used only in Asian populations; the efficacy of tegafur needs further testing.
Here, our patient received IRE combined with gemcitabine and tegafur. To our surprise, she was evaluated as having CR after four cycles of treatment and has survived for 12 mo after treatment. Meanwhile, no serious clinical adverse reactions occurred.
The combination of IRE with gemcitabine and tegafur may be a viable option for treating metastatic pancreatic cancer, with acceptable adverse effects. However, large prospective studies should be carried out to clarify the efficiency, safety, and OS benefit.
The authors thank the patient for participating in the study and for agreeing to the publication of this report.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Demetrashvili Z S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Yeo TP. Demographics, epidemiology, and inheritance of pancreatic ductal adenocarcinoma. Semin Oncol. 2015;42:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Ferrone CR, Pieretti-Vanmarcke R, Bloom JP, Zheng H, Szymonifka J, Wargo JA, Thayer SP, Lauwers GY, Deshpande V, Mino-Kenudson M, Fernández-del Castillo C, Lillemoe KD, Warshaw AL. Pancreatic ductal adenocarcinoma: long-term survival does not equal cure. Surgery. 2012;152:S43-S49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 3. | Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 1120] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 4. | Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, Leach SD, Stanger BZ. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1400] [Cited by in RCA: 1664] [Article Influence: 128.0] [Reference Citation Analysis (0)] |
| 5. | Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2129] [Cited by in RCA: 2112] [Article Influence: 150.9] [Reference Citation Analysis (3)] |
| 6. | Gleisner AL, Assumpcao L, Cameron JL, Wolfgang CL, Choti MA, Herman JM, Schulick RD, Pawlik TM. Is resection of periampullary or pancreatic adenocarcinoma with synchronous hepatic metastasis justified? Cancer. 2007;110:2484-2492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Edd JF, Horowitz L, Davalos RV, Mir LM, Rubinsky B. In vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE Trans Biomed Eng. 2006;53:1409-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 335] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 8. | Ansari D, Kristoffersson S, Andersson R, Bergenfeldt M. The role of irreversible electroporation (IRE) for locally advanced pancreatic cancer: a systematic review of safety and efficacy. Scand J Gastroenterol. 2017;52:1165-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Stillström D, Nilsson H, Jesse M, Peterhans M, Jonas E, Freedman J. A new technique for minimally invasive irreversible electroporation of tumors in the head and body of the pancreas. Surg Endosc. 2017;31:1982-1985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Liu S, Qin Z, Xu J, Zeng J, Chen J, Niu L, Xu M. Irreversible electroporation combined with chemotherapy for unresectable pancreatic carcinoma: a prospective cohort study. Onco Targets Ther. 2019;12:1341-1350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Nakamura K, Yamaguchi T, Ishihara T, Sudo K, Kato H, Saisho H. Phase II trial of oral S-1 combined with gemcitabine in metastatic pancreatic cancer. Br J Cancer. 2006;94:1575-1579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Zhong S, Qie S, Yang L, Yan Q, Ge L, Wang Z. S-1 monotherapy versus S-1 combination therapy in gemcitabine-refractory advanced pancreatic cancer: A meta-analysis (PRISMA) of randomized control trials. Medicine (Baltimore). 2017;96:e7611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Kim MK, Lee KH, Jang BI, Kim TN, Eun JR, Bae SH, Ryoo HM, Lee SA, Hyun MS. S-1 and gemcitabine as an outpatient-based regimen in patients with advanced or metastatic pancreatic cancer. Jpn J Clin Oncol. 2009;39:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21550] [Article Influence: 1346.9] [Reference Citation Analysis (1)] |
| 15. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15461] [Article Influence: 2576.8] [Reference Citation Analysis (2)] |
| 16. | Lu F, Poruk KE, Weiss MJ. Surgery for oligometastasis of pancreatic cancer. Chin J Cancer Res. 2015;27:358-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 17. | Lu 2nd, Kwon D, Chalikonda S, Sellers M, Kotz E, Scoggins C, McMasters KM, Watkins K. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Ann Surg. 2015;262:486-94; discussion 492-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 294] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 18. | Cannon R, Ellis S, Hayes D, Narayanan G, Martin RC 2nd. Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J Surg Oncol. 2013;107:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 245] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 19. | Hong Y, Rice J, Sharma D, Martin RCG 2nd. The use of IRE in multi-modality treatment for oligometastatic pancreatic cancer. Am J Surg. 2018;216:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Bhutiani N, Philips P, Scoggins CR, McMasters KM, Potts MH, Martin RC. Evaluation of tolerability and efficacy of irreversible electroporation (IRE) in treatment of Child-Pugh B (7/8) hepatocellular carcinoma (HCC). HPB (Oxford). 2016;18:593-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Martin RC 2nd, Durham AN, Besselink MG, Iannitti D, Weiss MJ, Wolfgang CL, Huang KW. Irreversible electroporation in locally advanced pancreatic cancer: A call for standardization of energy delivery. J Surg Oncol. 2016;114:865-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Li Y, Sun J, Jiang Z, Zhang L, Liu G. Gemcitabine and S-1 combination chemotherapy versus gemcitabine alone for locally advanced and metastatic pancreatic cancer: a meta-analysis of randomized controlled trials in Asia. J Chemother. 2015;27:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |