Published online Sep 6, 2020. doi: 10.12998/wjcc.v8.i17.3743
Peer-review started: April 14, 2020
First decision: May 15, 2020
Revised: May 25, 2020
Accepted: August 12, 2020
Article in press: August 12, 2020
Published online: September 6, 2020
Processing time: 142 Days and 22.4 Hours
Pancreatic cancer is one of the common malignant tumors of the digestive system, and radical resection is the first choice of treatment for pancreatic cancer. If patients with locally advanced pancreatic cancer cannot be treated in time and effectively, their disease often develops rapidly and their survival period is very short.
To evaluate the therapeutic effect of 125I seed implantation in patients with locally advanced pancreatic cancer.
The demographics and perioperative outcomes of a consecutive series of patients who underwent 125I seed implantation to treat locally advanced pancreatic cancer between January 1, 2017 and June 30, 2019 were retrospectively analyzed. According to the results of preoperative computed tomography or magnetic resonance imaging, the treatment planning system was used to determine the area and number of 125I seeds implanted. During the operation, 125I seeds were implanted into the tumor under the guidance of intraoperative ultrasound, with a spacing of 1.5 cm and a row spacing of 1.5 cm. For patients with obstructive jaundice and digestive tract obstruction, choledochojejunostomy and gastroenterostomy were performed simultaneously. After operation, the patients were divided into a non-chemotherapy group and a chemotherapy group that received gemcitabine combined with albumin-bound paclitaxel treatment.
Among the 50 patients, there were 29 males and 21 females, with a mean age of 56.9 ± 9.8 years. The main reason for the failure of radical resection was superior mesenteric artery invasion (37, 74%), followed by superior mesenteric vein invasion (33, 66%). Twenty-one (62%) patients underwent palliative surgery and postoperative pain relief occurred in 40 (80%) patients. The estimated blood loss in operation was 107.4 ± 115.3 mL and none of the patient received blood transfusion. The postoperative hospital stay was 7.5 ± 4.2 d; one patient had biliary fistula and three had pancreatic fistula, all of whom recovered after conservative treatment. After operation, 26 patients received chemotherapy and 24 did not. The 1-year survival rate was significantly higher in patients who received chemotherapy than in those who did not (60.7% vs 35.9%, P = 0.034). The mean overall survival of patients of the chemotherapy group and non-chemotherapy group was 14 and 11 mo, respectively (χ2 = 3.970, P = 0.046).
Radioactive 125I seed implantation combined with postoperative chemotherapy can prolong the survival time, relieve pain, and improve the quality of life of patients with locally advanced pancreatic cancer.
Core tip: The survival period of patients with locally advanced pancreatic cancer is very short. Radioactive 125I seed implantation can prolong the survival time, relieve pain, and improve the quality of life of patients with locally advanced pancreatic cancer.
- Citation: Li CG, Zhou ZP, Jia YZ, Tan XL, Song YY. Radioactive 125I seed implantation for locally advanced pancreatic cancer: A retrospective analysis of 50 cases. World J Clin Cases 2020; 8(17): 3743-3750
- URL: https://www.wjgnet.com/2307-8960/full/v8/i17/3743.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i17.3743
Pancreatic cancer is one of the common malignant tumors of the digestive system, which has the characteristics of late detection, early metastasis, rapid progression, and poor prognosis. In recent decades, the incidence of pancreatic cancer has been increasing year by year in the world. Although the surgical resection rate has been improved, the overall prognosis and survival of patients have not improved significantly[1-3]. Radical resection is the first choice of treatment for pancreatic cancer, which is considered to be the best choice to prolong the survival of patients. However, due to the atypical early symptoms of pancreatic cancer, most of the patients were diagnosed at an advanced stage. About 10%-30% of all pancreatic cancer patients could receive radical resection, and the 5-year survival rate was less than 10%[4-6].
If patients with pancreatic cancer cannot undergo radical resection and be treated in time and effectively, their condition often deteriorates rapidly and their survival time is very short. The mean survival time of those patients is only 6-10 mo[7]. Radioactive seed implantation is an alternative treatment for locally advanced pancreatic cancer. In 1965, radioactive 125I seed came out. 125I seeds were first used in the treatment of prostate cancer patients and achieved success[8]. Up to now, 125I seeds have been used as the first choice for the treatment of early prostate cancer in developed countries such as Europe and the United States. Its curative effect is equivalent to that of radical resection[9,10].
In recent years, with the rapid development of computer technology, imaging, and radiation physics, radioactive seed implantation has been widely used. At the same time, the physical and biological characteristics of 125I seeds have been studied intensely. In particular, the clinical efficacy and complications of 125I seed implantation in the treatment of tumors have been considered and discussed, and a large amount of valuable experience has been accumulated. These results promoted the rapid development of 125I seed implantation technology, and provided a new choice for the treatment of patients with locally advanced pancreatic cancer[11-13]. The purpose of this study was to summarize a single center experience with 125I seed implantation in the treatment of locally advanced pancreatic cancer.
The clinical data of 50 patients with locally advanced pancreatic cancer who underwent 125I seed implantation between January 1, 2017 and June 30, 2019 were retrospectively analyzed. This study was approved by the Institutional Review Board of Chinese PLA General Hospital.
The inclusion criteria were: (1) Advanced pancreatic cancer confirmed by pathology; (2) No distant metastasis detected by preoperative imaging; and (3) Anastomosis was performed and 125I seed were implanted in the tumor to relieve jaundice and obstruction of the digestive tract. The exclusion criteria were: (1) Karnofsky Performance Scale score < 70; (2) Systemic failure symptoms; and (3) Other medical conditions that contraindicated anesthesia and surgery.
The physical half-life of the radioactive 125I seeds (China Isotope and Radiation Corporation, Beijing, China) used in this study is 59.6 d, the diameter is 0.8 mm, the length is 4.5 mm, and the wall thickness is 0.05 mm. The half value layer of the seeds for lead is 0.025 mm and it is 20.0 mm for soft tissue in human body. Activity range of a single seed is 11.1-37 MBq and it can radiate 27.4 and 31.4 keV X-ray and 35.5 keV γ-ray.
Magnetic resonance imaging (MRI) or contrast-enhanced computed tomography (CT) was performed as a routine diagnostic procedure. According to the results of preoperative CT or MRI, the treatment planning system (TPS) was used to determine the area and number of 125I seeds implanted. During the operation, 125I seeds were implanted into the tumor under the guidance of intraoperative ultrasound, with a spacing of 1.5 cm and a row spacing of 1.5 cm. The matched peripheral dose of 125I seeds implanted in patients in this study was 110-160 Gy.
The baseline demographics and perioperative and pathology data were obtained from the electronic medical records. The clinical outcomes, including estimated blood loss (EBL), postoperative complications, and postoperative hospital stay (PHS), were analyzed retrospectively. Postoperative biliary fistula was defined as the outflow of bile or bile containing fluid from the abdominal drainage tube. Postoperative pancreatic fistula was defined as pancreatic juice flowing out of the body through abdominal drainage tube or incision, and the amylase content in the drainage fluid is three times higher than that in blood.
During the operation, the resectability of the tumor was explored first. If the tumor invades the superior mesenteric vein or the superior artery or locally invades the retroperitoneum, the radical resection cannot be performed and frozen pathology was performed by puncture biopsy. 125I seeds were implanted into the tumor under the guidance of intraoperative ultrasound after being confirmed by pathology as pancreatic cancer. For patients with obstructive jaundice or digestive tract obstruction, choledochojejunostomy and gastroenterostomy were performed simultaneously. After operation, the patients were divided into a non-chemotherapy group and a chemotherapy group that received gemcitabine combined with albumin-bound paclitaxel treatment.
All patients were followed 1 mo after discharge and then at 3-mo intervals thereafter.
Continuous data are presented as the mean ± SD or the median and interquartile range according to their distributions. The Student’s t-test was used to compare normally distributed variables between groups, whereas the Mann–Whitney U test was used for non-normally distributed variables. Overall survival (OS) was estimated using the Kaplan-Meier method, and comparison of OS between subgroups was analyzed using the log-rank test. A P value of < 0.05 was considered statistically significant. All analyses were performed with the IBM SPSS statistical software, version 22 (SPSS, Chicago, IL, United States).
Table 1 shows the detailed characteristics of the 50 patients. The patients included 29 men and 21 women with a mean age of 56.9 years. The most common tumor site was the pancreatic head (28, 56%), followed by the pancreatic neck and body (17, 34%) and pancreatic tail (5, 10%). The main reason for the failure of radical resection was superior mesenteric artery invasion (37, 74%), followed by excellent mesenteric vein invasion (33, 66%), and tumor invaded the artery and vein at the same time in half of all the patients. Twenty-one (62%) patients underwent palliative surgery and postoperative pain relief occurred in 40 (80%) patients. All the tumors were pancreatic adenocarcinoma on final histopathological examination.
| Clinicopathologic feature | Value |
| Mean age (range), yr | 56.9 ± 9.8 (38-80) |
| Sex | |
| Male | 29 (58) |
| Female | 21 (42) |
| Tumor location | |
| Head | 28 (56) |
| Neck and body | 17 (34) |
| Tail | 5 (10) |
| Vascular involvement | |
| Any superior mesenteric vein | 33 (66) |
| Any superior mesenteric artery | 37 (74) |
| Both venous and arterial | 25 (50) |
| Palliative operation | 21 (62) |
| Choledochojejunostomy | 15 (71.4) |
| Choledochojejunostomy and gastroenterostomy | 6 (28.6) |
| Postoperative pain relief | |
| Complete remission | 10 (20) |
| Partial remission | 30 (60) |
| No relief | 10 (20) |
| Postoperative hospital stay, d | 7.5 ± 4.2 (4-25) |
| Estimated blood loss, mL | 107.4 ± 115.3 (10-600) |
| Postoperative complications | |
| Biliary fistula | 1 (2) |
| Pancreatic fistula | 3 (6) |
| Vital status at last follow-up | |
| Alive | 11 (22) |
| Dead | 39 (78) |
| Median overall survival (range), mo | 12.0 (4-24) |
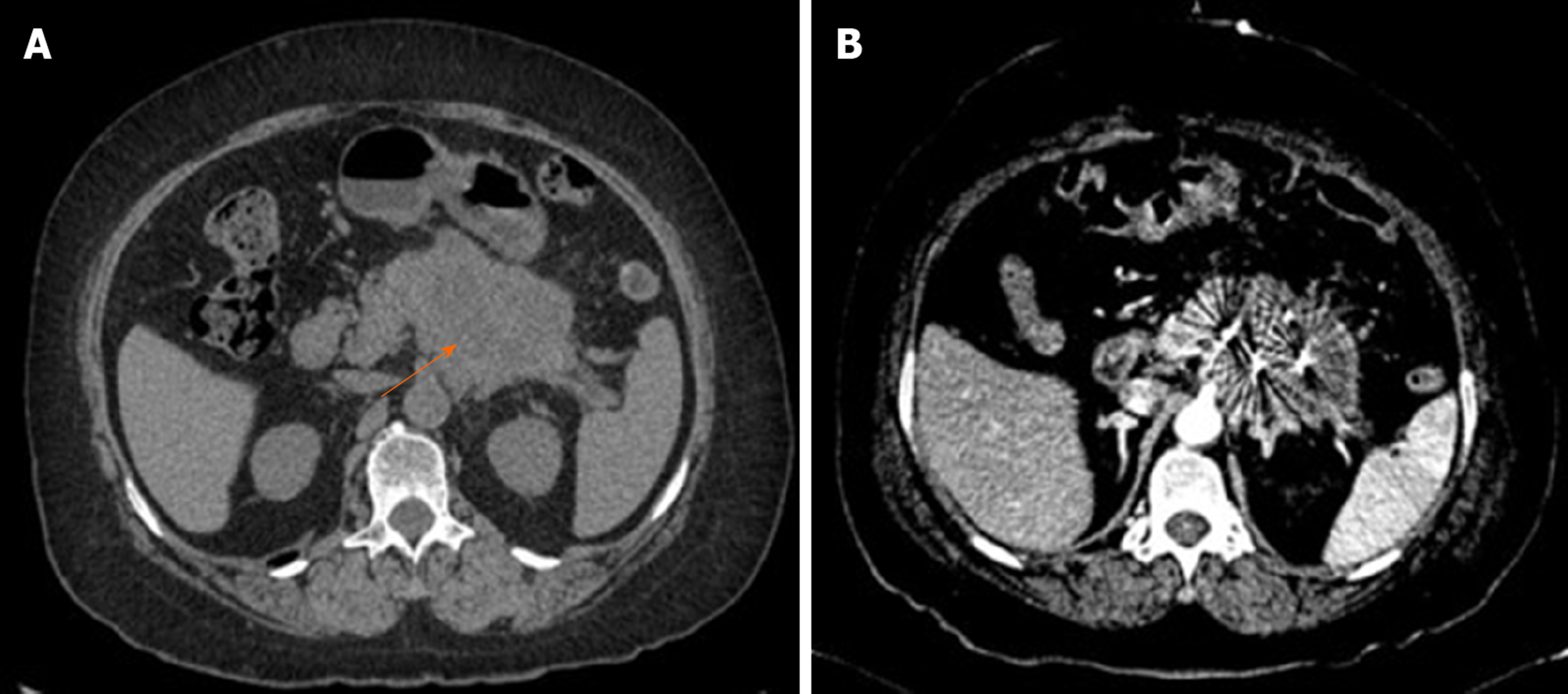
All patients were successfully implanted with 125I seeds; 15 patients underwent choledochojejunostomy, and six underwent choledochojejunostomy combined with gastroenterostomy. The EBL in operation was 107.4 ± 115.3 mL and none of the patient received blood transfusion. The PHS was 7.5 ± 4.2 d; one patient had biliary fistula and three had pancreatic fistula, all of whom recovered after conservative treatment. Figure 1 shows that the 125I seeds were evenly distributed in tumor as revealed by postoperative CT reexamination.
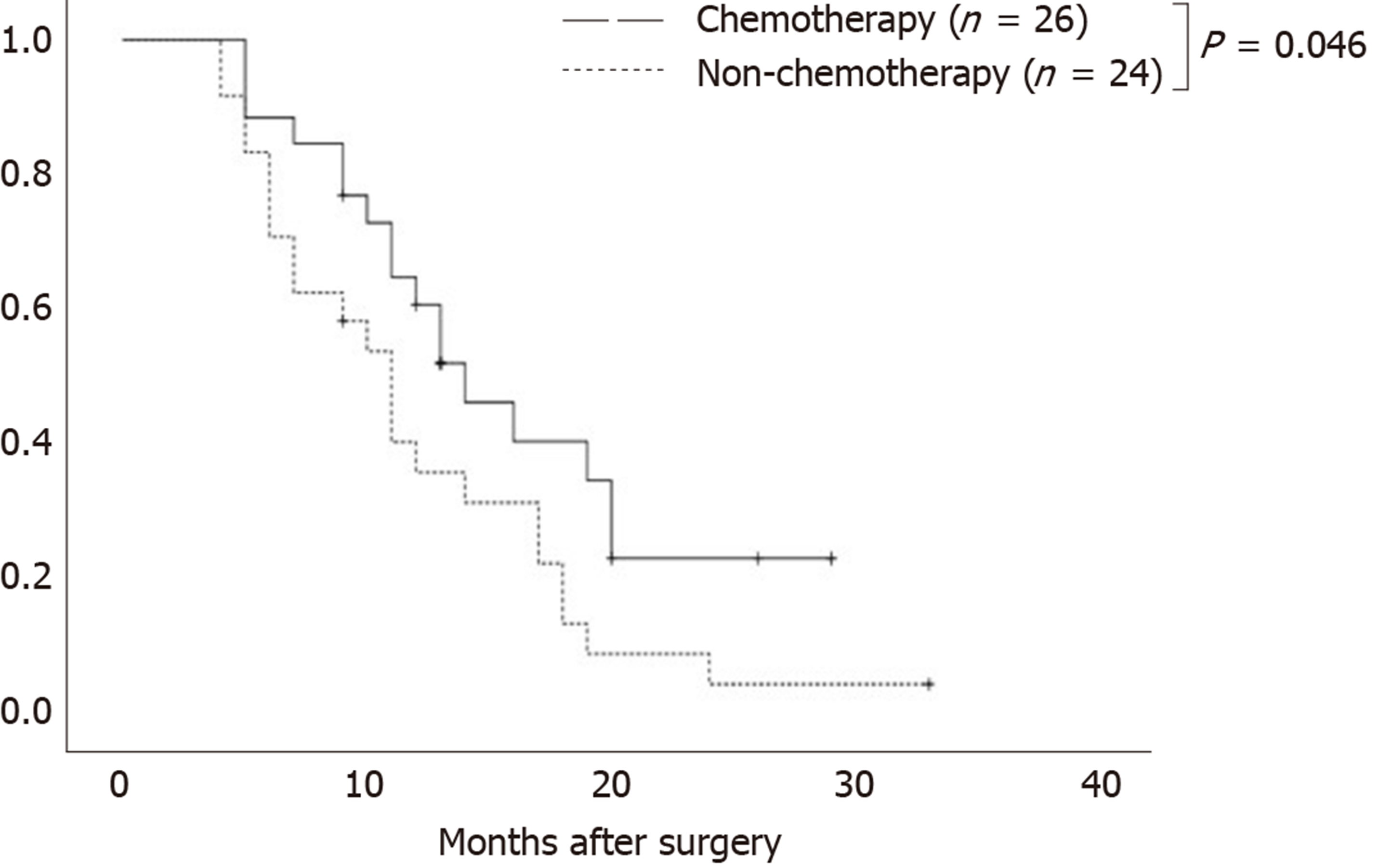
As of the last follow-up, 11 patients were still alive and 39 died. After operation, 26 patients received chemotherapy and 24 did not. Table 2 shows the baseline data of the two groups of patients, and there was no significant difference between the two groups in age, gender, tumor location, or operation mode. The 1-year survival rate was significantly higher in patients who received chemotherapy than in those who did not (60.7% vs 35.9%, P = 0.034). Figure 2 shows that the mean OS of patients of the chemotherapy group and non-chemotherapy group was 14 and 11 mo, respectively (χ2 = 3.970, P = 0.046).
| Clinicopathologic feature | Non-chemotherapy (n = 24) | Chemotherapy (n = 26) | P value |
| Mean age (range), yr | 58.75 (38-80) | 55.31 (38-69) | 0.220 |
| Sex | |||
| Male | 14 | 15 | 0.595 |
| Female | 10 | 11 | |
| Tumor location | |||
| Head | 15 | 13 | 0.645 |
| Neck and body | 7 | 10 | |
| Tail | 2 | 3 | |
| Palliative operation | |||
| Choledochojejunostomy | 10 | 5 | 1.000 |
| Choledochojejunostomy and gastroenterostomy | 4 | 2 | |
| 1-year survival rate (%) | 35.9 | 60.7 | 0.034 |
The pancreas is a typical retroperitoneal organ with a complex and special anatomic location that is deeply surrounded by gastrointestinal organs, the liver, the kidney, the spinal cord, and other organs. The biological behavior of pancreatic cancer is not sensitive to radiotherapy, and the radiation tolerance of surrounding tissues is low. Conventional external radiotherapy cannot accurately locate the lesion area so that the therapeutic dose of radiation reaches the non-target area or even the normal tissue area, causing severe damage to surrounding organs[14-16].
Local adaptation and low-dose continuous therapy are the main and superior characteristics of 125I seeds in the treatment of pancreatic cancer. 125I seeds have beneficial biological characteristics and regulatory ability for the distribution ratio of the radiation dose between the treatment target and normal tissue. They are suitable for clinical application in implantable radiotherapy and are effective in the treatment of various malignant tumors[17-19]. For locally advanced pancreatic cancer, the TPS combined with ultrasound guidance can ensure the reasonable space location of 125I seeds and maximize their killing effect.
The invasion and growth of pancreatic cancer are mainly caused by continuous proliferation of tumor cells. The DNA of tumor cells in the proliferative period is in the late stage of synthesis and mitosis and is extremely sensitive to gamma rays. A small number of gamma rays can destroy DNA, preventing tumor cell proliferation[20,21]. After implantation into tumor tissue, 125I seeds can continuously release low-dose gamma rays to kill tumor cells, damage the DNA of tumor cells, inhibit tumor cell proliferation, and induce tumor cell apoptosis. At the same time, studies have shown that 125I seeds can enhance the sensitivity of hypoxic cells in tumors and enhance their ability to kill tumor cells. Continuous irradiation in tumor tissue can significantly improve its biological effect and has the advantages of minor damage to surrounding normal tissues and a low incidence of adverse reactions[22,23].
Our results showed that patients with locally advanced pancreatic cancer who received radioactive particle implantation had improved pain and quality of life. The combination of postoperative chemotherapy is helpful to prolong the survival period of patients. We recommend that all patients have a biopsy during or before surgery. If possible, gene detection should be carried out on the patient biopsy samples to choose a possible and effective scheme for further chemotherapy after surgery.
Complications of 125I seed implantation in the treatment of pancreatic cancer can occur not only during implantation but also after implantation. Complications during surgery are rare, mainly due to direct damage caused by the puncture needle accidentally penetrating the surrounding blood vessels and tissues during the implantation of particles, leading to bleeding and pancreatic fistula at the puncture site. The needle channel should be carefully adjusted under ultrasound guidance to avoid puncture into the blood vessels and dilated pancreatic duct[24,25]. Our experience is that a 4/0 Prolene suture should be used to suture the puncture point after removing the puncture needle after each particle implantation to reduce the incidence of bleeding and pancreatic leakage. Postoperative complications include seed displacement, local embolism, pain, liver dysfunction, and pancreatic fistula. The complications of radiation inflammation and bleeding and obstruction of the gastrointestinal tract reported in the literature have not occurred in this study.
In conclusion, our experience shows that 125I seed implantation not only is effective for patients with unresectable local advanced pancreatic cancer but can also reduce the clinical symptoms and prolong the relative survival time of those patients.
Pancreatic cancer has the characteristics of late detection, early metastasis, rapid progression, and poor prognosis.
Application of 125I seeds in the treatment of pancreatic cancer.
To summarize a single center experience with 125I seed implantation in the treatment of locally advanced pancreatic cancer.
The demographics and perioperative outcomes of a consecutive series of patients who underwent 125I seed implantation to treat locally advanced pancreatic cancer were retrospectively analyzed. According to the results of preoperative computed tomography or magnetic resonance imaging, the treatment planning system was used to determine the area and number of 125I seeds implanted.
Among the 50 patients, there were 29 males and 21 females, with a mean age of 56.9 ± 9.8 years. The main reason for the failure of radical resection was superior mesenteric artery invasion, followed by superior mesenteric vein invasion. Twenty-one patients underwent palliative surgery and postoperative pain relief occurred in 40 patients. The estimated blood loss in operation was 107.4 ± 115.3 mL and none of the patient received blood transfusion. After operation, 26 patients received chemotherapy and 24 patients did not. The 1-year survival rate was significantly higher in patients who received chemotherapy than in those who did not. The mean OS of patients of the chemotherapy group and non-chemotherapy group was 14 mo and 11 mo, respectively.
Our experience shows that 125I seed implantation is not only effective for unresectable local advanced pancreatic cancer patients, but can also reduce the clinical symptoms and prolong the relative survival time of those patients.
The diversification of cancer treatments has contributed to its survival rate.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Griglione N, Theiss AL S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10:10-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 1511] [Article Influence: 251.8] [Reference Citation Analysis (1)] |
| 2. | Noel M, Fiscella K. Disparities in Pancreatic Cancer Treatment and Outcomes. Health Equity. 2019;3:532-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 3. | Zhou B, Wu D, Liu H, Du LT, Wang YS, Xu JW, Qiu FB, Hu SY, Zhan HX. Obesity and pancreatic cancer: An update of epidemiological evidence and molecular mechanisms. Pancreatology. 2019;19:941-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Wegner RE, Verma V, Hasan S, Schiffman S, Thakkar S, Horne ZD, Kulkarni A, Williams HK, Monga D, Finley G, Kirichenko AV. Incidence and risk factors for post-operative mortality, hospitalization, and readmission rates following pancreatic cancer resection. J Gastrointest Oncol. 2019;10:1080-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Furukawa K, Shiba H, Hamura R, Haruki K, Fujiwara Y, Usuba T, Nakabayashi Y, Misawa T, Okamoto T, Yanaga K. Prognostic Factors in Patients With Recurrent Pancreatic Cancer: A Multicenter Database Analysis. Anticancer Res. 2020;40:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Shridhar R, Takahashi C, Huston J, Meredith KL. Neoadjuvant therapy and pancreatic cancer: a national cancer database analysis. J Gastrointest Oncol. 2019;10:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Gai B, Zhang F. Chinese expert consensus on radioactive 125I seeds interstitial implantation brachytherapy for pancreatic cancer. J Cancer Res Ther. 2018;14:1455-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Beyer DC, Priestley JB. Biochemical disease-free survival following 125I prostate implantation. Int J Radiat Oncol Biol Phys. 1997;37:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Ennis RD, Hu L, Ryemon SN, Lin J, Mazumdar M. Brachytherapy-Based Radiotherapy and Radical Prostatectomy Are Associated With Similar Survival in High-Risk Localized Prostate Cancer. J Clin Oncol. 2018;36:1192-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 10. | Wallis CJD, Saskin R, Choo R, Herschorn S, Kodama RT, Satkunasivam R, Shah PS, Danjoux C, Nam RK. Surgery Versus Radiotherapy for Clinically-localized Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol. 2016;70:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 11. | Xu W, Liu Y, Lu Z, Jin ZD, Hu YH, Yu JG, Li ZS. A new endoscopic ultrasonography image processing method to evaluate the prognosis for pancreatic cancer treated with interstitial brachytherapy. World J Gastroenterol. 2013;19:6479-6484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Huang ZM, Pan CC, Wu PH, Zhao M, Li W, Huang ZL, Yi RY. Efficacy of minimally invasive therapies on unresectable pancreatic cancer. Chin J Cancer. 2013;32:334-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Du YQ, Li ZS, Jin ZD. Endoscope-assisted brachytherapy for pancreatic cancer: From tumor killing to pain relief and drainage. J Interv Gastroenterol. 2011;1:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Zhang ZK, Yang YM. [Current research status and progress in comprehensive diagnosis and treatment of pancreatic cancer in the era of targeted therapy]. Zhonghua Wai Ke Za Zhi. 2020;58:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Fujinaga H, Sakai Y, Yamashita T, Arai K, Terashima T, Komura T, Seki A, Kawaguchi K, Nasti A, Yoshida K, Wada T, Yamamoto K, Kume K, Hasegawa T, Takata T, Honda M, Kaneko S. Biological characteristics of gene expression features in pancreatic cancer cells induced by proton and X-ray irradiation. Int J Radiat Biol. 2019;95:571-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Li D, Su D, Xue L, Liu Y, Pang W. Establishment of pancreatic cancer stem cells by flow cytometry and their biological characteristics. Int J Clin Exp Pathol. 2015;8:11218-11223. [PubMed] |
| 17. | Kou F, Gao S, Liu S, Wang X, Chen H, Zhu X, Guo J, Zhang X, Feng A, Liu B. Preliminary clinical efficacy of iodine-125 seed implantation for the treatment of advanced malignant lung tumors. J Cancer Res Ther. 2019;15:1567-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Song Z, Ye J, Wang Y, Li Y, Wang W. Computed tomography-guided iodine-125 brachytherapy for unresectable hepatocellular carcinoma. J Cancer Res Ther. 2019;15:1553-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Liu SF, Lu J, Wang H, Han Y, Wang DF, Yang LL, Li ZX, Hu XK. Computed tomography-magnetic resonance imaging fusion-guided iodine-125 seed implantation for single malignant brain tumor: Feasibility and safety. J Cancer Res Ther. 2019;15:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Ghaly M, Gogineni E, Saif MW. The Evolving Field of Stereotactic Body Radiation Therapy in Pancreatic Cancer. Pancreas (Fairfax). 2019;3:9-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Nichols RC, Rutenberg M. Optimizing neoadjuvant radiotherapy for resectable and borderline resectable pancreatic cancer using protons. World J Gastrointest Surg. 2019;11:303-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Palta M, Godfrey D, Goodman KA, Hoffe S, Dawson LA, Dessert D, Hall WA, Herman JM, Khorana AA, Merchant N, Parekh A, Patton C, Pepek JM, Salama JK, Tuli R, Koong AC. Radiation Therapy for Pancreatic Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract Radiat Oncol. 2019;9:322-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 23. | Hama Y. Locally advanced pancreatic cancer successfully treated with high-dose helical tomotherapy. Int Cancer Conf J. 2018;7:152-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Lee JM, Choi HS, Chun HJ, Kim ES, Keum B, Seo YS, Jeen YT, Lee HS, Um SH, Kim CD, Kim HB. EUS-guided irreversible electroporation using endoscopic needle-electrode in porcine pancreas. Surg Endosc. 2019;33:658-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Hicks AM, Chou J, Capanu M, Lowery MA, Yu KH, O'Reilly EM. Pancreas Adenocarcinoma: Ascites, Clinical Manifestations, and Management Implications. Clin Colorectal Cancer. 2016;15:360-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |