Published online Jan 26, 2020. doi: 10.12998/wjcc.v8.i2.313
Peer-review started: September 26, 2019
First decision: October 24, 2019
Revised: December 25, 2019
Accepted: January 1, 2020
Article in press: January 1, 2020
Published online: January 26, 2020
Processing time: 112 Days and 11.1 Hours
Dystonic gait (DG) is one of clinical symptoms associated with functional dystonia in the functional movement disorders (FMDs). Dystonia is often initiated or worsened by voluntary action and associated with overflow muscle activation. There is no report for DG in FMDs caused by an abnormal pattern in the ankle muscle recruitment strategy during gait.
A 52-year-old male patient presented with persistent limping gait. When we requested him to do dorsiflexion and plantar flexion of his ankle in the standing and seating positions, we didn’t see any abnormality. However, we could see the DG during the gait. There were no evidences of common peroneal neuropathy and L5 radiculopathy in the electrodiagnostic study. Magnetic resonance imaging of the lumbar spine, lower leg, and brain had no definite finding. No specific finding was seen in the neurologic examination. For further evaluation, a wireless surface electromyography (EMG) was performed. During the gait, EMG amplitude of left medial and lateral gastrocnemius (GCM) muscles was larger than right medial and lateral GCM muscles. When we analyzed EMG signals for each muscle, there were EMG bursts of double-contraction in the left medial and lateral GCM muscles, while EMG analysis of right medial and lateral GCM muscles noted regular bursts of single contraction. We could find a cause of DG in FMDs.
We report an importance of a wireless surface EMG, in which other examination didn’t reveal the cause of DG in FMDs.
Core tip: Dystonic gait occur as one of clinical symptoms associated with functional dystonia in the functional movement disorders. Dystonia is often initiated by voluntary action and associated with persistent muscle activation. In the functional movement disorders, mere presence of an abnormal gait does not confirm a functional etiology. We report an importance of a wireless surface electromyography when it comes to diagnosis the dystonic gait in functional movement disorders, in which other examination didn’t reveal the cause of dystonic gait.
- Citation: Oh MK, Kim HS, Jang YJ, Lee CH. Role of a wireless surface electromyography in dystonic gait in functional movement disorders: A case report. World J Clin Cases 2020; 8(2): 313-317
- URL: https://www.wjgnet.com/2307-8960/full/v8/i2/313.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i2.313
Functional dystonia is the second most common type of functional movement disorder (FMD)[1]. It may affect any part of the body or be generalized. Dystonic gait (DG) occur as one of clinical symptoms associated with functional dystonia in the FMDs. Dystonia is often initiated by voluntary action and associated with persistent muscle activation. DG can be manifested in the limping gait[2,3]. In functional gait orders of FMDs, there are many types of gait patterns such as excessive trunk sway, too much slowness and stiffness, tightrope walking, knock-kneed, and habitual limping[2,4]. The mere presence of an abnormal gait does not confirm a functional etiology. And walking is often bizarre and dose not conform to any of the usual patterns observed with neurologic gait diseases[5]. So there is no gold standard test for confirming the diagnosis of DG in FMD.
So far, DG caused by an abnormal pattern in the ankle muscle recruitment strategy during gait has never been reported through a wireless surface electromyography (EMG) findings. Herein we report an importance of a wireless surface EMG when it comes to diagnosis the DG in FMD, in which other examination didn’t reveal the cause of DG.
A 52-year-old male patient was referred by the neurology department to the rehabilitation department because of a 6-mo history of left limping gait.
The abrupt onset of his symptom started after severe stress. Since then his symptom persisted but He didn’t complain any numbness or tingling sensation on the left lateral side of the lower leg and dorsum of the foot.
He had no history of any tumor, trauma, and other diseases.
He had no specific personal or family history of neurologic disease.
The Medical Research Council scale for weakness showed the normal grade. When we requested him to do dorsiflexion and plantarflexion of his ankle in the standing and seating positions, we didn’t see the any abnormality. However, we could see the DG during the gait. He had no lower back pain and the straight leg raise reached 80° on both sides. No specific finding occurred in the neurologic examination.
Laboratory finding, including a complete blood count, erythrocyte sedimentation rate, C-reactive protein, creatine kinase, lactic dehydrogenase, and liver function tests were within normal ranges.
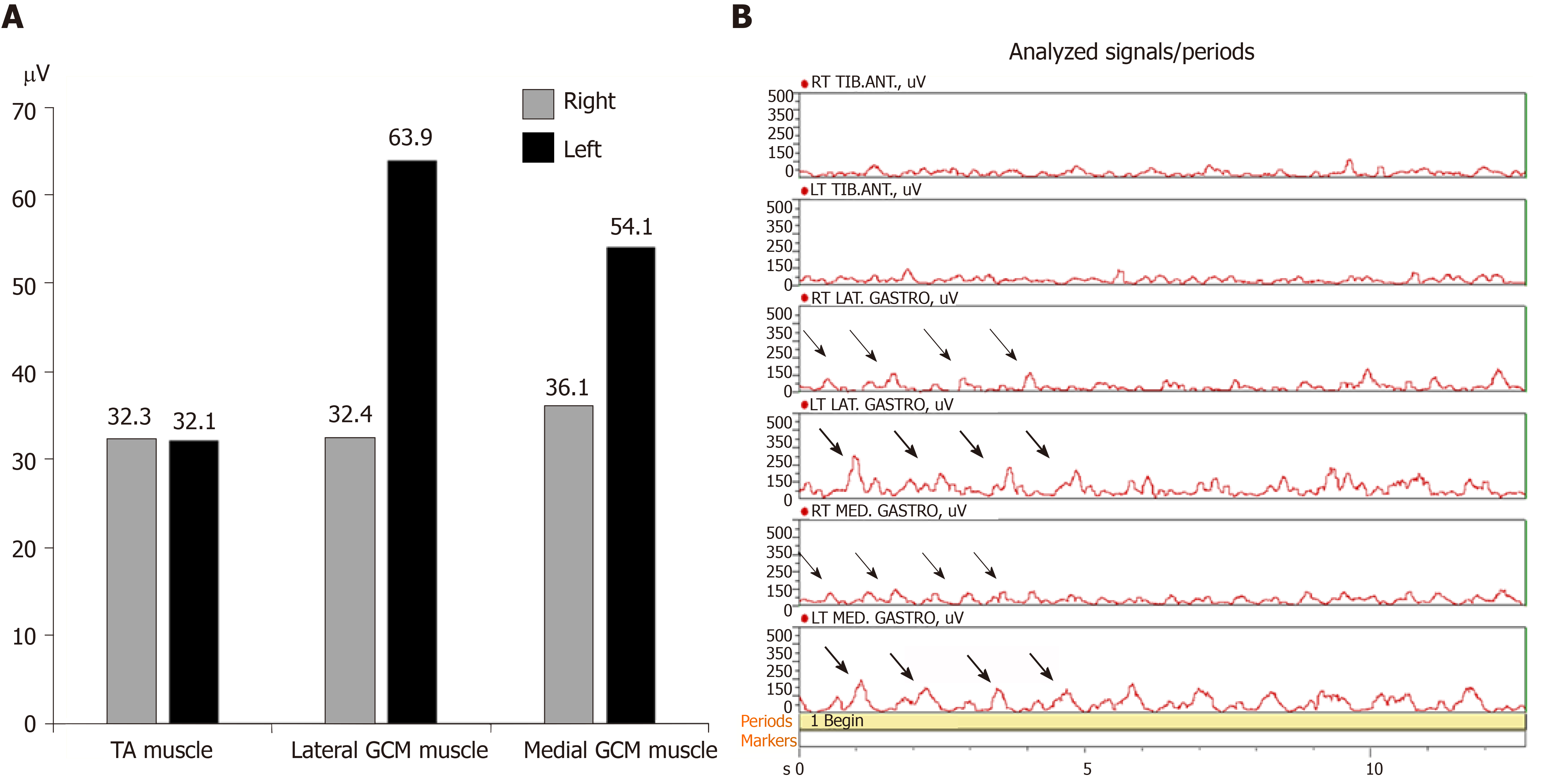
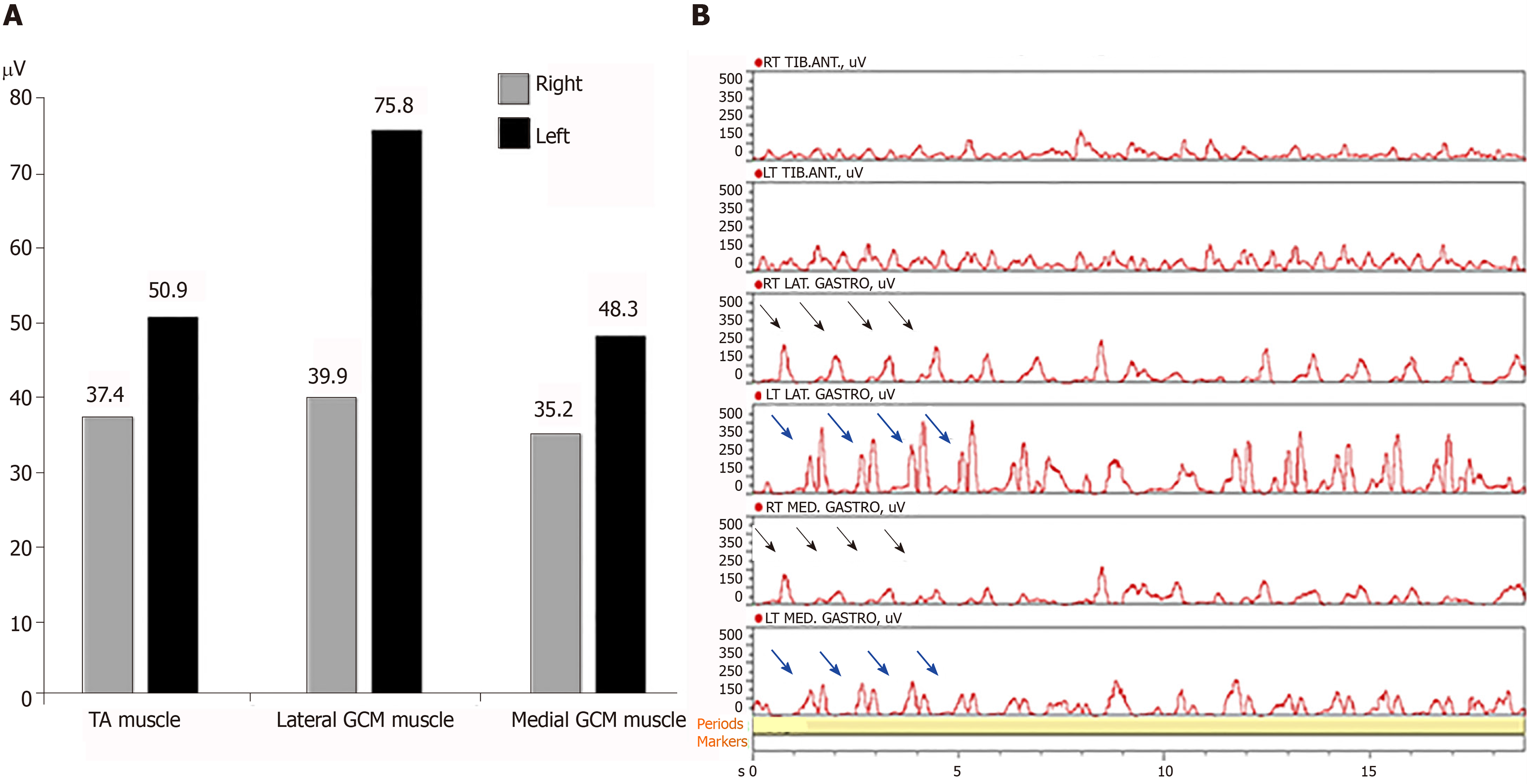
We requested the nerve conduction studies with needle EMG. There were no evidences of common peroneal neuropathy and L5 radiculopathy in nerve conduction studies and needle EMG. Magnetic resonance imaging (MRI) of the lumbar spine showed no remarkable finding. In addition, lower leg MRI was conducted to rule out the muscle origin. There was no definite finding. MRI of brain was performed to rule out a central origin. There was no specific finding except a few tiny unidentified bright object in the both cerebral white matters. For further evaluation, a wireless surface EMG (Noraxon, TELEmyo DTS, USA) was performed. Gel-type, 20-mm diameter, Ag/AgCl alloy dual electrodes (Noraxon Dual EMG Electrode, USA) were attached to tibialis anterior (TA), medial gastrocnemius (GCM), and lateral GCM muscles of both sides (left and right) after cleaning the sites with alcohol to reduce skin impedance. The surface EMGs were amplified 500 times, sampled at 1.5 kHz and digitized using a 16-bit analogue to digital converter. Signals were filtered at a bandwidth of 10-500 Hz to eliminate noise recorded during the data collection process, and root mean square window of 50 ms was used for signal smoothing. Mean of each period was calculated during the test. At first he was told to walk on the spot from right foot during 13 s. EMG amplitude of left medial and lateral GCM muscles was larger than right medial and lateral GCM muscles. EMG amplitude was similar at the both TA muscles. When we analyzed EMG signals for each muscle during 13 s, there were EMG bursts of double-contraction in the left medial and lateral GCM muscles, while EMG analysis of right medial and lateral GCM muscles noted regular bursts of single contraction. EMG analysis of both TA muscles were shown in similar bursts (Figure 1). Secondly he was told to walk forward from right foot during 20 s. EMG amplitude of left medial and lateral GCM muscles was also larger than right medial and lateral GCM muscles. EMG amplitude of left TA muscle was slightly larger than the one of right TA muscle. EMG analysis of both GCM muscles were alike in that walking on the spot. The bursts of left TA muscle were slightly higher and more than the ones of right TA muscle (Figure 2).
We could find a cause of DG associated FMDs with a wireless surface EMG. We recommended motion analysis for more precise analysis and department of psychiatry to rule out for a psychiatric cause. He refused our suggestion.
He received an intramuscular injection with botulinum toxin A 30 U to left medial and lateral GCM under ultrasound guidance to minimize discomfort in walking.
After botulinum toxin injection, his symptom improved after about 30 min. We recommended again a department of psychiatry to rule out for a psychiatric cause in the 3 mo follow-up. He refused our suggestion and did not come to our clinic after that time.
FMDs are clinical syndromes defined by the occurrence of abnormal involuntary movements that are incongruent with a known neurologic cause[5]. DG is one of clinical symptoms associated with functional dystonia in the FMDs[2]. In a retrospective review of 279 patients with various types of FMD, 118 patients had a functional gait disturbance. Among these patients, excessive slowness was the most frequent gait patterns, followed by DG, bizarre gait, astasia-abasia, and knee buckling[6]. There is no gold standard test for confirming the diagnosis of FMD. However, we had a few examinations such as the nerve conduction studies with needle EMG and MRI of the lumbar spine, lower leg, and brain to diagnose other organic or neurologic disease. No definite abnormality was not present. In the neurologic examination, there was no abnormality in our case although patients with a functional gait disturbance had more frequent excessive slowing of movements on finger to nose testing and finger or foot tapping.
Surface EMG is a non-invasive technique for measuring muscle electrical activity that occurs during muscle contraction and relaxation cycles. It is the electromyographic analysis that makes it possible to obtain an electrical signal from a muscle in a moving body[7,8]. A few studies for surface EMG reported normative data in adults during walking[9,10]. Although a recent analysis including TA and GCM showed that amplitude and timing of muscle co-contraction are correlated with age and velocity in healthy adult walking, a wide literature reported that TA and GCM act as pure agonist/antagonists for ankle plantar/dorsiflexion (no co-contractions) in about 20% of strides and co-contractions appear in early stance, mid stance, and swing phase of strides[11]. Muscle co-contraction is the stimultaneous contraction of agonist and antagonist muscles crossing a joint. It is commonly to augment function in maintenance of joint stability, providing resistance to rotation at a joint[12]. Significantly increased complexity in muscle recruitment strategy beyond the activation as pure ankle plantar/dorsiflexors suggests that co-contractions are likely functional to further physiological tasks as foot inversion, balance improvement, control of ankle stability and knee flexion. So when looking at the gait cycle, the part of the gait cycle that involves most dorsiflexion action would be heel contact of the foot at 10% of gait cycle, and the entire swing phase. During propulsive phase (toe off) of the gait cycle the plantar flexor muscles contract concentrically, pushing the foot into plantar flexion and propelling the body forward[9]. In our case, we could assess an abnormal pattern of the ankle muscle recruitment strategy during normal gait through a wireless surface EMG. Although motion analysis has not been conducted, we could identify EMG bursts of double-contraction in the left medial and lateral GCM muscles immediately after bursts of single contraction in the right medial and lateral GCM muscles in the analyzed EMG signals. It was the reason why the patient had presented left DG. Contrary to walking on the spot, the bursts of left TA muscle were slightly higher and more than the ones of right TA muscle while the patient was walking forward. It was interpreted as a compensatory action of left TA muscle to move forward in the period of push off in the gait cycle.
DG is a rare condition. This case illustrates the need to consider a wireless surface EMG for diagnosis of cause in a patient presenting with DG in FMDs, particularly in the absence of other obvious causes.
Manuscript source: Unsolicited Manuscript
Specialty type: Medicine, research and experimental
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Karayiannakis AJ S-Editor: Wang YQ L-Editor: A E-Editor: Xing YX
| 1. | Portera-Cailliau C, Victor D, Frucht S, Fahn S. Movement disorders fellowship training program at Columbia University Medical Center in 2001-2002. Mov Disord. 2006;21:479-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Peckham EL, Hallett M. Psychogenic movement disorders. Neurol Clin. 2009;27:801-819, vii. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Fahn S, Williams DT. Psychogenic dystonia. Adv Neurol. 1988;50:431-455. [PubMed] |
| 4. | Keane JR. Hysterical gait disorders: 60 cases. Neurology. 1989;39:586-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 63] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Snijders AH, van de Warrenburg BP, Giladi N, Bloem BR. Neurological gait disorders in elderly people: clinical approach and classification. Lancet Neurol. 2007;6:63-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 281] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 6. | Baik JS, Lang AE. Gait abnormalities in psychogenic movement disorders. Mov Disord. 2007;22:395-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Basmajian JV, de Luca CJ. Muscles Alive: their functions revealed by electromyography. Postgrad Med J. 1963;39:162. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 8. | Massó N, Rey F, Romero D, Gual G, Costa L, Germán A. Surface electromyography applications. Apunts Med Esport. 2010;45:127-136. |
| 9. | Perry J, Burnfield JM. Gait Analysis: Normal and Pathological Function. J Sports Sci Med. 2010;9:353. [RCA] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 481] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 10. | Sutherland DH. The evolution of clinical gait analysis part l: kinesiological EMG. Gait Posture. 2001;14:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Hortobágyi T, Solnik S, Gruber A, Rider P, Steinweg K, Helseth J, DeVita P. Interaction between age and gait velocity in the amplitude and timing of antagonist muscle coactivation. Gait Posture. 2009;29:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Di Nardo F, Mengarelli A, Maranesi E, Burattini L, Fioretti S. Assessment of the ankle muscle co-contraction during normal gait: a surface electromyography study. J Electromyogr Kinesiol. 2015;25:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |