Published online Sep 26, 2020. doi: 10.12998/wjcc.v8.i18.4034
Peer-review started: April 18, 2020
First decision: June 8, 2020
Revised: June 8, 2020
Accepted: September 3, 2020
Article in press: September 3, 2020
Published online: September 26, 2020
Processing time: 156 Days and 15.2 Hours
Acute kidney injury (AKI) after liver transplantation (LT) is a frequent and multifactorial event related to increased morbidity and mortality. Risk factors for AKI after LT still need to be clarified.
To identify the predictors of acute kidney injury after liver transplantation.
The frequency and pre- and intraoperative predictors of AKI within the first 7 d after LT were evaluated in adult liver transplant candidates in a single LT center in Croatia. AKI was defined according to the Kidney Disease: Improving Global Outcomes criteria.
Out of 205 patients (mean age 57 ± 10 years; 73.7% males, 52.7% with alcohol-related liver disease) 93 (45.36%) developed AKI, and the majority of them (58.06%) had stage 1. Only 5.38% of patients required renal replacement therapy after LT. The majority of patients (82.8%) developed AKI within the first two days after the procedure. Multivariate logistic regression identified pre-LT body mass index (OR = 1.1, 95%CI: 1.05-1.24) and red blood cell transfusion (OR = 1.66, 95%CI: 1.09-2.53) as independent predictors of early post-LT AKI occurrence. 30-d survival after LT was significantly better for patients without AKI (P = 0.01).
Early AKI after LT is a frequent event that negatively impacts short-term survival. The pathogenesis of AKI is multifactorial, but pre-LT BMI and intraoperative volume shifts are major contributors.
Core Tip: Acute kidney injury after liver transplantation is a frequent complication, but severe forms of AKI requiring dialysis are infrequent. The pathogenesis of acute kidney injury is multifactorial, with pre-LT BMI and intraoperative volume shifts being among major contributors.
- Citation: Mrzljak A, Franusic L, Pavicic-Saric J, Kelava T, Jurekovic Z, Kocman B, Mikulic D, Budimir-Bekan I, Knotek M. Pre- and intraoperative predictors of acute kidney injury after liver transplantation. World J Clin Cases 2020; 8(18): 4034-4042
- URL: https://www.wjgnet.com/2307-8960/full/v8/i18/4034.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i18.4034
There has been an increasing interest in acute kidney injury (AKI) in the setting of liver transplantation (LT), in particular given its high incidence rates up to 60% and its unfavourable prognosis. AKI after LT encompasses a heterogeneous group of events, differing in etiology, reversibility of kidney damage, and short- and long-term consequences.
Post-transplant AKI has been associated with increased acute rejection rates, infectious complications, prolonged hospital stays, and higher hospital costs[1-5]. In particular, AKI has been associated with the development of chronic kidney disease (CKD) and poorer long-term graft and/or patient survival[6,7]. In addition, post-transplant CKD is independently associated with late mortality and cardiovascular events[8,9].
According to the International Registry in Organ Donation and Transplantation, in the last five years (2014-2019), Croatia has one of the highest liver transplantation rates in the world, estimated between 27.35-32.2 per million population[10]. As the transplant population increases, it is of paramount importance to address multiple modifiable short and long-term risk factors out of which kidney disease has a significant influence on post-transplant outcomes.
The study aims to assess the frequency and pre- and intraoperative predictors of AKI within the first 7 d after LT and its impact on the short-term outcome in a single high-volume liver transplant center in Croatia.
All consecutive adult patients who underwent primary, deceased-donor liver transplantation between October 2014 and October 2016 at Merkur University Hospital (MUH), Zagreb, Croatia were included in the study. Patients with prior solid-organ transplants, candidates for simultaneous liver-kidney transplants, and patients on dialysis before LT were excluded. MUH is a high-volume transplant center with approximately 120 liver transplants performed per year, representing over 90% of the LT program in the country[11]. Patients’ clinical and laboratory data were retrospectively retrieved from the Electronic Medical Records. Approval for the study was obtained from the Hospital Ethics Committee.
The primary outcome measure was AKI. Post-transplant AKI was defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria[12]; an increase in serum creatinine by ≥ 26.5 µmol/L within 48 h or an increase in creatinine to ≥ 1.5 times baseline within the first 7 post-operative days. AKI was classified into three stages: stage 1, creatinine increase to ≥ 26.5 µmol/L or increase to 1.5-1.9-fold from baseline; stage 2, increase to 2–2.9-fold; stage 3, increase > 3-fold or increase in serum creatinine to ≥ 354 µmol/L or the initiation of renal replacement therapy (RRT).
Preoperative and intraoperative parameters were used to predict AKI after LT and they included age, gender, primary diagnosis, co-morbidities before LT, and Model for End-Stage Liver Disease (MELD). MELD was calculated according to formula: MELDScore = 10*((0.957*ln(Creatinine))+(0.378*ln(Bilirubin))+(1.12*ln(INR)))+6.43 available at: https://reference.medscape.com/calculator/meld-score-end-stage-liver-disease[13]. The last laboratory values for bilirubin, sodium and creatinine levels and international normalized ratio (INR) were obtained before transplant. We determined the estimated GFR (eGFR) using the Abbreviated Modification of Diet in Renal Disease (aMDRD) formula: eGFR = 186 × (SCr mg dl-1)-1.154 × age-0.203 × 0.742 if a patient is female × 1.21[14]. All biochemical parameters were analyzed in an accredited (ISO 15189:2012 “Medical laboratories -- Requirements for quality and competence”) clinical laboratory.
Chronic kidney disease was defined as histology-proven kidney disease and/or the presence of pathological proteinuria or erythrocyturia and/or eGFR < 60 mL/min/1.732 calculated by the aMDRD formula, for at least three months before LT.
According to our center protocol, whenever not contraindicated (most frequently because of uncorrectable coagulopathy), kidney biopsies were performed in patients with suspected parenchymal renal disease (e.g., proteinuria > 1 g/d and/or glomerular erythrocyturia), or in patients with stage 3-4 chronic kidney disease to determine the extent of interstitial fibrosis/tubule atrophy (IF/TA) and global glomerulosclerosis, as criteria for simultaneous liver and kidney transplantation. Patients with IF/TA, or glomerulosclerosis > 30% were considered candidates for liver and kidney transplants.
In addition, warm and cold ischemia time, intraoperative parameters of red blood cells (RBCs) transfusion (homologous and/or autologous), fresh frozen plasma (FFP), and total fluids were analyzed. Postoperative data; creatinine levels, RRT, date of death were used to define the end-point and outcome analysis within 30 days after liver transplant.
All patients underwent first-time orthotopic liver transplantation with whole grafts from donors after brain death (DBD) using the piggy back technique without venovenous bypass. If autologous RBC transfusion was used, cell salvage technique was employed: The blood was collected from the suction, surgical drains, or both and re-transfused back to the patient after filtration or washing.
Immunosuppression was provided per standard hospital protocol using triple immunosuppressive regimens. Methylprednisolone (500 mg) was given before reperfusion of the graft, followed by a gradual taper until 20 mg by the end of the first week. The oral dose for mycophenolate mofetil was 1 gram twice a day in combination with tacrolimus or cyclosporine (at the discretion of the transplant physician) starting within the first 24 h post-LT. The initial oral dose of tacrolimus or cyclosporine was 0.5 mg or 25 mg twice a day, respectively, starting within the first 24 h post-LT. Daily blood trough levels guided subsequent dosing with a targeted level of 8–10 mg/dL or 150-200 µg/dL for tacrolimus and cyclosporine, respectively. In addition to immunosuppression, all patients received antimicrobial prophylaxis in the form of amoxicillin and clavulanic acid or piperacillin tazobactam for 48 h after LT, followed by valganciclovir 450 mg once daily and trimethoprim-sulphomethoxasole 480 mg three times per week, starting within the first week.
Statistical analysis was performed using MedCalc and GraphPad Prism. Differences between the groups were tested using χ2 - and Fischer’s exact test (categorical variables) or Student's t-test and Mann-Whitney U tests (ordinal or numerical variables). The strength of the association between AKI and potential risk factors was tested using univariate and multivariate logistic regression. Two variables that did not follow a normal distribution (RBC and bilirubin) were logarithmized (base 2) to achieve normal distribution before inclusion to multivariate analysis. The Kaplan–Meier method was used to estimate patient survival; the log-rank test was used to compare survival between patients with or without early post-transplant AKI. The level of statistical significance was set at α = 0.05.
In the study period, 234 patients underwent liver transplantation, out of which 205 were included. Twenty-six patients did not meet inclusion criteria (3 on dialysis before LT). The mean age of the cohort was 57 ± 10 years; 151 (73.7%) were males, and alcohol-related liver disease 108 (52.7%) was the main etiology. At the time of transplant, more than one-third of the patients 74 (36.1%) had accompanying liver tumors, almost one third had type 2 diabetes (T2D) 58 (28.3 %), and arterial hypertension 57 (27.8%) and 21 (10.2%) had chronic kidney disease. The characteristics of the patients are presented in Table 1.
| Characteristics | All (n = 205) | AKI (n = 93) | non-AKI (n = 112) | P value |
| Male/female, n (%) | 151 (73.7)/54 (26.3) | 72 (77.4)/21 (22.6) | 79 (70.5)/33 (77.4) | 0.265 |
| Age, yr | 57 ± 10 | 56.51 ± 8.83 | 56.87 ± 11.38 | 0.803 |
| BMI, kg/m2 | 26.26 ± 4.46 | 27.51 ± 4.93 | 25.22 ± 3.76 | < 0.001 |
| Etiology of liver disease1 | ||||
| Alcohol-related, n (%) | 108 (52.7) | 54 (58.1) | 54 (48.2) | 0.16 |
| Viral diseases, n (%) | ||||
| Hepatitis C, n (%) | 31 (15.1) | 15 (16.1) | 16 (14.3) | 0.714 |
| Hepatitis B, n (%) | 17 (18.3) | 5 (5.4) | 12 (10.7) | 0.168 |
| Autoimmune diseases n (%) | 19 (19.3) | 7 (7.5) | 12 (10.7) | 0.433 |
| Other liver disease n (%) | 33 (16.1) | 14 (15.1) | 19 (17) | 0.711 |
| Co-morbidites, n (%) | ||||
| Tumors, n (%) | 74 (36.1) | 27 (29) | 47 (42) | 0.055 |
| Arterial hypertension, n (%) | 57 (27.8) | 24 (25.8) | 33 (29.5) | 0.561 |
| CKD, n (%) | 21 (10.2) | 7 (7.5) | 14 (12.5) | 0.242 |
| T2D, n (%) | 58 (28.3) | 27 (29) | 31 (27.7) | 0.83 |
| Laboratory values preLT, mean ± SD | ||||
| Hb (g/L) | 114.26 ± 22.84 | 110.76 ± 22.6 | 117.17 ± 22.72 | 0.045 |
| Bilirubin, µmol/L | 40 (23-103) | 48.5 (26.8-112.5) | 33.5(21.3-88.5) | 0.075 |
| Creatinine, µmol/L | 98.28 ± 75.05 | 96.16 ± 64.08 | 100.04 ± 83.31 | 0.714 |
| INR | 1.61 ± 0.56 | 1.68 ± 0.56 | 1.55 ± 0.55 | 0.115 |
| Na, mmol/L | 135.3 ± 4.87 | 134.78 ± 5.27 | 135.73 ± 4.48 | 0.18 |
| eGFR, mL/min/1.73 m² | 90.61 ± 47.27 | 90.64 ± 51.59 | 90.58 ± 43.59 | 0.993 |
| MELD | 16.94 ± 7.58 | 17.66 ± 7.2 | 16.35 ± 7.86 | 0.22 |
| MELD-Na | 19.39 ± 7.76 | 20.45 ± 7.32 | 18.49 ± 8.04 | 0.08 |
| Intraoperative parameters | ||||
| FFP, mL | 2112.19 ± 1393.22 | 2388.4 ± 1389.4 | 1873.2 ± 1358.2 | 0.01 |
| Cristalloids, mL | 5020.67 ± 1506.97 | 4941.54 ± 1281.1 | 5090.58 ± 1684.74 | 0.493 |
| Colloids, mL | 1724.77 ± 925.12 | 1891.76 ± 913.24 | 1578.65 ± 914.9 | 0.018 |
| Albumins, mL | 741.79 ± 378.36 | 823.08 ± 404.65 | 670.67 ± 340.02 | 0.005 |
| CIT, h | 437.67 ± 135.74 | 452.3 ± 136.26 | 427.42 ± 135.31 | 0.304 |
| WIT, min | 27.29 ± 6.93 | 28 ± 7.72 | 26.59 ± 6.13 | 0.425 |
| RBCs, L | 5.7 (3.7-8.2) | 6 (4.9-9.9) | 4.71 (3-7.5) | 0.001 |
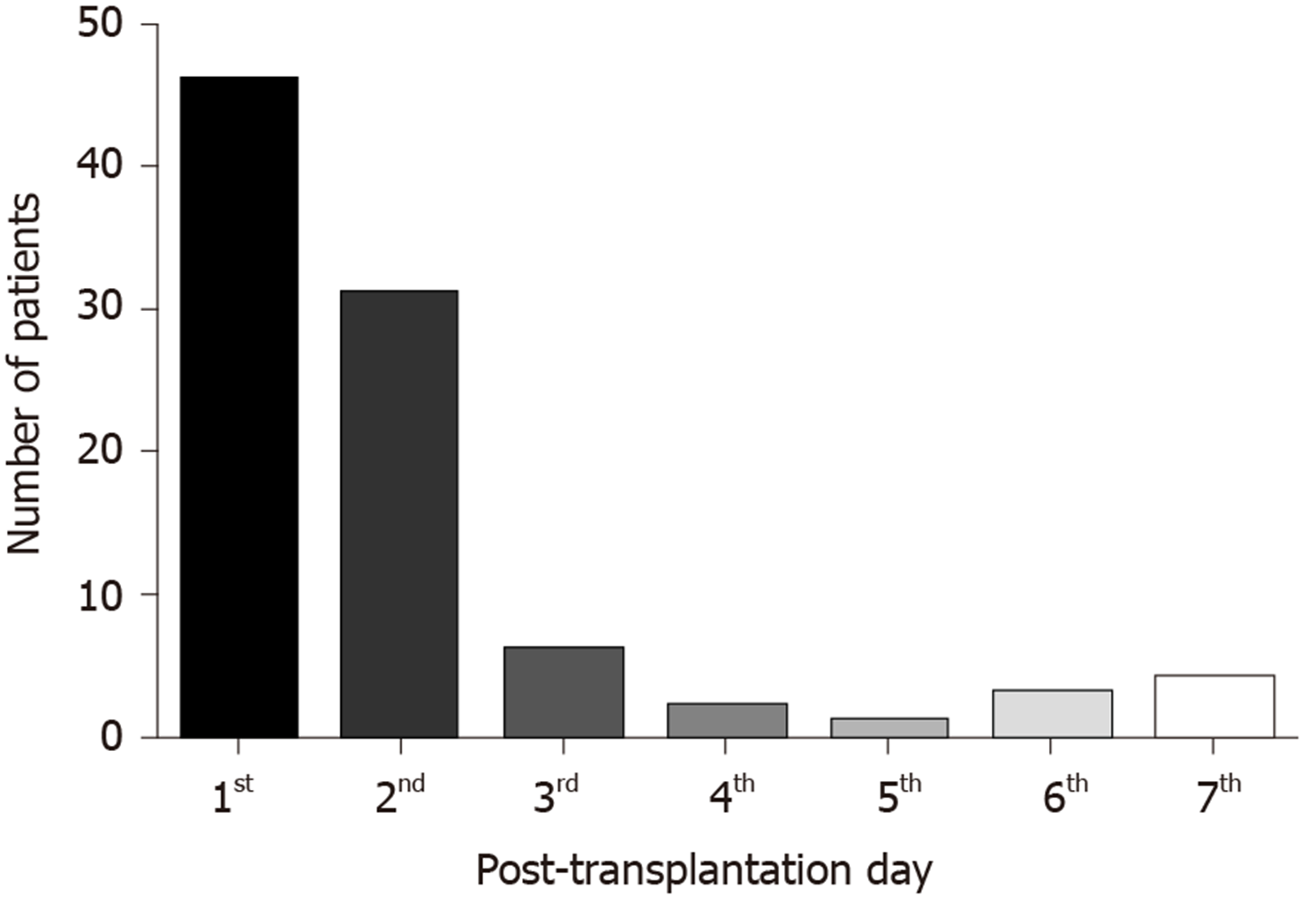
Within the first 7 d after liver transplant, acute kidney injury developed in 93 (45.36%) of patients. The distribution of the stages of AKI is presented in Table 2. The majority of patients, 54 (58.06%), had stage 1. Only 5 (5.38%) patients required renal replacement therapy within the first week after LT. The majority of patients (82.8%) developed AKI within the first two days after the procedure, Figure 1.
| n (%) | |
| No AKI | 112 |
| AKI stage 1 | 54 (58.06) |
| AKI stage 2 | 24 (25.81) |
| AKI stage 3 | 15 (16.13) |
| Postop HD | 5 (5.38) |
| Severe AKI (stage 2/3) | 39 (41.94) |
| Overall AKI | 93 (45.36) |
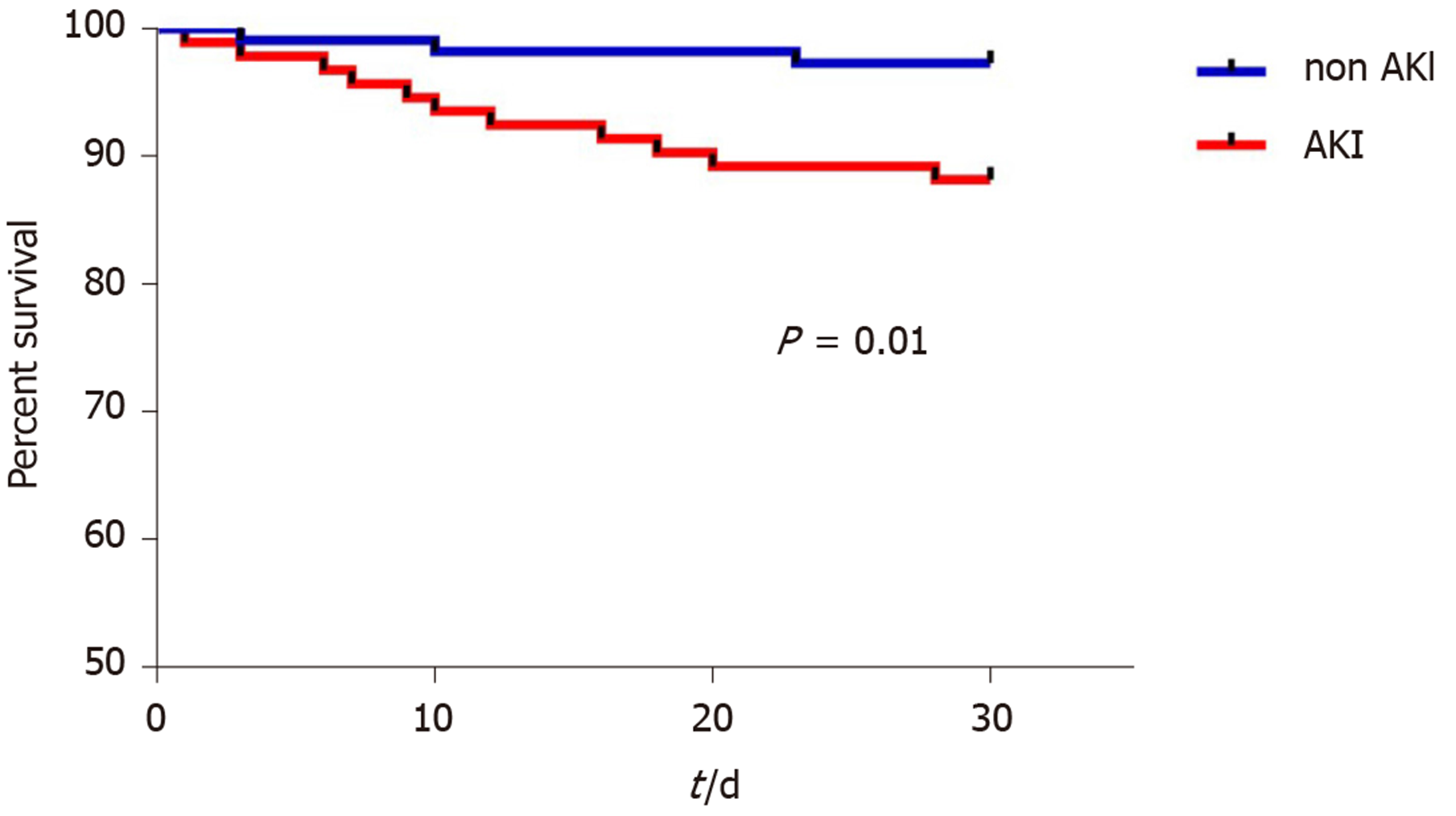
Patients who developed AKI had higher BMI pre-LT (27.51 ± 4.93 vs 25.22 ± 3.76, P < 0.001), and higher intraoperative blood loss reflected in the requirements of red blood cell transfusion (P = 0.001), fresh frozen plasma (P = 0.01), albumin (P = 0.005), and colloids (P = 0.018) during the procedure (Table 1). There were no differences between the groups regarding age, gender, etiology of liver disease, co-morbidities, preoperative laboratory parameters or cold and warm ischemia times (Table 1). In univariate logistic regression, factors associated with AKI within the first 7 days after LT were pre-LT BMI (P < 0.001), pre-LT hemoglobin (P = 0.047), and intraoperative RBC transfusion (P = 0.001). Multivariate logistic regression identified pre-LT BMI (OR = 1.1, 95%CI: 1.05-1.24) and RBCs transfusion (OR = 1.66, 95%CI: 1.09-2.53) as independent predictors of early post-LT AKI occurrence (Table 3). 30-d patient survival after LT was significantly better in patients without AKI (log-rank, P = 0.01) (Figure 2).
| Characteristics | OR | 95%CI (OR) | P value | OR | 95%CI (OR) | P value |
| RBCa | 1.84 | 1.3-2.6 | 0.001 | 1.66 | 1.09-2.53 | 0.018 |
| BMI | 1.13 | 1.06–1.21 | < 0.001 | 1.14 | 1.05-1.24 | 0.001 |
| Hb | 0.99 | 0.98–1.00 | 0.047 | 0.98 | 0.97-1 | 0.053 |
| NaMELD | 1.03 | 0.99–1.07 | 0.082 | 0.93 | 0.83-1.04 | 0.195 |
| Tumor, yes vs no | 0.57 | 0.32–1.02 | 0.056 | 1.51 | 0.71-3.23 | 0.288 |
| HBV, yes vs no | 0.47 | 0.16–1.40 | 0.176 | 0.98 | 0.24-4.01 | 0.976 |
| Bilirubina | 1.14 | 0.97–1.34 | 0.123 | 1.39 | 0.96-2.02 | 0.079 |
| ALC, yes vs no | 1.49 | 0.86–2.59 | 0.16 | 1.47 | 0.69-3.14 | 0.315 |
| Na | 0.96 | 0.91–1.02 | 0.182 | 0.96 | 0.88-1.05 | 0.415 |
| INR | 1.49 | 0.90–2.47 | 0.119 | 0.87 | 0.35-2.15 | 0.762 |
Our study showed that in the early postoperative period, AKI represents a significant clinical burden in transplant recipients, as almost half of them (47.8%) developed some stage of acute kidney injury. However, the majority (54.1%) had stage 1 AKI, and only 2.4% patients required renal replacement therapy within the first week after LT. Similar extent of kidney injury was noted in previous studies which demonstrated that every second liver recipient encountered at least transient renal injury early after transplantation[15,16].
Post-transplant AKI likely results from a combination of pre-transplant and procedure-related events, in addition to post-transplant interventions (e.g., immunosuppression). We assessed pre- and intra-operative parameters and showed that patients who developed AKI had significantly higher pre-LT BMI, lower pre-LT hemoglobin and higher intraprocedural requirements of red blood cells, fresh frozen plasma, albumin and colloids reflecting in particular severity of the disease and demanding procedure-related events. However, the differences regarding MELD scores, etiology of liver disease, co-morbidities, cold and warm ischemia times, in addition to age and gender between patients with and without AKI did not reach statistical significance. Previous studies have shown that patients with high MELD score at the time of transplant are at higher risk of developing AKI after the transplant[1,17]. Namely, the severity of the liver disease is associated with post-LT AKI[17], as decompensated liver patients are more susceptible to kidney ischemia by the activation of endogenous vasoactive substances released during the procedure[18]. Given Croatia’s high liver transplantation rates, the average MELD score at the time of transplant is low (MELD 16.94 ± 7.58, Table 1), and the waiting time is short. High laboratory MELD patients, as well as the patients who are prioritized (standard exception) according to the Eurotransplant allocation system (e.g., hepatocellular carcinoma)[19], have particularly short waiting times. Therefore, in general, our liver patients are not “too sick” at the time of transplant, which favourably influences the outcomes and might have led to observed lack of association of the MELD with AKI in our study. The majority of our patients (82.8%) developed AKI within the first two days after the procedure implicating that procedure-related events are the major determinants in the development of early AKI. Indeed, any event during the procedure resulting in kidney hypoperfusion may result in post-LT AKI[20]. From our data, it is difficult to assess the etiopathogenesis of AKI in our cohort. Presumably, a significant proportion of patients developed pre-renal azotemia, due to large intraoperative fluid shifts and blood loss (as evidenced by the relationship between AKI and RBCs transfusion), with rapid renal function improvement in the early post-operative period. On the other hand, transfusion of RBCs may play an additional role in AKI development by inducing a pro-inflammatory state and increasing the concentrations of nephrotoxic free hemoglobin and iron[21,22].
In our study, in addition to transfusion of RBCs, the pre-transplant BMI has been identified as an independent predictor of AKI in the early post-LT period. A substantial body of evidence supports that higher body mass index in critically ill patients is associated with the development of AKI and increased short- and long-term mortality[23-25]. However, this should be taken with caution in patients with end-stage liver disease, as BMI is limited by the inability to assess body composition (the relative proportions of fat and lean mass), and even more so in patients with decompensated cirrhosis where further limitations arise from fluid retention[26]. Indeed, Leonard and colleagues showed on 1313 patients awaiting LT that after correction for ascites volume, 11%-20% of patients fall into a lower BMI classification and that BMI can no longer serve as an independent predictor of a post-transplant patient or graft survival[27]. In our study, it is interesting that decreased pre-transplant kidney function was not related to the development of subsequent AKI, unlike some previous reports, where pre-transplant AKI or CKD were risk factors for post-transplant AKI[28,29].
AKI, as a risk factor for mortality after LT, needs more investigation. Although it is clear from our results that the patients who developed AKI had lower 30-d survival, further research is required to assess whether AKI is an independent causative factor contributing to decreased survival. Similarly, the long-term risk of developing CKD needs to be evaluated in patients with post-transplant kidney injury.
Our study has several limitations that need to be pointed out. This is a retrospective study. The majority of patients were male, and the leading etiology was alcoholic liver disease, representing a typical LT population in Croatia. Also, the study did not include donor-related parameters, which might have contributed to the AKI occurrence. The use of nephrotoxic drugs was not explicitly analysed, but our center tends to avoid pre-transplant or post-transplant use of nephrotoxic drugs. No kidney histology data are included. Special care is taken to avoid nephrotoxic drugs in patients with renal impairment, as stated in our protocol in the Methods. In addition, the lack of an exact measure of body constitution and a more precise assessment of kidney function by creatinine clearance in end-stage liver disease patients can be regarded as important study limitations.
In conclusion, despite these limitations, our study offers valuable insight into the epidemiology of AKI and identifies potential risk factors for its development after liver transplantation. Early AKI after LT is a frequent event that negatively impacts short-term survival. The pathogenesis of AKI is multifactorial, but pre-LT BMI and intraoperative volume shifts are major contributors.
Acute kidney injury (AKI) after liver transplantation (LT) is a frequent and multifactorial event related to increased morbidity and mortality.
Risk factors for AKI after LT still need to be clarified.
To identify the predictors of acute kidney injury after liver transplantation.
The frequency and pre- and intraoperative predictors of AKI within the first 7 d after LT were evaluated in adult liver transplant candidates in a single LT center in Croatia. AKI was defined according to the Kidney Disease: Improving Global Outcomes criteria.
Out of 205 patients, 45.36% developed AKI, and the majority of them (58.06%) had stage 1. Only 5.38% of patients required renal replacement therapy after LT. Pre-LT body mass index (OR = 1.1, 95%CI: 1.05-1.24) and red blood cell transfusion (OR = 1.66, 95%CI: 1.09-2.53) were identified as independent predictors of early post-LT AKI occurrence. 30-d survival after LT was significantly better for patients without AKI (P = 0.01).
Early AKI after LT is a frequent event that negatively impacts short-term survival. The pathogenesis of AKI is multifactorial, but pre-LT BMI and intraoperative volume shifts are major contributors.
Acute kidney injury, as a risk factor for mortality after LT, needs more investigation. Although it is clear from our results that the patients who developed AKI had lower 30-d survival, further research is required to assess whether AKI is an independent causative factor contributing to decreased survival. Similarly, the long-term risk of developing CKD needs to be evaluated in patients with post-transplant kidney injury.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Casanova Rituerto D, Isaji S, Pop TL S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Nadim MK, Genyk YS, Tokin C, Fieber J, Ananthapanyasut W, Ye W, Selby R. Impact of the etiology of acute kidney injury on outcomes following liver transplantation: acute tubular necrosis versus hepatorenal syndrome. Liver Transpl. 2012;18:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 2. | Gonwa TA, Mai ML, Melton LB, Hays SR, Goldstein RM, Levy MF, Klintmalm GB. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation. 2001;72:1934-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 379] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 3. | Wong LP, Blackley MP, Andreoni KA, Chin H, Falk RJ, Klemmer PJ. Survival of liver transplant candidates with acute renal failure receiving renal replacement therapy. Kidney Int. 2005;68:362-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, Thottakkara P, Efron PA, Moore FA, Moldawer LL, Segal MS, Bihorac A. Cost and Mortality Associated With Postoperative Acute Kidney Injury. Ann Surg. 2015;261:1207-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 294] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 5. | Wyatt CM, Arons RR. The burden of acute renal failure in nonrenal solid organ transplantation. Transplantation. 2004;78:1351-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1703] [Cited by in RCA: 1637] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 7. | Hussaini T, Yoshida EM, Partovi N, Erb SR, Scudamore C, Chung S, Marquez V. Early Persistent Progressive Acute Kidney Injury and Graft Failure Post Liver Transplantation. Transplant Direct. 2019;5:e429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Rubín A, Sánchez-Montes C, Aguilera V, Juan FS, Ferrer I, Moya A, Montalva E, Pareja E, López-Andujar R, Prieto M, Berenguer M. Long-term outcome of 'long-term liver transplant survivors'. Transpl Int. 2013;26:740-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. 2010;10:1420-1427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 587] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 10. | International Registry in Organ Donation and Transplantation. Accessed April 2020. Available from: http://www.irodat.org/. |
| 11. | Republic of Croatia, Ministry of Health, National Transplantation Program. Results; [cited 2020 Mar 25]. Database: [internet]. Available from: https://zdravlje.gov.hr/UserDocsImages/dokumenti/Tekstovi%20razni/Preliminarno%20izvje%C5%A1%C4%87e%20NTP%202019..pdf. |
| 12. | Kellum J, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Herzog CA, Joannidis M, Kribben A, Levey AS, MacLeod AM, Mehtla RL, Murray PT, Naicker S, Opal SM, Schaefer F, Schetz M, Uchino S. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;1-138. |
| 13. | Kamath PS, Kim WR; Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD). Hepatology. 2007;45:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1228] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 14. | Pöge U, Gerhardt T, Palmedo H, Klehr HU, Sauerbruch T, Woitas RP. MDRD equations for estimation of GFR in renal transplant recipients. Am J Transplant. 2005;5:1306-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Hilmi IA, Damian D, Al-Khafaji A, Planinsic R, Boucek C, Sakai T, Chang CC, Kellum JA. Acute kidney injury following orthotopic liver transplantation: incidence, risk factors, and effects on patient and graft outcomes. Br J Anaesth. 2015;114:919-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 205] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 16. | Angeli P, Bezinover D, Biancofiore G, Bienholz A, Findlay J, Paugam Burtz C, Reyntjens K, Sakai T, Saner FH, Tomescu D, Wagener G, Weiss E. Acute kidney injury in liver transplant candidates: a position paper on behalf of the Liver Intensive Care Group of Europe. Minerva Anestesiol. 2017;83:88-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Romano TG, Schmidtbauer I, Silva FM, Pompilio CE, D'Albuquerque LA, Macedo E. Role of MELD score and serum creatinine as prognostic tools for the development of acute kidney injury after liver transplantation. PLoS One. 2013;8:e64089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Durand F, Graupera I, Ginès P, Olson JC, Nadim MK. Pathogenesis of Hepatorenal Syndrome: Implications for Therapy. Am J Kidney Dis. 2016;67:318-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Eurotransplant. Accessed April 2020. Available from: https://www.eurotransplant.org/. |
| 20. | Goren O, Matot I. Update on perioperative acute kidney injury. Curr Opin Crit Care. 2016;22:370-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | de Haan JE, Hoorn EJ, de Geus HRH. Acute kidney injury after liver transplantation: Recent insights and future perspectives. Best Pract Res Clin Gastroenterol. 2017;31:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Karkouti K. Transfusion and risk of acute kidney injury in cardiac surgery. Br J Anaesth. 2012;109 Suppl 1:i29-i38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 23. | Ju S, Lee TW, Yoo JW, Lee SJ, Cho YJ, Jeong YY, Lee JD, Kim JY, Lee GD, Kim HC. Body Mass Index as a Predictor of Acute Kidney Injury in Critically Ill Patients: A Retrospective Single-Center Study. Tuberc Respir Dis (Seoul). 2018;81:311-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Gorordo-Delsol LA, Jiménez-Ruiz A, Zamora-Gómez SE, Castañón-González JA. Body Mass Index and Acute Kidney Injury. Crit Care Med. 2016;44:e767-e768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Danziger J, Chen KP, Lee J, Feng M, Mark RG, Celi LA, Mukamal KJ. Obesity, Acute Kidney Injury, and Mortality in Critical Illness. Crit Care Med. 2016;44:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 26. | Eslamparast T, Montano-Loza AJ, Raman M, Tandon P. Sarcopenic obesity in cirrhosis-The confluence of 2 prognostic titans. Liver Int. 2018;38:1706-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 27. | Leonard J, Heimbach JK, Malinchoc M, Watt K, Charlton M. The impact of obesity on long-term outcomes in liver transplant recipients-results of the NIDDK liver transplant database. Am J Transplant. 2008;8:667-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 28. | Suzuki M, Mujtaba MA, Sharfuddin AA, Yaqub MS, Mishler DP, Faiz S, Vianna RM, Mangus RS, Tector JA, Taber TE. Risk factors for native kidney dysfunction in patients with abdominal multivisceral/small bowel transplantation. Clin Transplant. 2012;26:E351-E358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Bilbao I, Charco R, Balsells J, Lazaro JL, Hidalgo E, Llopart L, Murio E, Margarit C. Risk factors for acute renal failure requiring dialysis after liver transplantation. Clin Transplant. 1998;12:123-129. [PubMed] |