Published online Aug 6, 2020. doi: 10.12998/wjcc.v8.i15.3355
Peer-review started: April 5, 2020
First decision: April 24, 2020
Revised: May 5, 2020
Accepted: July 15, 2020
Article in press: July 15, 2020
Published online: August 6, 2020
Processing time: 123 Days and 0.5 Hours
Coronavirus disease 2019 (COVID-19) is a public health emergency of international concern. The global population lacks immunity to COVID-19 and is generally susceptible. Underlying conditions, especially chronic respiratory diseases, may affect progression, treatment and prognosis of COVID-19.
We report a patient with confirmed COVID-19 combined with asthma. It took 41 d from disease onset to discharge to obtain two negative tests for this coronavirus.
This case indicates the dynamic clinical characteristics, laboratory and computed tomography findings and adjustment of treatment, and the possible relationship between glucocorticoid therapy and coronavirus clearance.
Core tip: This is a case of confirmed coronavirus disease 2019 with asthma. We reported the whole course. It took 41 d from disease onset to discharge. This case indicated dynamic clinical characteristics, laboratory findings, computed tomography images and adjustment of treatment and discussed the possible relation between glucocorticoids therapy and virus clearance.
- Citation: Liu AL, Xu N, Li AJ. COVID-19 with asthma: A case report. World J Clin Cases 2020; 8(15): 3355-3364
- URL: https://www.wjgnet.com/2307-8960/full/v8/i15/3355.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i15.3355
In December 2019, a cluster of acute respiratory illnesses, now known as coronavirus disease 2019 (COVID-19), occurred in Wuhan, Hubei Province, China[1-5]. The disease has rapidly spread worldwide from Wuhan. The World Health Organization declared COVID-19 a public health emergency of international concern on January 30, 2020[6]. As of May 5, 2020, a total of 3489053 confirmed cases had been documented globally[7-9].
It is reported that at least 25% of patients with COVID-19 had at least one chronic disease[10]. The existence of underlying conditions, especially chronic respiratory diseases, with long-term drug treatment, may affect the progress, treatment and prognosis of COVID-19.
Here, we report the course of a patient with COVID-19 combined with asthma. It took 41 d from disease onset to discharge to obtain two negative tests for this coronavirus.
A 48-year-old man returned to Weihai from Wuhan on January 10, 2020. He developed recurrent fever on January 20, 2020 with a maximum temperature of 38.2 °C with mild cough, without sputum, expectoration or chest tightness. Only oral antipyretics were taken. He went to the clinic on January 23, 2020 where real-time reverse-transcription polymerase chain reaction of pharyngeal swab specimens confirmed COVID-19[11]. He was admitted to the hospital.
The patient had asthma for 10 years that was stable in normal circumstances, without affecting daily life and activity. He inhaled salmeterol/fluticasone powder (Xeretide, 50/500 μg) twice daily. Smoking or other medical history was denied.
On admission, temperature was 37.3 °C, respiratory rate was 23 breaths/min, and a few wheezing rales were heard in both lungs. Arterial blood gases at 21% fraction of inspiration O2 (FiO2) showed partial pressure of oxygen (PaO2) 97 mmHg, partial arterial pressure of carbon dioxide (PaCO2) 39.5 mmHg, pH 7.393, and oxygenation index (OI, PaO2/FiO2) was 465 mmHg [normal range (NR) 400-500 mmHg].
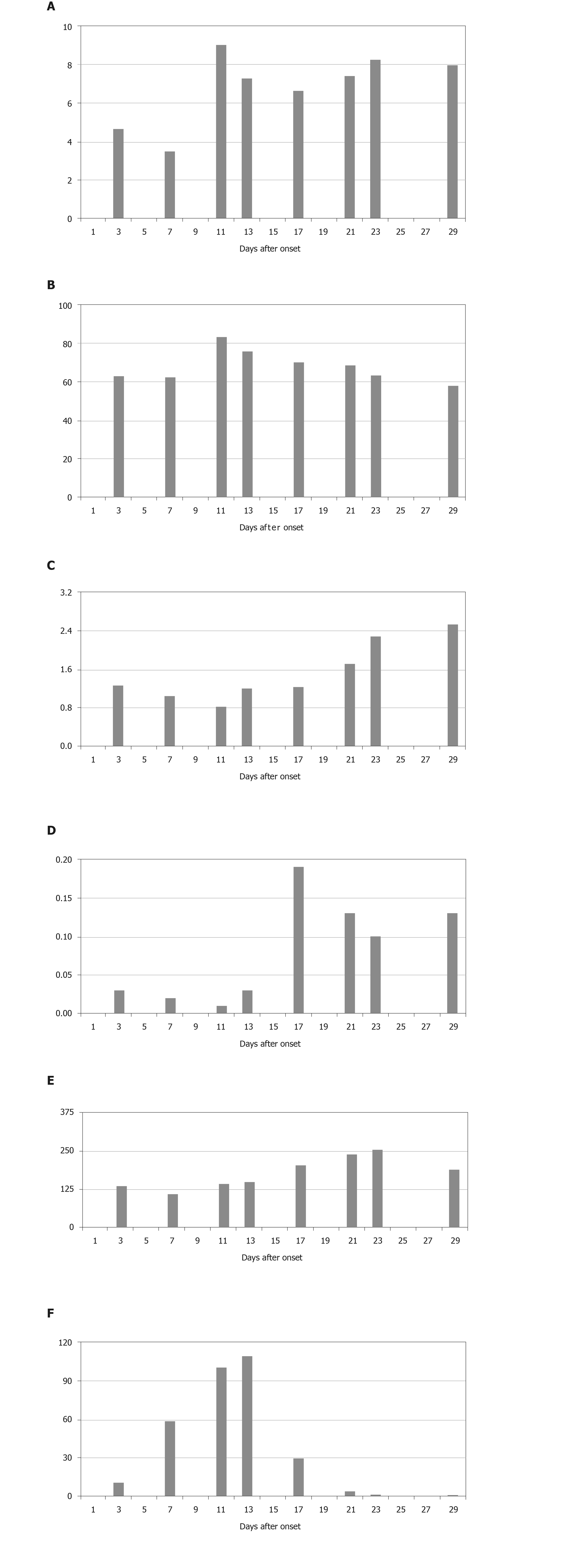
Laboratory parameters (Figure 1) were as follows: White blood cell count 4.66 × 109/L (NR, 3.3 × 109–9.5 × 109/L), neutrophil count 3.0 × 109/L (NR, 2 × 109–7.7 × 109/L), lymphocyte count 1.27 × 109/L (NR, 0.8 × 109–4 × 109/L), CD4 T lymphocyte count 542/μL (NR, 500–1440/μL), monocyte count 0.35×109/L (NR, 0.12 × 109–0.8 × 109/L), eosinophil count 0.03 × 109/L (NR, 0.05 × 109–0.5 × 109/L), platelet count 135 × 109/L (NR, 125 × 109–350 × 109/L), lactate dehydrogenase (LDH) 166 U/L (NR, 100–190 U/L), alanine transaminase (ALT) 15 U/L (NR, 0–50 U/L), aspartate transaminase (AST) 20 U/L (NR, 5–35U/L), albumin 39.8 g/L(NR, 38–55 g/L), C-reactive protein (CRP) 10.94 mg/L (NR, < 4 mg/L). Procalcitonin, myocardial enzymes, serum electrolytes and renal and liver function tests were normal. Detection of antigen and antibody of respiratory tract pathogenic spectrum (including influenza virus A/B) was negative. Chest X-ray showed increased lung markings in both lungs.
Confirmed COVID-19 combined with asthma.
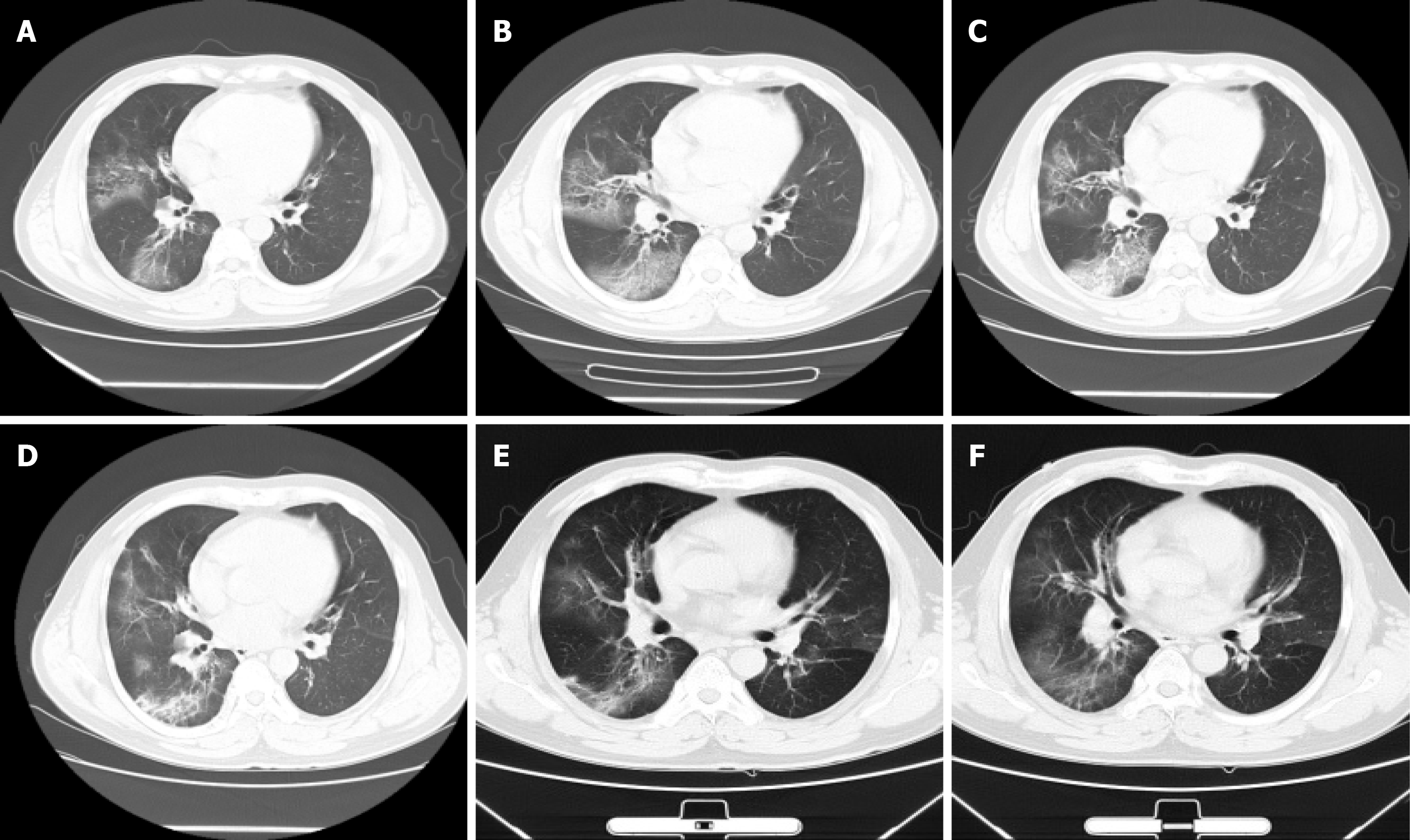
Lopinavir/ritonavir (Kaletra/Aluvia) and moxifloxacin were given orally. The patient’s condition gradually worsened with repeated fever, chest distress, wheezing and diarrhea. Chest computed tomography (CT) images (Figure 2) on day 6 (Figure 2A) after symptom onset (from January 20, 2020) showed ground-glass opacity (GGO) in the right lung. Neutrophil count, CRP, ALT, AST, LDH and fibrinogen increased, and platelet count, OI and albumin decreased. Procalcitonin was normal. He was treated with oxygen, intravenous methylprednisolone (40 mg q12h), aerosol inhalation of terbutaline combined with budesonide, interferon and traditional Chinese medicine.
The disease peaked on day 12 after symptom onset. CT images on day 13 (Figure 2C) indicated that the density of lesions was higher than before. After treatment, the patient’s temperature gradually stabilized, and his symptoms improved. His condition was stable from day 17 without fever, cough, chest pain, chest tightness or diarrhea. Laboratory parameters gradually improved. CD4 T lymphocyte count was 793/μL. Therapy was changed to inhaled salmeterol/fluticasone powder (Xeretide, 50/500 μg) and symptomatic treatment. CT images on day 20 (Figure 2D) after symptom onset showed visible absorption. Virus detection by nasopharyngeal swab or sputum was persistently positive. We tested by PCR every 2-4 d. On day 41 (March 1, 2020) after symptom onset (January 20, 2020) the patient was discharged after two consecutive negative tests from nasopharyngeal swabs and sputum (> 24 h interval).
COVID-19 is a public health emergency of international concern[6]. The global population lacks immunity to COVID-19 and is generally susceptible. The existence of underlying conditions, especially chronic respiratory diseases, may affect the progress, treatment and prognosis of COVID-19[12].
The Chinese Center for Disease Control and Prevention traced back to February 11, 2020 that there were 20982 new cases of COVID-19 reported that were combined with underlying diseases. The 511 patients with chronic respiratory diseases (2.4% in all) had a crude mortality rate of 6.3%, higher than 6% in patients with hypertension and 5.6% in cancer patients, and much higher than 0.9% of patients without underlying diseases[13].
Therefore, it is important to pay attention to the observation and active treatment of chronic underlying diseases in COVID-19 patients. Although the patient reported here suffered from asthma, he usually had good control and regular inhalation medicine. We continued his usual treatment when admitted.
The period between exposure and onset was 10 d, and the early clinical manifestations were fever and dry cough. There was no obvious severe dyspnea in the early stage of the disease. It is reported that most patients have dyspnea during disease progression, and a few patients have hemoptysis, diarrhea and other manifestations[11,14]. Laboratory findings showed that routine blood analysis, blood gas analysis, myocardial enzymes, serum electrolytes and renal and liver function tests were normal, and CRP increased. X-rays showed no sign of pneumonia. The clinical characteristics of this case were consistent with previous reports[10].
The patient’s condition gradually worsened with repeated fever, chest distress, wheezing and diarrhea. It is necessary to distinguish whether the aggravation of symptoms was caused by an acute attack of asthma or progression of pneumonia. CT confirmed progression of pneumonia, and exacerbation of infection induced an acute attack of asthma.
After aggravation, chest CT indicated diffuse GGO, increased neutrophil count, CRP, ALT, AST, LDH and fibrinogen and decreased platelet count, OI and albumin. Procalcitonin was normal.
The changes in chest CT imaging can be divided into different periods. Early in the disease, GGO is found. After progression of the disease, diffuse GGO and consolidation develop. In the absorption period, the consolidation is gradually absorbed. After dissipation, the lesion is absorbed or some fiber strip shadow remains. Pan et al[15] divided the dynamic changes in CT into four different periods according to the days after initial symptoms, in a similar manner to the present case.
Our patient had a normal peripheral blood leukocyte count. Increased neutrophil count and CRP were mainly associated with bacterial infection. Neutrophilia may be related to the coronavirus-induced cytokine storm, but in some severe cases, leukocytopenia may occur. Lymphocyte count was lower than at admission. But as the patient recovered, it gradually increased. Lymphocytopenia is common in patients with COVID-19, and in some cases it is severe. Our finding was consistent with recent reports[16]. Eosinophil and platelet counts decreased as the disease progressed, which was related to myelosuppression. ALT, AST and LDH fluctuated in different periods, under the influence of COVID-19 or some drugs with hepatotoxicity. Lower albumin showed poor nutritional status as the disease progressed. In patients with COVID-19, prolonged PT, increased D-dimer and fibrinogen have been described and are more common in severe cases[10,12,16]. Coagulation may be related to sustained inflammatory response. We found increased D-dimer and fibrinogen, consistent with previous studies[10].
The final diagnosis was confirmed as COVID-19 combined with asthma. There was no evidence of other virus infections. The patient had higher neutrophil count and CRP, CT indicated diffuse GGO, and bacterial infection was considered. Moxifloxacin was given as an antibacterial agent. Lopinavir/ritonavir (Kaletra/Aluvia) and interferon were used as antiviral agents on recommendation of the diagnosis and treatment plan formulated by the Chinese National Health Commission[17]. There was not much experience in antiviral treatment at the beginning of the coronavirus epidemic. Now there are a variety of potentially effective recommended drugs, such as lopinavir/ritonavir, interferon, ribavirin, chloroquine phosphate and arbidol[17].
Glucocorticoids are the basic drug therapy for asthma, but their application in viral pneumonia is controversial. Glucocorticoids are believed to be able to antagonize some pathophysiological processes of acute respiratory distress syndrome, including excessive inflammatory response, excessive cell proliferation and abnormal collagen deposition. A review of the Cochrane system showed that glucocorticoids reduce mortality of patients with severe community-acquired pneumonia[18]. For coronavirus infection, glucocorticoids were widely used during the epidemic of severe acute respiratory syndrome (SARS) in 2003. A retrospective cohort study showed that glucocorticoids reduce mortality and hospital stay[19]. However, some studies showed that glucocorticoids might increase the mortality of SARS and delay virus clearance[20]. Therefore, glucocorticoids should be used cautiously in patients with coronavirus pneumonia, and the indications and dosage should be strictly limited. Nevertheless, it is essential for the control and maintenance of underlying diseases. In the present patient, methylprednisolone was used for 3 d, followed by inhaled corticosteroid (ICS). His condition was stable from day 17, but virus detection was persistent until day 41 after symptom onset. Intravenous or oral glucocorticoids may inhibit the immune response and delay virus clearance[21], but it is not known whether ICSs have the same effect.
The respiratory mucosa immune system (RMIS) refers to the lymphoid tissue distributed in the respiratory mucosa and some exocrine glands. The RMIS makes contact with external antigens (such as symbiotic bacteria and harmful pathogens) directly on the surface of the mucous membrane. It is the first line of defense against invasion by respiratory pathogens. It can recognize and respond to inhaled antigens, generate effective immunity for rejection or elimination of harmful antigens or pathogens[22]. The RMIS consists of cells and molecules such as T lymphocytes, B lymphocytes, natural killer cells, bronchial epithelial cells, dendritic cells and secretory IgA[22,23]. ICSs can inhibit expression of inflammatory factors and chemokines, inhibit leukocyte infiltration and enhance intercellular adhesion so as to maintain epithelial integrity[24]. Prosperini et al[25] showed that the number of epithelial cells in sputum decreased after treatment with ICSs. Fukushima et al[26] found that after treatment with ICS total IgA in saliva of asthmatic patients with positive oral candidiasis decreased. The efficacy and adverse reactions of ICSs are closely related to the RMIS. ICSs may affect immunity and virus clearance from local bronchi and alveoli. At present, no randomized controlled trial has confirmed this.
We have reported a typical case of COVID-19 with asthma. This case indicates the dynamic clinical characteristics, laboratory results, CT findings and adjustment of treatment. We have discussed the possible relationship between glucocorticoid therapy and virus clearance. We hope that this case provides more ideas for future research.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu SX S-Editor: Ma YJ L-Editor: Filipodia E-Editor: Wang LL
| 1. | Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. 2020;92:401-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1670] [Cited by in RCA: 1769] [Article Influence: 353.8] [Reference Citation Analysis (0)] |
| 2. | Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, Ippolito G, Mchugh TD, Memish ZA, Drosten C, Zumla A, Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2274] [Cited by in RCA: 1962] [Article Influence: 392.4] [Reference Citation Analysis (0)] |
| 3. | Wuhan Municipal Health Commission. Report of novel coronavirus-infected pneumonia in China. Published January 20, 2020. Accessed January 31, 2020. Available from: URL: http://wjw.wuhan.gov.cn/front/web/showDetail/2020012009077. |
| 4. | Paules CI, Marston HD, Fauci AS. Coronavirus Infections-More Than Just the Common Cold. JAMA. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1204] [Cited by in RCA: 1135] [Article Influence: 227.0] [Reference Citation Analysis (0)] |
| 5. | Wuhan Municipal Health Commission. Report of cluste ring pneumonia of unknown etiology in Wuhan City. Published December 31, 2019. Accessed January 31, 2020. Available from: URL: http://wjw.wuhan.gov.cn/front/web/showDetail/2019123108989. |
| 6. | World Health Organization. Coronavirus disease (COVID-19) outbreak. Available from: URL: https://www.who.int. |
| 7. | Phan LT, Nguyen TV, Luong QC, Nguyen TV, Nguyen HT, Le HQ, Nguyen TT, Cao TM, Pham QD. Importation and Human-to-Human Transmission of a Novel Coronavirus in Vietnam. N Engl J Med. 2020;382:872-874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 758] [Cited by in RCA: 695] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 8. | Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, Zimmer T, Thiel V, Janke C, Guggemos W, Seilmaier M, Drosten C, Vollmar P, Zwirglmaier K, Zange S, Wölfel R, Hoelscher M. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020;382:970-971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2799] [Cited by in RCA: 2491] [Article Influence: 498.2] [Reference Citation Analysis (0)] |
| 9. | Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK; Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929-936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4155] [Cited by in RCA: 3822] [Article Influence: 764.4] [Reference Citation Analysis (1)] |
| 10. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18878] [Article Influence: 3775.6] [Reference Citation Analysis (7)] |
| 11. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30123] [Article Influence: 6024.6] [Reference Citation Analysis (3)] |
| 12. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14767] [Article Influence: 2953.4] [Reference Citation Analysis (0)] |
| 13. | Epidemiology of novel coronavirus pneumonia response mechanism in China Center for Disease Control and prevention. Epidemiological characteristics of new coronavirus pneumonia. Zhonghua Liuxingbing Xue Zazhi. 2020;41:145-151. |
| 14. | Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11224] [Cited by in RCA: 9319] [Article Influence: 1863.8] [Reference Citation Analysis (0)] |
| 15. | Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, Wang J, Hesketh RL, Yang L, Zheng C. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology. 2020;295:715-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1617] [Cited by in RCA: 1761] [Article Influence: 352.2] [Reference Citation Analysis (0)] |
| 16. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12977] [Article Influence: 2595.4] [Reference Citation Analysis (1)] |
| 17. | National Health Commission of the Peopleâs Republic of China home page. Available from: URL: http://www.nhc.gov.cn. |
| 18. | Stern A, Skalsky K, Avni T, Carrara E, Leibovici L, Paul M. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2017;12:CD007720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 19. | Chen RC, Tang XP, Tan SY, Liang BL, Wan ZY, Fang JQ, Zhong N. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129:1441-1452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 218] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 20. | Lee N, Allen Chan KC, Hui DS, Ng EK, Wu A, Chiu RW, Wong VW, Chan PK, Wong KT, Wong E, Cockram CS, Tam JS, Sung JJ, Lo YM. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 455] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 21. | Auyeung TW, Lee JS, Lai WK, Choi CH, Lee HK, Lee JS, Li PC, Lok KH, Ng YY, Wong WM, Yeung YM. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J Infect. 2005;51:98-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Lusuardi M, Capelli A, Di Stefano A, Donner CF. Lung mucosal immunity: immunoglobulin-A revisited. Eur Respir J. 2002;19:785; author reply 785-785; author reply 786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Del Donno M, Bittesnich D, Chetta A, Olivieri D, Lopez-Vidriero MT. The effect of inflammation on mucociliary clearance in asthma: an overview. Chest. 2000;118:1142-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Stellato C. Glucocorticoid actions on airway epithelial responses in immunity: functional outcomes and molecular targets. J Allergy Clin Immunol. 2007;120:1247-63; quiz 1264-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Prosperini G, Rajakulasingam K, Cacciola RR, Spicuzza L, Rorke S, Holgate ST, Di Maria GU, Polosa R. Changes in sputum counts and airway hyperresponsiveness after budesonide: monitoring anti-inflammatory response on the basis of surrogate markers of airway inflammation. J Allergy Clin Immunol. 2002;110:855-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Fukushima C, Matsuse H, Saeki S, Kawano T, Machida I, Kondo Y, Kohno S. Salivary IgA and oral candidiasis in asthmatic patients treated with inhaled corticosteroid. J Asthma. 2005;42:601-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |