Published online Sep 26, 2020. doi: 10.12998/wjcc.v8.i18.3971
Peer-review started: February 28, 2020
First decision: April 22, 2020
Revised: May 10, 2020
Accepted: June 29, 2020
Article in press: June 29, 2020
Published online: September 26, 2020
Processing time: 206 Days and 16.6 Hours
As a form of artificial intelligence, artificial neural networks (ANNs) have the advantages of adaptability, parallel processing capabilities, and non-linear processing. They have been widely used in the early detection and diagnosis of tumors. In this article, we introduce the development, working principle, and characteristics of ANNs and review the research progress on the application of ANNs in the detection and diagnosis of gastrointestinal and liver tumors.
Core Tip: This paper describes the application of artificial neural networks (ANNs) in the detection and diagnosis of gastrointestinal and liver tumors. We review the artificial intelligence, ANNs and their ability, parallel processing capability, and nonlinear processing. We also discuss the working principle and characteristics of ANNs.
- Citation: Mao WB, Lyu JY, Vaishnani DK, Lyu YM, Gong W, Xue XL, Shentu YP, Ma J. Application of artificial neural networks in detection and diagnosis of gastrointestinal and liver tumors. World J Clin Cases 2020; 8(18): 3971-3977
- URL: https://www.wjgnet.com/2307-8960/full/v8/i18/3971.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i18.3971
With the development of computer and communication technology, artificial intelligence (AI) has been applied in various industries in recent years. We are in the era of big data, and the effective use of these data in combination with existing AI technologies is a hot research topic at present[1]. Machine learning (ML) can help people transform enormous data resources into useful knowledge and information and help in decision-making[2].
ML is now widely applied in the healthcare industry. In the traditional medical diagnosis process, the diagnosis of a disease mainly depends on the judgment by a doctor. The doctor makes a diagnosis based on a patient’s symptoms combined with his or her accumulated experience over the years. This may lead to subjective judgment errors, while the use of ML classification technology to simulate decision-making capabilities can be efficiently used for automatic diagnosis of diseases and assist doctors in making decisions[3]. In many remote and impoverished areas of the world, there are inadequacies in medical care services and the necessary medical resources. Many people miss the best chance to cure the disease due to a delay in medical care, resulting in more deaths. According to statistics, approximately 1.2 billion people in the world are still living in extreme poverty, and more than 2.2 billion people live on less than 1.25 dollars a day[4]. In some regions such as Africa and India, the number of deaths due to diseases is high. Under these circumstances, modern scientific and technological progress can promote the development of the medical industry, and ML technology can be used to develop low-cost, high-accuracy automatic diagnostic equipment, to provide high-quality diagnostic and therapeutic solutions for deprived areas, and to effectively solve common medical problems worldwide.
In the context of big data, prediction techniques based on mathematical statistics and computer analysis have been applied in medical diagnosis[5]. In recent years, research methods for applying multiple ML techniques to diagnose and predict diseases and comparing their performance have been gradually used, such as the use of logistic regression, support vector machine (SVM), and random forest classification algorithms to predict the presence of breast cancer[6]. Ramana et al[7] used SVM, Bayesian, C4.5, and BP neural network algorithms for diagnosis classification and so on.
Whether or not it involves medical text data or imaging data, such as computed tomography (CT) and X-rays, the essential ML method used to diagnose diseases is the supervised classification using the automatic learning ability of ML. ML has been widely applied in the field of intelligent diagnostics. The diagnostic models are not only efficient but have also made considerable achievements with regard to accuracy and precision. With the development of the computer in the late 19th century, algorithm development also emerged, which facilitated the use of ML for modeling and analysis of extensive data sets. However, the application of ML for medical diagnoses in the 1990s was inadequate. To obtain optimum performance, an ML system must have the abilities to appropriately deal with insufficient or noisy data (flaws in data), and the transparency of diagnostic information, to explain decisions, and to reduce the number of tests necessary to obtain a reliable diagnosis. The naive and semi-naive Bayesian classifiers demonstrated the best performance[8].
At present, ML methods used in the intelligent diagnosis of diseases include linear regression models, SVMs, artificial neural networks (ANNs), decision trees, Bayesian classification, ensemble learning, k-nearest neighbor classification algorithms, and deep reinforcement learning. The improved models also achieved better prediction results for some diagnostic problems. The characteristics of different classification models vary, and the performance in different scenarios and different data sets may not be optimum. Algorithms need to proceed according to specific tasks to effectively solve problems. In general, the ML process in disease diagnosis is consistent with the above-mentioned methods and classification linear regression models.
Computer-aided diagnosis (CAD) is used in many medical departments such as radiology, but has not been widely used in gastrointestinal (GI) endoscopy. Due to the necessity for high-resolution imaging studies and an increased number of images per case, the development and implementation of CAD are essential. Ultrasound CAD systems using ML technology have been applied in recent years. However, the implementation of CAD has also encountered several challenges and computational power is one of its most significant limitations. As the GI endoscopist is dependent on real-time input from the system, integration of CAD in the workflow is vital. The CAD system enables the endoscopist to decide whether a particular treatment, such as polypectomy, is essential at the time of endoscopy. A portable and inexpensive high-resolution microendoscopy system should be used to integrate the CAD algorithm for image interpretation of biopsies. The United States Food and Drug Administration has approved many CAD systems for the detection of several different types of lesions, such as screening of cervical smears, detecting polyps on CT colonography, and detecting nodules on chest CT scans. Recent studies and developments have demonstrated that there is growing interest in the implementation of CAD systems in GI endoscopy. Deep learning has been applied in the ultrasound CAD system and the deep neural network has been shown to be more effective than the feature designed by humans. The application of CAD has resulted in significant improvements in diagnosis, i.e., a reduction in time for diagnosis, decreased workload for doctors, and improved diagnostic accuracy. The narrow-band imaging magnifying colonoscopy utilizing the CAD system classified colorectal polyps with a sensitivity of 95% and specificity of 93.3%. With the development of CAD systems, there is potential to integrate these systems into clinical practice to improve and refine diagnostic endoscopy[9,10].
An ANN is a theoretical mathematical model of the human neural network. It is an information processing system based on the structure and function of the human neural network. With the development of neural network technology, the use of ANNs is becoming more and more extensive, and their application fields are also expanding. An ANN is a computer network system which imitates the human neural structure, and many relatively independent artificial neurons are connected to form a network, which mimics the way that biological nerves process information to solve problems. McCulloch and Pitts proposed the earliest ANN in 1943 for logic operations. In 1949, Hebb proposed a set of rules that mimic the way that the human nervous system learns[11].
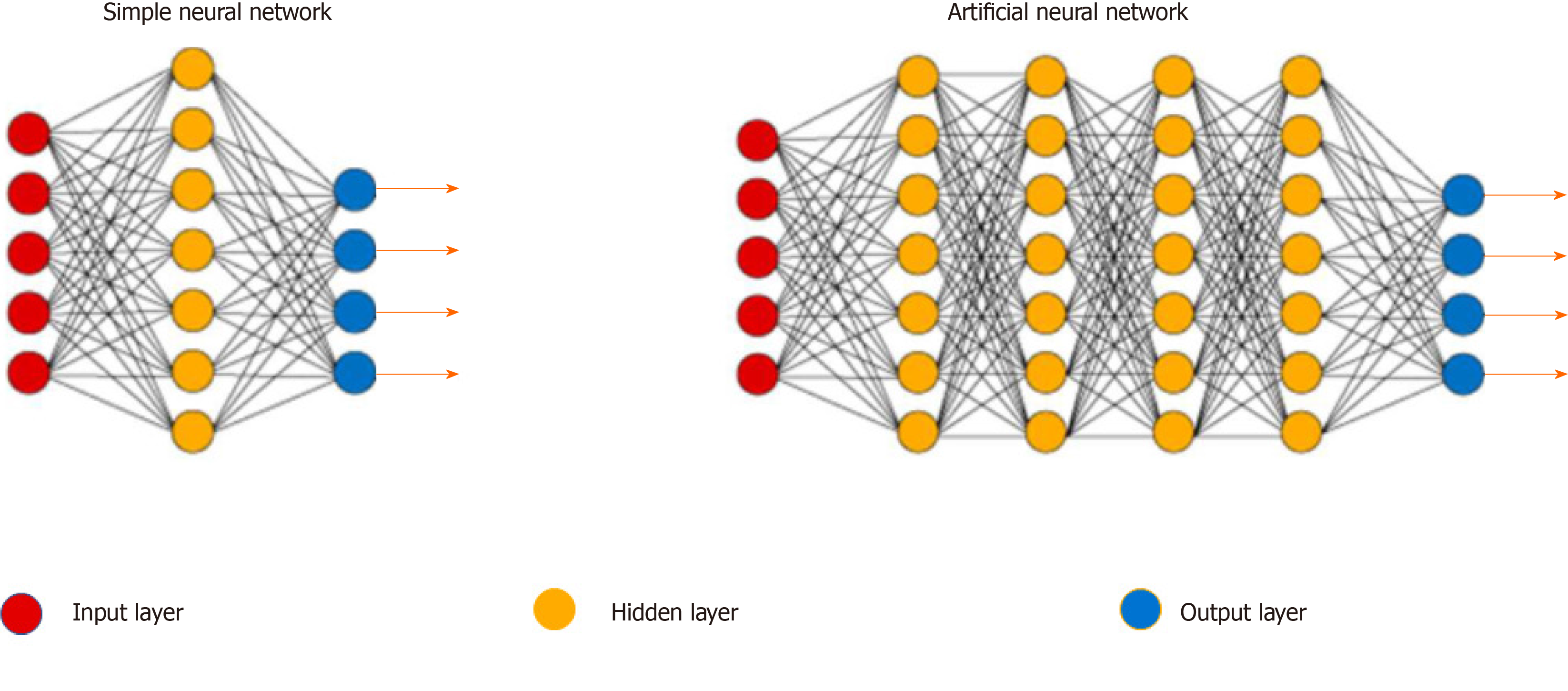
An ANN is a hierarchical network structure made up of multiple neurons connected by a specific rule, which are divided into an input layer, hidden layer, and output layer[12]. The working performance of an ANN is directly related to the training samples. If the training samples are incorrect, too few, or too similar, the working range and ability of the ANN are significantly reduced. In other words, the training sample is the teacher for the ANN. Therefore, the more training samples, the more correct and stronger the ANN's ability (Figures 1 and 2).
An ANN is a network of interconnected neurons obtained by studying the human brain. Research on ANNs has organically integrated many related disciplines and these networks have extensive application potential. An ANN has the following features: (1) Self-organizing and self-learning ability: It can change and adjust its structure by interacting with the environment; (2) Strong promotion ability; (3) Highly parallel: A large number of similar or independent operations can be performed simultaneously; and (4) Strong information synthesis ability: It can process both quantitative and qualitative information, and can coordinate multiple input information fusion and multimedia technologies[13].
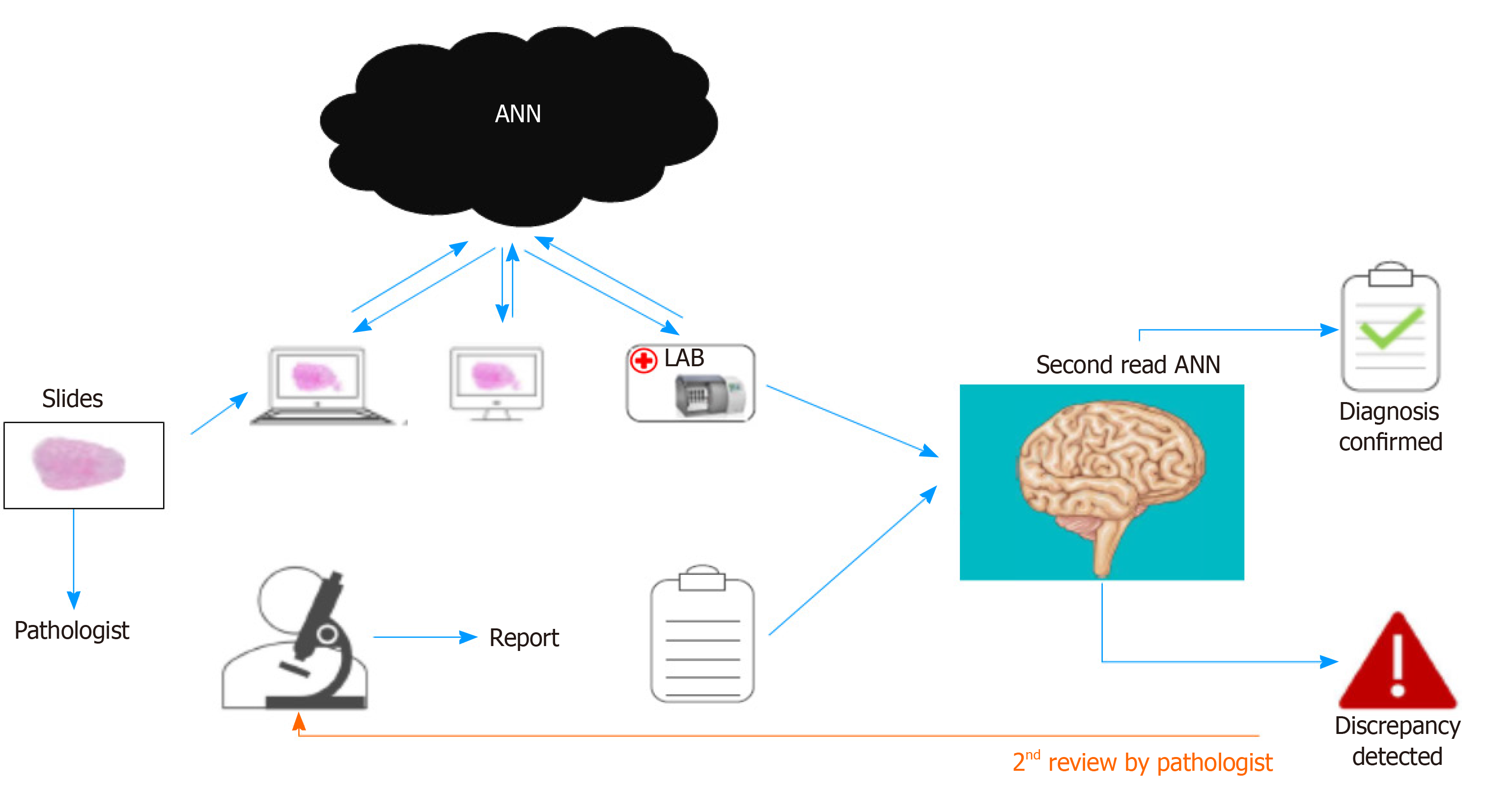
ANNs are a form of AI. Unlike other AI methods, ANNs can learn on their own. Users do not need to design complicated programs to solve problems, but only need to provide data. At present, the etiology of most diseases is unclear, and the manifes-tations of various diseases are ever-changing. In medical practice, judgment of the disease and the corresponding treatment is based on experience. Therefore, the learning, memory, and induction functions of an ANN determine its good application potential in the medical field (Figure 3).
Gastric cancer accounts for 23% of all deaths from malignant tumors, and is the most frequent cause of cancer-related death in China. The prognosis of gastric cancer depends mainly on early detection, early diagnosis, early treatment, and follow-up monitoring after treatment[14,15]. Conventional endoscopy is not suitable for general screening and follow-up and can cause pain. The detection of serum tumor markers is convenient and has important clinical significance in the diagnosis of gastric cancer[16]. In recent years, research on gastric cancer markers has developed rapidly, but these markers lack high specificity and sensitivity; thus, early diagnosis and screening of gastric cancer are not satisfactory[17]. Thara[18] and Kather et al[19] used a deep learning-based ANN to establish a variety of serum tumor marker ANN models for gastric cancer, which improved the diagnostic sensitivity while ensuring a higher specificity, proving that the ANN model has high value in the early diagnosis of gastric cancer.
The mortality rate of liver cancer in China ranks third among all malignant tumors, after gastric cancer and esophageal cancer[20]. Alpha fetoprotein (AFP) is the best indicator for early diagnosis of liver cancer, but some types of liver cancer such as highly differentiated and poorly differentiated liver cancer cells often do not produce AFP; thus, the positive rate of AFP for liver cancer diagnosis is generally 60%-70%. AFP is also positive in patients with liver cirrhosis[21,22], which can result in false positive findings in the diagnosis of primary liver cancer. Therefore, a simple, fast, sensitive, and specific early diagnostic technique is urgently required in clinical medicine. Luk et al[23] used ANN models to analyze the data of protein fingerprints in serum samples from liver cancer patients, liver cirrhosis patients, and healthy subjects, and found that the sensitivity and specificity of this method for liver cancer diagnosis were 96.97% and 87.88%, respectively.
CT, with its excellent low contrast resolution and rich image post-processing functions, has been rapidly popularized and developed in recent years and has become one of the primary methods used for the diagnosis of liver cancer[24,25]. However, the quality of CT images is determined and disturbed by many factors, especially in the early stage of liver cancer, when spatial morphological changes are relatively small, which makes it extremely difficult for the radiologist to diagnose and leads to misdiagnosis and missed diagnosis[26]. Therefore, it is necessary to use appropriate techniques and methods to process and analyze CT images of liver cancer to improve the identification and diagnosis of these lesions. Based on CT image preprocessing of liver cancer and other liver occupying diseases, Chlebus et al[27] extracted CT imaging features and used these as training parameters to establish an ANN-assisted diagnosis model for CT images of liver cancer, and simulated the network. The computed neural network was used for CT diagnosis of liver cancer, and the sensitivity and specificity of liver cancer diagnosis were significantly improved.
Colorectal cancer is a common malignant tumor with the third highest mortality rate worldwide and the second highest in the West[28,29]. At present, conventional methods for the diagnosis of colorectal cancer are still colonic barium double radiography and electronic colonoscopy, but they are both invasive diagnostic methods, and there are problems with cost and risk. Blood tumor marker detection is more straight-forward[30,31]. The diagnostic rate of a single serum marker is too low; for example, the sensitivity of carcinoembryonic antigen (CEA) in diagnosing colorectal cancer is only 55.2%[32]. Colorectal cancer is a complex disease, and its occurrence and development are related to changes in multiple genes; thus, it is necessary to combine multiple markers to detect and diagnose colorectal cancer. Scientists have now screened the optimal marker combination for detecting colorectal cancer using bioinformatics methods: CEA, CA199, CA242, CA211, and CA724, confirming that the ANN model is more sensitive in predicting colorectal cancer than the single serum marker CEA. At the same time, they used a variety of bioinformatics methods to analyze the tumor marker data for colorectal cancer and used the decision tree and SVM to verify the reliability of the screening results and the established model. During evaluation of the model, they applied a cross-validation method and estimated the predictive effect of the model on the test set. In this way, the blind test and the randomness of the results were avoided; thus, evaluation using the model was more reliable[13,33,34].
ANNs have many advantages that are comparable to the human brain. In addition, they can perform statistical analyses of linear or nonlinear multiple variables without setting prerequisites, and certain variables that are required to be analyzed by traditional statistical methods. A good ANN can make correct predictions even when the data are incomplete or biased. Although ANNs have many advantages, they are still not well known and used as traditional statistical methods. Before ANNs are widely accepted, more work is required, problems with some network entities need to be solved, and it is difficult for domain experts to describe the problems that they want to solve with data-based nodes, weights, and connections. Besides, the network is "black box reasoning" and all knowledge is stored inside the network, making it challenging to provide credible explanations. Finally, during the training phase, there are problems such as long training time, overfitting or insufficient training, network paralysis, and local minimums[13]. However, it should be acknowledged that with further ANN research, ANNs will be recognized by clinicians and become an effective tool for the diagnosis and prediction of diseases (especially tumors).
At present, data acquisition is still a major problem, especially when it is used for the medical diagnostics industry, as the data involve personal privacy, which makes it challenging to obtain. The availability of public data is limited, which restricts the research on deep learning. Therefore, obtaining larger-scale and more effective data, and establishing a suitable model under limited data conditions are issues that need to be studied in practical work.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hori K S-Editor: Liu M L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Yu KH, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng. 2018;2:719-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 1086] [Article Influence: 155.1] [Reference Citation Analysis (0)] |
| 2. | Guarin DL, Dusseldorp J, Hadlock TA, Jowett N. A Machine Learning Approach for Automated Facial Measurements in Facial Palsy. JAMA Facial Plast Surg. 2018;20:335-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 3. | Khattri S, Shemer A, Rozenblit M, Dhingra N, Czarnowicki T, Finney R, Gilleaudeau P, Sullivan-Whalen M, Zheng X, Xu H, Cardinale I, de Guzman Strong C, Gonzalez J, Suárez-Fariñas M, Krueger JG, Guttman-Yassky E. Cyclosporine in patients with atopic dermatitis modulates activated inflammatory pathways and reverses epidermal pathology. J Allergy Clin Immunol. 2014;133:1626-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 4. | Cecere D. New study finds 45, 000 deaths annually linked to lack of health coverage. Harvard Gazette. 2009;17. |
| 5. | LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36149] [Cited by in RCA: 20034] [Article Influence: 2003.4] [Reference Citation Analysis (0)] |
| 6. | Patrício M, Pereira J, Crisóstomo J, Matafome P, Gomes M, Seiça R, Caramelo F. Using Resistin, glucose, age and BMI to predict the presence of breast cancer. BMC Cancer. 2018;18:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 7. | Ramana BV, Babu MSP, Venkateswarlu N. A critical study of selected classification algorithms for liver disease diagnosis. Int J Database Manage Syst. 2011;3:101-114. [RCA] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Kononenko I. Machine learning for medical diagnosis: history, state of the art and perspective. Artif Intell Med. 2001;23:89-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 860] [Cited by in RCA: 510] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 9. | Huang Q, Zhang F, Li X. Machine Learning in Ultrasound Computer-Aided Diagnostic Systems: A Survey. Biomed Res Int. 2018;2018:5137904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Leggett CL, Wang KK. Computer-aided diagnosis in GI endoscopy: looking into the future. Gastrointest Endosc. 2016;84:842-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Hassoun MH. Fundamentals of artificial neural networks: MIT press. P IEEE. 1996;84:906. [DOI] [Full Text] |
| 12. | Ramesh AN, Kambhampati C, Monson JR, Drew PJ. Artificial intelligence in medicine. Ann R Coll Surg Engl. 2004;86:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 390] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 13. | Ahmed FE. Artificial neural networks for diagnosis and survival prediction in colon cancer. Mol Cancer. 2005;4:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 14. | Wu CH, Lin SR, Hsieh JS, Chen FM, Lu CY, Yu FJ, Cheng TL, Huang TJ, Huang SY, Wang JY. Molecular detection of disseminated tumor cells in the peripheral blood of patients with gastric cancer: evaluation of their prognostic significance. Dis Markers. 2006;22:103-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Ebert MP, Röcken C. Molecular screening of gastric cancer by proteome analysis. Eur J Gastroenterol Hepatol. 2006;18:847-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Shah MA, Khanin R, Tang L, Janjigian YY, Klimstra DS, Gerdes H, Kelsen DP. Molecular classification of gastric cancer: a new paradigm. Clin Cancer Res. 2011;17:2693-2701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 17. | Kanda M, Kodera Y. Recent advances in the molecular diagnostics of gastric cancer. World J Gastroenterol. 2015;21:9838-9852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Thara L. Gastric Cancer Prediction: A Comparative Analysis of Methodologies and Performances in Deep Learning Perspective. J Gujarat Res Soc. 2019;21:147-153. |
| 19. | Kather JN, Pearson AT, Halama N, Jäger D, Krause J, Loosen SH, Marx A, Boor P, Tacke F, Neumann UP, Grabsch HI, Yoshikawa T, Brenner H, Chang-Claude J, Hoffmeister M, Trautwein C, Luedde T. Deep learning can predict microsatellite instability directly from histology in gastrointestinal cancer. Nat Med. 2019;25:1054-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 992] [Cited by in RCA: 755] [Article Influence: 125.8] [Reference Citation Analysis (0)] |
| 20. | Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1799] [Cited by in RCA: 1816] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 21. | Notarpaolo A, Layese R, Magistri P, Gambato M, Colledan M, Magini G, Miglioresi L, Vitale A, Vennarecci G, Ambrosio CD, Burra P, Di Benedetto F, Fagiuoli S, Colasanti M, Maria Ettorre G, Andreoli A, Cillo U, Laurent A, Katsahian S, Audureau E, Roudot-Thoraval F, Duvoux C. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. J Hepatol. 2017;66:552-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 22. | Lemoine A, Le Bricon T, Salvucci M, Azoulay D, Pham P, Raccuia J, Bismuth H, Debuire B. Prospective evaluation of circulating hepatocytes by alpha-fetoprotein mRNA in humans during liver surgery. Ann Surg. 1997;226:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Luk JM, Lam BY, Lee NP, Ho DW, Sham PC, Chen L, Peng J, Leng X, Day PJ, Fan ST. Artificial neural networks and decision tree model analysis of liver cancer proteomes. Biochem Biophys Res Commun. 2007;361:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Hamami ME, Poeppel TD, Müller S, Heusner T, Bockisch A, Hilgard P, Antoch G. SPECT/CT with 99mTc-MAA in radioembolization with 90Y microspheres in patients with hepatocellular cancer. J Nucl Med. 2009;50:688-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Outwater EK. Imaging of the liver for hepatocellular cancer. Cancer Control. 2010;17:72-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Takayasu K, Muramatsu Y, Mizuguchi Y, Ojima H. CT Imaging of early hepatocellular carcinoma and the natural outcome of hypoattenuating nodular lesions in chronic liver disease. Oncology. 2007;72 Suppl 1:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 27. | Chlebus G, Schenk A, Moltz JH, van Ginneken B, Hahn HK, Meine H. Automatic liver tumor segmentation in CT with fully convolutional neural networks and object-based postprocessing. Sci Rep. 2018;8:15497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (1)] |
| 28. | Brenner H, Chen C. The colorectal cancer epidemic: challenges and opportunities for primary, secondary and tertiary prevention. Br J Cancer. 2018;119:785-792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 193] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 29. | Colditz GA. Cancer culture: epidemics, human behavior, and the dubious search for new risk factors. Am J Public Health. 2001;91:357-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Winawer SJ. Screening of colorectal cancer: progress and problems. Recent Results Cancer Res. 2005;166:231-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Mladen DM, Dragoslav MP, Sanja Z, Bozidar B, Snezana D. Problems in screening colorectal cancer in the elderly. World J Gastroenterol. 2003;9:2335-2337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Griesenberg D, Nürnberg R, Bahlo M, Klapdor R. CEA, TPS, CA 19-9 and CA 72-4 and the fecal occult blood test in the preoperative diagnosis and follow-up after resective surgery of colorectal cancer. Anticancer Res. 1999;19:2443-2450. [PubMed] |
| 33. | Ward DG, Suggett N, Cheng Y, Wei W, Johnson H, Billingham LJ, Ismail T, Wakelam MJ, Johnson PJ, Martin A. Identification of serum biomarkers for colon cancer by proteomic analysis. Br J Cancer. 2006;94:1898-1905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 163] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 34. | Zhong W, Yu Z, Zhan J, Yu T, Lin Y, Xia ZS, Yuan YH, Chen QK. Association of serum levels of CEA, CA199, CA125, CYFRA21-1 and CA72-4 and disease characteristics in colorectal cancer. Pathol Oncol Res. 2015;21:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |