Published online Sep 6, 2020. doi: 10.12998/wjcc.v8.i17.3828
Peer-review started: June 29, 2020
First decision: July 24, 2020
Revised: August 5, 2020
Accepted: August 20, 2020
Article in press: August 20, 2020
Published online: September 6, 2020
Processing time: 66 Days and 22.2 Hours
Immunoglobulin A nephropathy (IgAN) is the most commonly encountered glomerular disease in Asian countries. It has a broad clinical presentation, and it is frequently associated with other conditions. Chronic liver disease is well recognized as the leading cause of secondary IgAN. However, cases of IgAN associated with autoimmune hepatitis (AIH) have seldom been reported.
A 63-year-old Korean woman was admitted to Pusan National University Hospital for an evaluation of abdominal pain and elevated liver enzymes. Two weeks prior, she had presented to our hospital with proteinuria of approximately 1350 mg/d and hematuria and was diagnosed with IgAN. Autoimmune profiles were highly positive for antinuclear antibodies, and symptoms related to portal hypertension including ascites and peripheral edema were present. A diagnosis of AIH was made according to the simplified scoring system of the International Autoimmune Hepatitis Group. Despite immunosuppression with prednisolone and azathioprine, rapid deterioration of liver function led to end-stage liver disease. After a living-donor liver transplantation, liver function gradually improved, and she had maintained stable liver and kidney function at the six months follow-up.
Cases of secondary IgAN with chronic liver disease have been frequently reported in the literature but are rarely associated with AIH. We encountered an IgAN patient with concurrent progressive liver failure due to AIH.
Core tip: Immunoglobulin A nephropathy (IgAN) is an autoimmune disease and may be related to other autoimmune conditions. To the best of our knowledge, only two cases of IgAN with autoimmune hepatitis have been reported, and the pathophysiological associations of both diseases have not been established.
- Citation: Jeon YH, Kim DW, Lee SJ, Park YJ, Kim HJ, Han M, Kim IY, Lee DW, Song SH, Lee SB, Seong EY. Autoimmune hepatitis in a patient with immunoglobulin A nephropathy: A case report. World J Clin Cases 2020; 8(17): 3828-3834
- URL: https://www.wjgnet.com/2307-8960/full/v8/i17/3828.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i17.3828
Immunoglobulin A nephropathy (IgAN) is the most prevalent glomerular disease worldwide and is an important cause of end-stage kidney disease. IgAN is an autoimmune disorder characterized by diffuse mesangial deposition of immunoglobulin A (IgA)[1]. The accumulation of mesangial IgA associated with chronic liver disease, such as alcoholic liver cirrhosis and chronic viral hepatitis, is the most common form of secondary IgAN[2]. Secondary IgAN is usually clinically silent, and the most common symptom is microscopic hematuria. IgAN has also been reported to be associated with other autoimmune conditions, including ankylosing spondylitis, psoriasis, inflammatory bowel disease and Hashimoto’s thyroiditis[2,3]. However, few cases have been reported in the literature of IgAN associated with autoimmune hepatitis (AIH).
A 63-year-old woman presented with right upper quadrant abdominal discomfort.
The patient’s symptoms developed insidiously over 3 d. She did not show any symptoms related to portal hypertension.
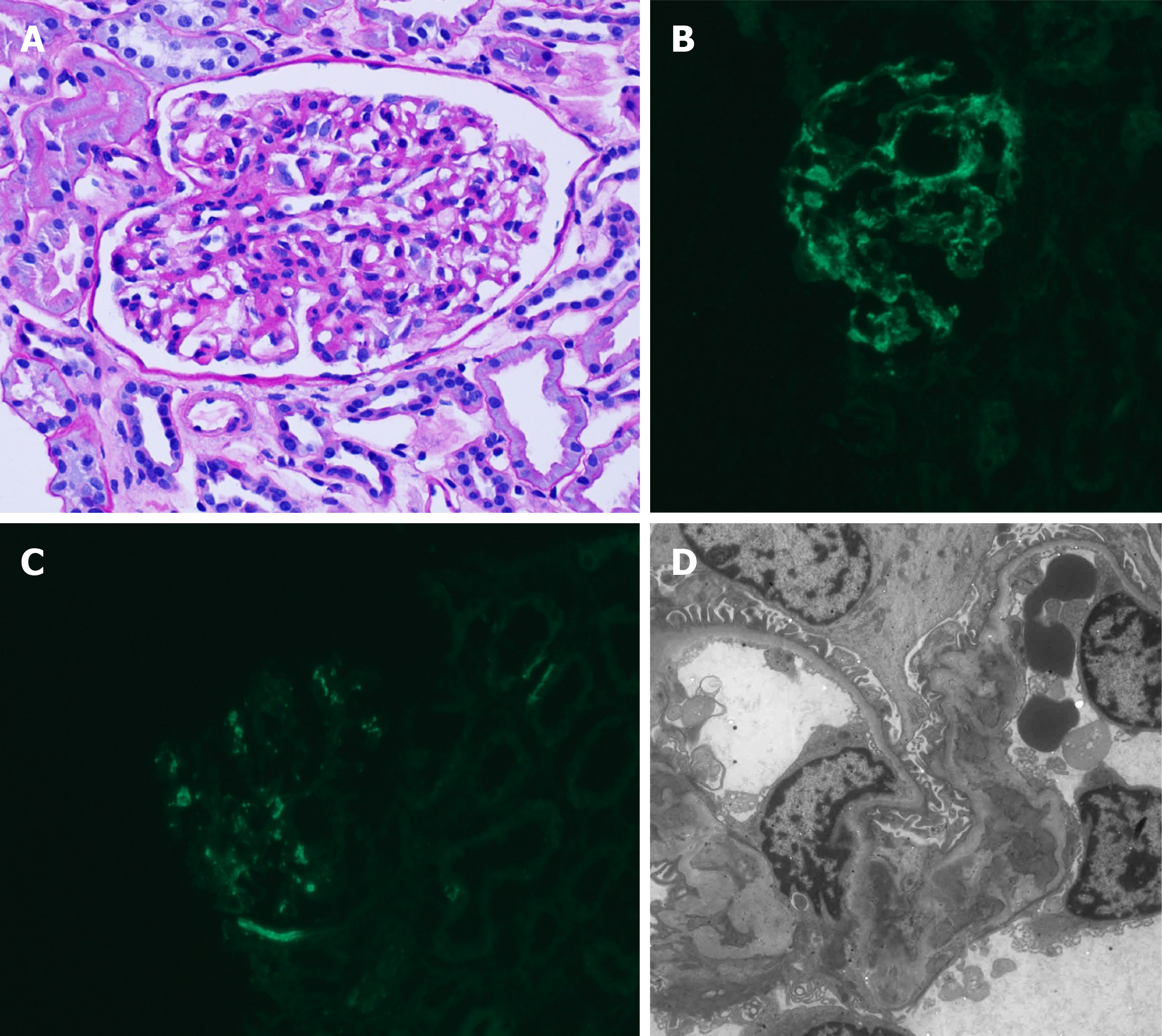
Two weeks ago, she presented to our hospital with proteinuria of approximately 1350 mg/d and hematuria and was diagnosed with IgAN. Light microscopic findings of kidney biopsy showed a moderate increase in mesangial matrix and mesangial cellularity (Figure 1A) with focal severe tubular atrophy and interstitial infiltration of mononuclear cells. Out of 37 glomeruli, 2 glomeruli (5%) showed global sclerosis. Immunofluorescence microscopy showed predominant mesangial IgA staining (Figure 1B) and C3 staining (Figure 1C). Electron microscopy revealed mesangial electron-dense deposits and focal effacement of the epithelial cell foot processes (Figure 1D). Laboratory findings were as follows: aspartate aminotransferase (AST), 20 IU/L; alanine aminotransferase (ALT), 8 IU/L; and serum creatinine, 1.21 mg/dL. She was not taking any other medications except an angiotensin II receptor blocker.
On admission, she had a height of 155.5 cm, a weight of 53.7 kg, a blood pressure of 120/70 mmHg, a regular heart rate of 64 bpm, and a temperature of 36.1 °C. The patient had mild right upper quadrant pain and tenderness and icteric sclera. She denied taking any new medication or alcohol consumption.
Laboratory findings were as follows: white blood cell count, 4090/µL; hemoglobin, 10.6 g/dL; platelet count, 99 × 10³/µL; AST, 771 IU/L; ALT, 488 IU/L; alkaline phosphatase, 92 IU/L; total bilirubin, 3.04 mg/dL; direct bilirubin, 1.86 mg/dL; total protein, 6.89 g/dL; albumin, 3.13 g/dL; and prothrombin international normalized ratio, 1.57. Serum creatinine and estimated glomerular filtration rate were 1.26 mg/dL and 45.6 mL/min per 1.73 m², respectively, similar to the values 2 wk prior. She was negative for anti-hepatitis B surface antigen, anti-hepatitis B core antibody, anti-hepatitis C virus antibody and anti-hepatitis A virus IgM. The results of immunological studies and serum iron and copper studies are shown in Tables 1 and 2.
| Reference values | ||
| IgG (mg/dL) | 1822.0 | 700-1600 |
| IgA (mg/dL) | 550.4 | 70-400 |
| IgM (mg/dL) | 144.3 | 40-230 |
| IgE (mg/dL) | 40.4 | 0-100 |
| C3 (mg/dL) | 123.7 | 90-180 |
| C4 (mg/dL) | 26.8 | 10-40 |
| FANA | 1:320 | Below 1:40 |
| Anti-smooth muscle Ab | Negative | Negative |
| Anti-mitochondrial Ab | Negative | Negative |
| Anti-LKM-1 Ab | Negative | Negative |
| Anti-ds DNA IgG | Negative | Negative |
| Anti-Sm Ab | Negative | Negative |
| Anti-cardiolipin Ab IgG | Negative | Negative |
| Anti-cardiolipin Ab IgM | Negative | Negative |
| Anti-Ro | Negative | Negative |
| Anti-La | Negative | Negative |
| Anti-centromere Ab | Negative | Negative |
| Anti-Scl-70 | Negative | Negative |
| Anti-RNP Ab | Negative | Negative |
| Reference values | ||
| Iron (µg/dL) | 88 | 33-193 |
| TIBC (µg/dL) | 260 | 264-448 |
| Transferrin saturation (%) | 33.85 | |
| Ferritin (ng/mL) | 128.0 | 6-282 |
| Copper (µg/dL) | 75 | 75-145 |
| Ceruloplasmin (mg/dL) | 25 | 16-45 |
| 24 h urinary copper excretion (µg/24 h) | 39.6 | 15-60 |
A contrast-enhanced computed tomography (CT) scan of the abdomen revealed a distal common bile duct (CBD) stone with mild upstream bile duct dilatation and an intrahepatic portosystemic shunt in the left lobe of the liver. There was not any evidence of chronic liver injury or portal hypertension such as esophageal varix, splenomegaly and ascites. Endoscopic retrograde cholangiopancreatography showed CBD stone without bile duct narrowing.
After endoscopic removal of CBD stones, liver enzyme levels started to decrease. On the fourth day, AST, ALT and total bilirubin gradually increased to 896 IU/L, 579 IU/L, and 5.05 mg/dL, respectively. Symptoms related to portal hypertension, including ascites and peripheral edema, occurred. Based on the abovementioned findings, the diagnosis of AIH was made according to the simplified scoring system of the International Autoimmune Hepatitis Group[4]. The score prior to steroid therapy was 6 points: Antinuclear antibodies (2), IgG (2) and absence of viral hepatitis (2).
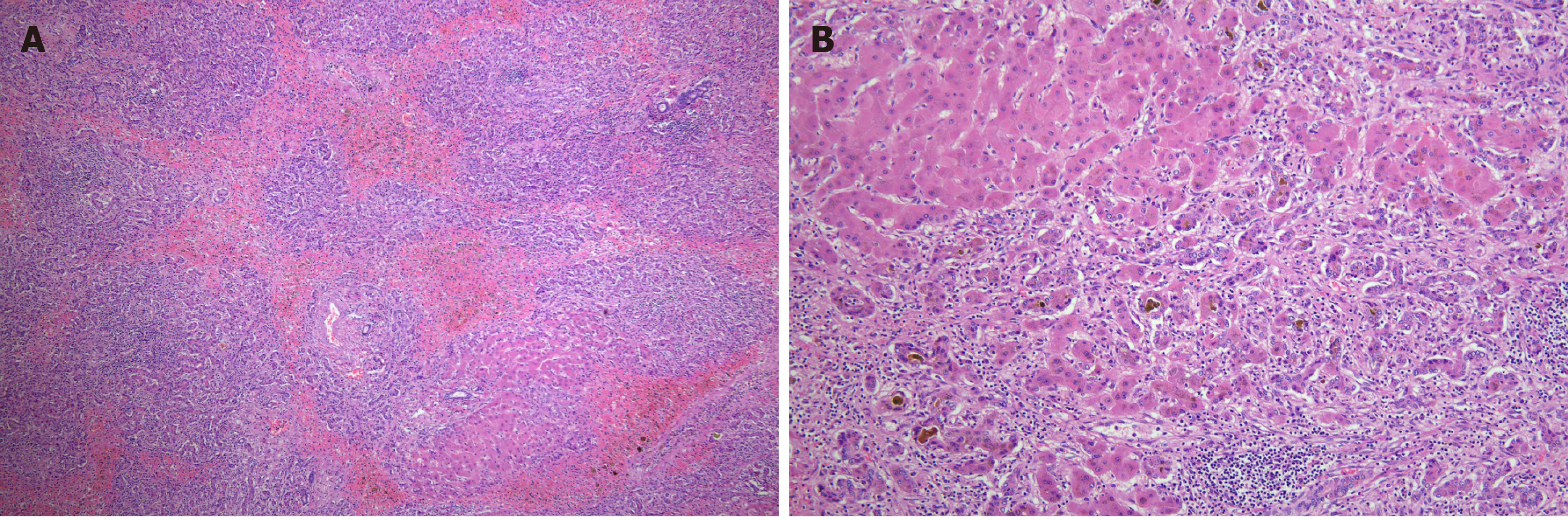
She was treated with prednisolone 0.5 mg/kg in combination with azathioprine for 2 wk. Despite the treatment, liver function rapidly deteriorated, and hepatorenal syndrome requiring renal replacement therapy developed subsequently. The patient was not exposed to the nephrotoxic agents and had no episode of bleeding or septic shock. The model for end-stage liver disease score was 37 points at the time of waiting list registration for liver transplantation. Approximately two months after hospitalization, she underwent living-donor liver transplantation from her son. Hepatectomy specimens showed submassive necrosis. Prominent necrosis involved entire lobules in most of the liver parenchyma (Figure 2A), and the remaining parenchyma showed canalicular type cholestasis (Figure 2B).
After having a well-functioning allograft, the general condition and laboratory findings improved. Immunosuppressive drugs, including corticosteroids, tacrolimus and mycophenolate, were used. Three weeks after surgery, hemodialysis was stopped because of improving renal function. Two months later, the serum creatinine level was 1.5 mg/dL, and the spot urine protein/creatinine ratio was 193.3 mg/g.
IgAN associated with chronic liver disease is the most prevalent pattern of secondary IgAN. Mesangial IgA deposition is frequently found in autopsy specimens in the general population without medical illness[5]. IgAN has been found in 25% of kidney biopsy specimens from 60 patients with end-stage liver disease[6]. Serum IgA levels are frequently elevated in patients with alcoholic liver cirrhosis. In an ex-vivo study, peripheral blood mononuclear cells of alcoholic liver cirrhosis patients secreted more IgA than those of healthy controls[7]. The accumulation of IgA is thought to result from decreased clearance of IgA, which can be explained in two ways. As cirrhosis progresses, the number of hepatocytes and Kupffer cells, which express the asialoglycoprotein receptor and Fc receptors that bind galactose residues of IgA and remove it from circulation, also decreases[8,9]. Portal hypertension may play a role in hyperimmunoglobulinemia because of the portal venous flow directly from the portal vein to the systemic circulation. Our patient had no clinical evidence of portal hypertension before fulminant hepatic failure, though she did have a portosystemic shunt on CT scan at the time of diagnosis of IgAN. A portosystemic shunt is a communication between the portal vein and the systemic vein, and it is caused by a congenital malformation, liver cirrhosis or trauma. Portosystemic shunts can be found incidentally in the absence of other signs of portal hypertension[10,11].
Secondary IgAN associated with autoimmune disorders, including Sjogren’s disease, ankylosing spondylitis and coeliac disease, has been reported in the literature[2,12,13]. However, IgAN with AIH has been reported in only a limited number of patients, and most of them had other autoimmune diseases such as Sjogren’s disease which is often reported to be associated with IgAN[14,15]. In our case, two different diseases that do not share a common pathogenesis were diagnosed sequentially. AIH is a chronic inflammatory liver disease and that may progress to liver cirrhosis or fulminant hepatitis[4]. The pathogenesis of AIH is not fully understood. The destruction of self-tolerance to hepatocyte antigen may play a key role in the pathogenesis of AIH. Autoimmune liver injury is characterized by autoreactive CD4 and CD8 T cells via cellular immune mechanisms. Currently, regulatory T cells that suppress excessive immune reactions are considered important mediator cells in the immunopathology of AIH[16].
Since there are a limited number of cases, the renal outcome of patients with secondary IgAN receiving liver transplantation is not clear. A retrograde observational study suggested that kidney function tends to be relatively favorable after liver transplantation. Among 7 patients who underwent liver transplantation, only one progressed to end-stage kidney disease during the 5 years follow-up period[17]. In this report, liver transplantation did not affect the disease progression of IgAN.
There is neither a clear definition of secondary IgAN, which is recognized when IgAN coexists with other conditions, nor a histopathological feature to distinguish primary IgAN from secondary IgAN[18]. Moreover, reported cases of IgAN concurrent with AIH are sparse, and the pathophysiological relationship between the two diseases has not been well established. In our patient, the clinical activity of IgAN did not correlate with the acute onset of AIH, which indicates that the possibility of the coexistence of primary IgAN with AIH cannot be excluded. Therefore, the analysis of additional cases is required in the future. Additional case reports are necessary to promote studies on the relevance of IgAN and AIH.
We report a rare case of IgAN associated with AIH presenting as fulminant hepatic failure treated with liver transplantation. Both diseases were diagnosed simultaneously, even though they were thought to have different pathogeneses. Whether the secondary IgAN was related to AIH or the two diseases coincidentally occurred remains uncertain. Further case reports and analyses of IgAN concurrent with other diseases, which is known to be rare, are needed to determine potential pathophysiological relationships.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ferreira GSA, Tanabe K S-Editor: Yan JP L-Editor: A P-Editor: Xing YX
| 1. | Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368:2402-2414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 914] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 2. | Saha MK, Julian BA, Novak J, Rizk DV. Secondary IgA nephropathy. Kidney Int. 2018;94:674-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 3. | Ambruzs JM, Walker PD, Larsen CP. The histopathologic spectrum of kidney biopsies in patients with inflammatory bowel disease. Clin J Am Soc Nephrol. 2014;9:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 4. | Manns MP, Lohse AW, Vergani D. Autoimmune hepatitis--Update 2015. J Hepatol. 2015;62:S100-S111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 252] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 5. | Sinniah R. Occurrence of mesangial IgA and IgM deposits in a control necropsy population. J Clin Pathol. 1983;36:276-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Calmus Y, Conti F, Cluzel P, Hill G, Antoine C, Scatton O, Soubrane O, Glotz D, Pillebout E, Nochy D. Prospective assessment of renal histopathological lesions in patients with end-stage liver disease: effects on long-term renal function after liver transplantation. J Hepatol. 2012;57:572-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Massonnet B, Delwail A, Ayrault JM, Chagneau-Derrode C, Lecron JC, Silvain C. Increased immunoglobulin A in alcoholic liver cirrhosis: exploring the response of B cells to Toll-like receptor 9 activation. Clin Exp Immunol. 2009;158:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Tomana M, Kulhavy R, Mestecky J. Receptor-mediated binding and uptake of immunoglobulin A by human liver. Gastroenterology. 1988;94:762-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Rifai A, Mannik M. Clearance of circulating IgA immune complexes is mediated by a specific receptor on Kupffer cells in mice. J Exp Med. 1984;160:125-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Kim M, Lee KY. Understanding the Pathophysiology of Portosystemic Shunt by Simulation Using an Electric Circuit. Biomed Res Int. 2016;2016:2097363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Remer EM, Motta-Ramirez GA, Henderson JM. Imaging findings in incidental intrahepatic portal venous shunts. AJR Am J Roentgenol. 2007;188:W162-W167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Chen Y, Zhao X, Tang D, Xu C, Sun L, Sun L, Wu J, Mei C. IgA nephropathy in two patients with Sjögren's syndrome: one with concomitant autoimmune hepatitis. Intern Med. 2010;49:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Habura I, Fiedorowicz K, Woźniak A, Idasiak-Piechocka I, Kosikowski P, Oko A. IgA nephropathy associated with coeliac disease. Cent Eur J Immunol. 2019;44:106-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Grønbaek L, Vilstrup H, Pedersen L, Jepsen P. Extrahepatic autoimmune diseases in patients with autoimmune hepatitis and their relatives: A Danish nationwide cohort study. Liver Int. 2019;39:205-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Singri N, Gleason B, Flamm SL, Kanwar YS, Ghossein C. Secondary IgA nephropathy presenting as nephrotic syndrome with glomerular crescentic changes and acute renal failure in a patient with autoimmune hepatitis. J Nephrol. 2004;17:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Wang M, Zhang H. The pathogenesis of autoimmune hepatitis. Front Lab Med. 2018;2:36-39. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Hommos MS, El-Zoghby ZM. Renal Outcomes in Patients With IgA Nephropathy Undergoing Liver Transplant: A Retrospective Cohort Study. Transplant Direct. 2017;3:e193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Obrișcă B, Ștefan G, Gherghiceanu M, Mandache E, Ismail G, Stancu S, Boitan B, Ion O, Mircescu G. "Associated" or "Secondary" IgA nephropathy? An outcome analysis. PLoS One. 2019;14:e0221014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |