Published online Aug 6, 2020. doi: 10.12998/wjcc.v8.i15.3320
Peer-review started: April 3, 2020
First decision: May 15, 2020
Revised: May 27, 2020
Accepted: July 15, 2020
Article in press: July 15, 2020
Published online: August 6, 2020
Processing time: 125 Days and 6.4 Hours
Isolated splenic metastasis is a rare clinical entity. Multiple metastases in the spleen after radical colon resection in a patient who subsequently underwent a second local resection for isolated metachronous splenic metastasis are exceedingly rare.
We report a colon cancer patient who underwent laparoscopic radical colon resection 14 mo previously, and subsequently underwent a second local resection due to local recurrence detected by elevated serum carcinoembryonic antigen (CEA) and positron emission tomography (PET). However, multiple metastases in the spleen were found 7 mo later by elevated serum CEA and PET-magnetic resonance imaging. Then the patient underwent total laparoscopic splenectomy. Local tumor recurrence and splenic metastasis from colorectal cancer (CRC) were found by postoperative pathology. Genetic analysis of these recurrent and metastatic tissues showed KRAS exon2, APC exon16 and TP53 exon6 missense mutations, but no mutations of NRAS, KRAF, EGFR, ERBB2, MET, MLH1, MSH2 and MSH6 were detected. Chemotherapy and target therapy were administered after multiple disciplinary team (MDT) consultation, and no tumor recurrence has been observed to date. We also reviewed the literature by conducting a search of the PubMed database using the following key words: CRC, splenic metastasis, isolated, and review. We identified 34 relevant papers, which included 28 cases of metachronous metastasis and 6 cases of simultaneous metastasis.
Close monitoring of serum CEA levels is crucial for the detection of isolated splenic metastases after colon surgery. In terms of overall survival and progression-free survival, MDT plays an important role in the entire process of disease management.
Core tip: Isolated splenic metastasis is a rare clinical entity. We report a case of 48-year-old woman with isolated splenic metastasis 21 mo after radical colon adenocarcinoma resection. Close monitoring of serum carcinoembryonic antigen levels is crucial after colon adenocarcinoma surgery. Splenectomy seems to be the preferred treatment and multiple disciplinary team plays an important role in the entire process of disease management.
- Citation: Hu L, Zhu JY, Fang L, Yu XC, Yan ZL. Isolated metachronous splenic multiple metastases after colon cancer surgery: A case report and literature review. World J Clin Cases 2020; 8(15): 3320-3328
- URL: https://www.wjgnet.com/2307-8960/full/v8/i15/3320.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i15.3320
Splenic metastases in patients with malignant tumors generally indicate multiple metastases[1,2]. Isolated splenic metastasis after radical colon adenocarcinoma resection is a rare clinical entity[3]. The mechanism of their rarity is unknown and many theories have focused on anatomical and histological aspects. We report a case of isolated splenic metastasis 21 mo after radical colon adenocarcinoma resection, which was successfully treated by totally laparoscopic splenectomy. We discuss the clinical and pathological aspects of the case, and consider the diagnostic and therapeutic options based on our observation of the case. We also reviewed the literature and identified 34 relevant papers, including 28 cases of metachronous metastasis and 6 cases of simultaneous metastasis.
A 48-year-old female patient was admitted to our hospital with elevated serum carcinoembryonic antigen (CEA). The patient had no symptoms of abdominal pain, diarrhea or bloody stools.
The patient underwent laparoscopic radical colon resection 14 mo previously and subsequently underwent a second local resection due to local recurrence detected by elevated serum CEA and positron emission tomography (PET). However, multiple metastases in the spleen were found 7 mo later by elevated serum CEA and PET-magnetic resonance imaging. The patient then underwent a totally laparoscopic splenectomy. Local tumor recurrence and splenic metastasis from the colorectal cancer (CRC) were found by postoperative pathology.
The patient denied a history of hypertension, diabetes, and other relevant illnesses.
The patient did not have a family history of cancer.
Physical examination was normal.
Complete blood count, liver and renal function tests, and alpha fetoprotein, carbohydrate antigen 19-9 (CA19-9), cancer antigen 125 (CA125), and CA153 were within normal limits, except for CEA which was 12.41 ng/mL (normal range 0-5.0 ng/mL), 51.49 ng/mL and 206.89 ng/mL, respectively.
The first endoscopy revealed a sigmoid colon mass. The second and third endoscopies were normal.
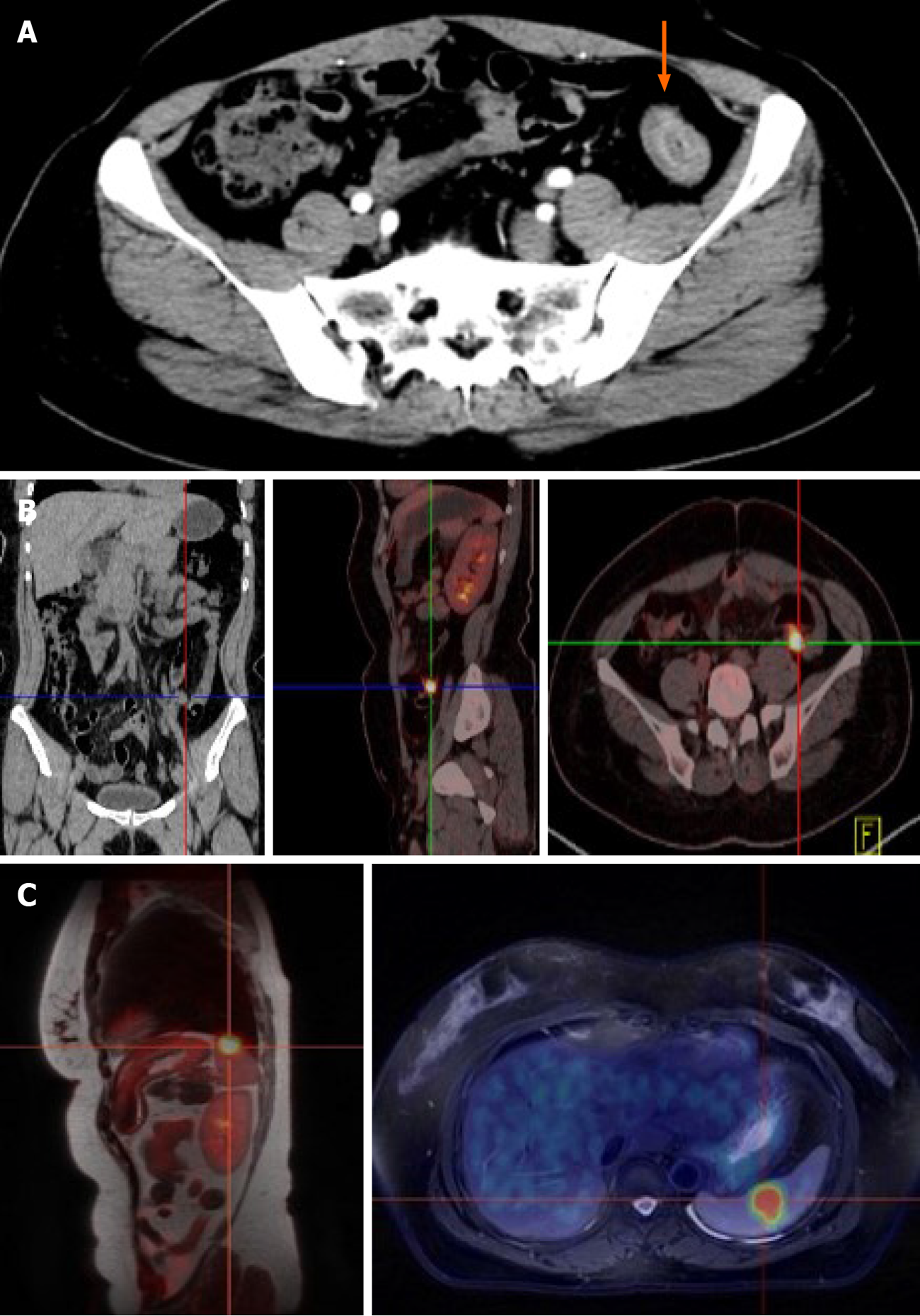
A sigmoid colon mass was found by abdomen enhanced computed tomography (CT) (Figure 1A). Local recurrence in the descending colon was found by positron emission tomography (PET)-CT (Figure 1B) and lymph nodes showed no high metabolic shadow. Isolated splenic metastasis was found by PET-magnetic resonance imaging (MRI) (Figure 1C) and lymph nodes also showed no high metabolic shadow.
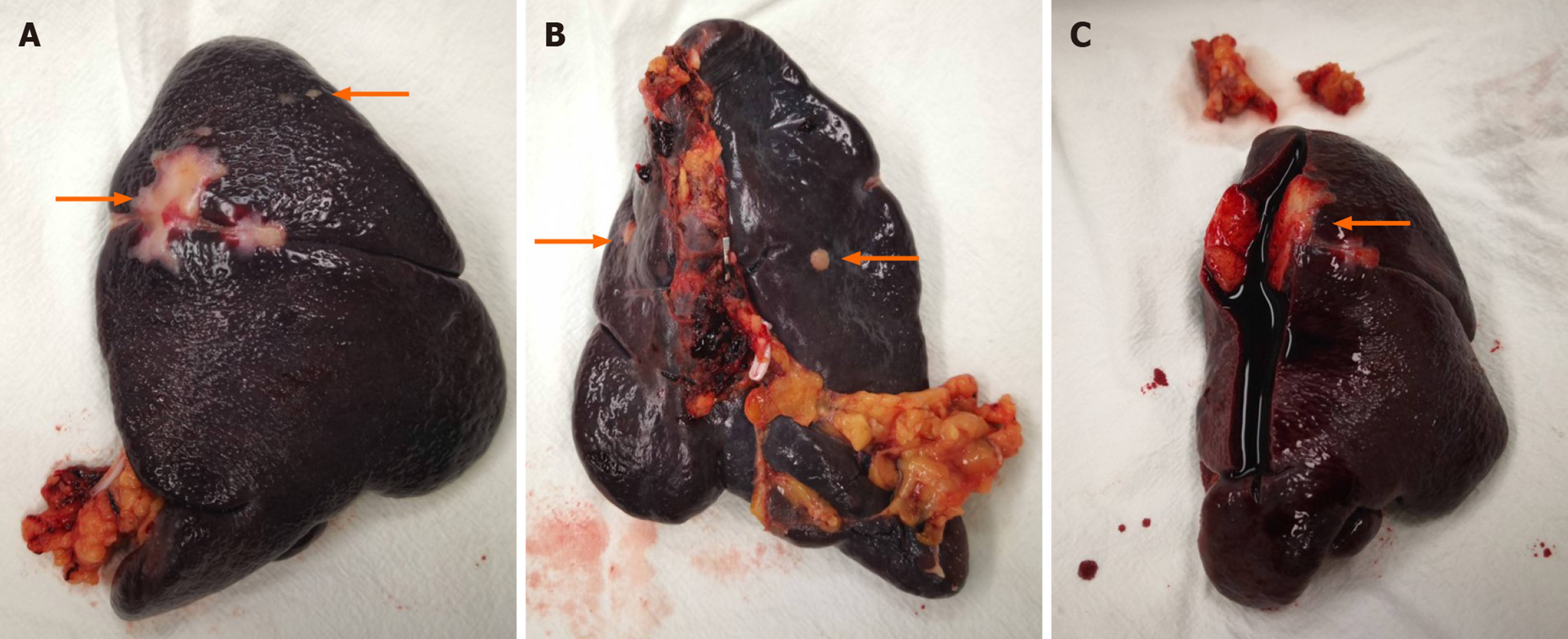
The clinical stage assessed by enhanced CT before surgery was cT4aN0M0 and postoperative pathological stage was pT4aN2M0 after laparoscopic radical resection of sigmoid adenocarcinoma and one-stage anastomosis. The patient received chemotherapy (Xelox regimen, capecitabine 1000 mg/m2) for 3 wk with a total of 8 treatment courses. However, the CEA level gradually increased 14 mo after surgery, and PET-CT showing a high metabolic shadow in the descending colon. Following MDT consultation, a second laparoscopic exploration was performed. The extent of local resection based on a laparoscopic exploration and no other recurrences were found. Local resection of the recurrence in the intestinal tract and one-stage anastomosis were carried out during surgery. The tumor edge is 5 cm away from the proximal and distal margin. Postoperative pathology confirmed a poorly differentiated colon adenocarcinoma (with extra-serosal and muscular is invasion).The circumferential, proximal, and distal margins were negative. Xelox chemotherapy was again administered for 3 wk per treatment course for almost 3 mo. At a subsequent examination, the CEA level had gradually increased over the 7-mo period after the second operation. After the second MDT consultation, laparoscopic exploration was performed, and no peritoneal metastases were observed and the spleen was removed via laparoscopy. The surgical specimen displayed multiple metastases (Figure 2). Chemotherapy with FOLFIRI (irinotecan 180 mg/m2 + 5-FU 400 mg/m2 d1, injection, +2400 mg/m2, injection, every 2 wk) and bevacizumab (5 mg/kg drop every 2 wk) was administered for 2 wk following the suggestions by a third MDT consultation.
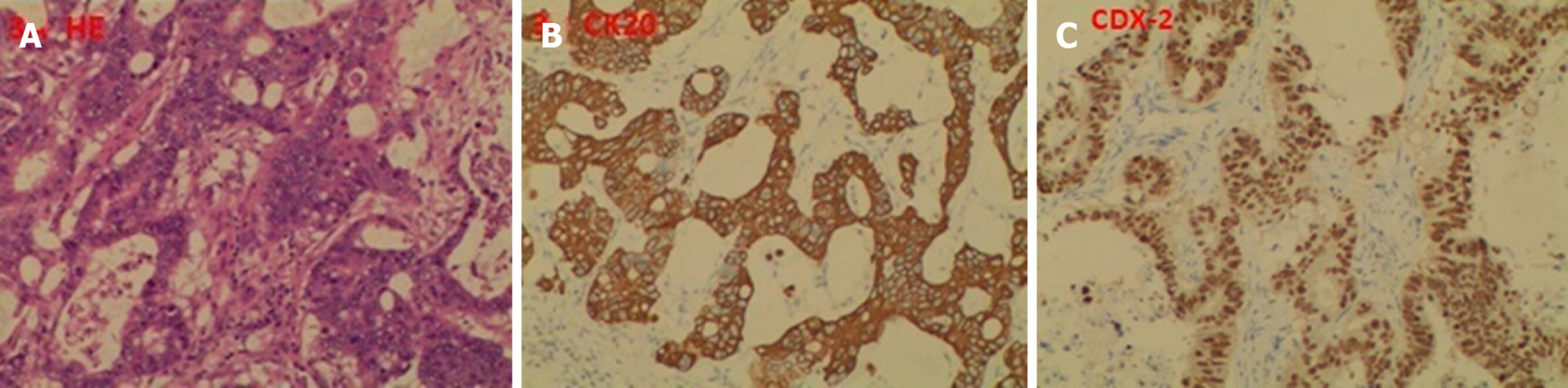
The first postoperative pathology indicated a poorly differentiated adenocarcinoma; 4 of 15 perirectal lymph nodes harvested were positive; and circumferential, proximal, and distal margins were negative. The second postoperative pathology showed the same results. The last postoperative pathology indicated multiple metastatic moderately differentiated adenocarcinoma of the spleen, with tumor sizes ranging from 0.4 to 3.0 cm, and originating from CRC via hematoxylin and eosin staining and immunohistochemistry (Figure 3). Genetic analysis of local recurrence and splenic metastasis showed KRAS exon2, APC exon16, and TP53 exon6 missense mutations, while no mutations of NRAS, KRAF, EGFR, ERBB2, MET, MLH1, MSH2 and MSH6 were detected.
The diagnosis of postoperative isolated metachronous splenic metastasis of CRC requires clear imaging, which can be obtained by CT examinations, and sometimes ultrasonography and PET are required. In our case, PET-CT was performed due to an elevated CEA level, which only revealed local colon cancer and not splenic metastasis (Figure 1B), and subsequent PET-MRI detected isolated splenic metastasis (Figure 1C). Pathological stage I was found in the primary focus in only 1 case (3.4%), stage II in 7 cases (24.1%), stage III in 18 cases (62.1%), and the stage in 3 cases (10.4%) could not be determined. Higher pathological staging suggests the possibility of splenic metastasis. However, stage I CRC can also metastasize to the spleen. Pathological biopsy was needed for the final diagnosis of postoperative splenic metastasis of CRC, 2 cases were confirmed by fine needle biopsy, and the others by postoperative pathology.
The treatment of postoperative isolated metachronous splenic metastasis of CRC includes surgery, chemotherapy combined with targeted therapy or radiotherapy. Surgery was performed in all cases, 2 cases underwent totally laparoscopic splenectomy (including our case), and the remaining cases underwent open total splenectomy. Laparoscopic resection of a splenic malignancy is controversial due to potential cancer cell implantation during laparoscopic surgery. Lopez Monclova et al[4] reported 6 cases of laparoscopic metastasis splenectomy, all of which had no postoperative complication sand the total survival time ranged from 2 mo to 11 years[4,5]. In terms of abdominal organ malignancy, laparoscopic surgery does not result in higher surgical complications than traditional laparotomy[6]. Therefore, laparoscopic total splenectomy is safe, reproducible and results in a faster postoperative recovery than traditional surgery. Our patient underwent three surgeries, all conducted under laparoscopy, and no postoperative complications were observed. Chemotherapy is considered a priority treatment for distant metastasis. However, only 4 patients received perioperative chemotherapy and 1 received targeted therapy after splenectomy of metastatic tumors, as shown in Table 1. It is possible to reach a state of No Evidence of Disease after excision of an isolated metastasis and most cases require continuous observation. The time to disease recurrence after splenectomy ranged from 3 to 84 mo (median time 18.0 mo) in these patients. Although few patients were treated with chemotherapy and targeted therapy, systemic chemotherapy still provides an essential contribution to the overall survival rate in the presence of distant metastasis. No relevant literature was identified on the use of radiotherapy in combination with chemotherapy. In our case, patient received chemotherapy combined with targeted therapy after splenectomy, following the suggestions of the MDT. The time without disease recurrence remains to be defined in this patient.
| No. | Age/sex | Primary | Stage | Size in cm | SO/M | CEA in ng/mL | Imaging | Treatment | 1st DFI in mo | 2nd DFI in mo | Ref. |
| 1 | 78/F | Rectum | III | 18 | SO | 64 | CT | S | 48 | 84 | [13] |
| 2 | 72/M | Sigmoid | III | 9 | SO | 106 | LSS | S | 48 | 6 | [14] |
| 3 | 81/M | Cecum | III | NA | SO | 7.5 | LSS | S | 30 | 12 | [15] |
| 4 | 51/F | Rectum | II | NA | SO | 13.5 | CT | S | 51 | 14 | [16] |
| 5 | 72/F | Descending | II | 3 | SO | 223 | CT | S | 144 | 12 | [17] |
| 6 | 62/F | Descending | III | 4 | SO | High | CT | S | 42 | 12 | [18] |
| 7 | 74/M | Sigmoid | II | 9.5 | SO | 23.4 | CT | S | 24 | 24 | [19] |
| 8 | 52/M | Ascending | NA | NA | SO | NA | US and CT | S | 12 | 6 | [20] |
| 9 | 48/M | Ascending | NA | NA | SO | NA | US and CT | S | 24 | 3 | [20] |
| 10 | 33/F | Sigmoid | III | 3.5 | SO | 9 | CT and MRI | S | 3 | 12 | [21] |
| 11 | 65/M | Ascending | III | 5 | SO | 14.9 | CT | S | 36 | 18 | [22] |
| 12 | 60/F | NA | NA | NA | SO | High | CT | S | 108 | 5 | [23] |
| 13 | 51/M | Sigmoid | III | 13 | SO | N | CT | S | 72 | 6 | [24] |
| 14 | 62/M | Sigmoid | III | 3 | SO | N | US and CT | S | 24 | 23 | [25] |
| 15 | 55/F | Sigmoid | III | 3 | SO | N | PET-CT | S | 21 | 12 | [26] |
| 16 | 52/F | Sigmoid | II | 13 | SO | 7.2 | US and CT | Cmt, S | 108 | 22 | [27] |
| 17 | 76/M | Descending | III | 6.5 | SO | High | PET-CT | S, Cmt | 17 | 12 | [28] |
| 18 | 72/M | Rectum | III | NA | SO | High | CT | S | 18 | NL | [29] |
| 19 | 69/F | Sigmoid | II | 4 | SO | High | CT | S | 24 | 60 | [30] |
| 20 | 52/F | Descending | III | 4.5 | SO | High | PET | S | 36 | NL | [31] |
| 21 | 80/F | Transverse | III | 8 | SO | 52 | CT | S, TT | 9 | 6 | [32] |
| 22 | 73/M | Hepatic flexure | II | 1.5 | SO | High | CT, PET | S | 60 | 40 | [33] |
| 23 | 70/M | Splenic flexure | III | 8 | SO | High | CT, US | S | 24 | 12 | [34] |
| 24 | 59/M | Ascending | III | 4 | SO | 37 | CT | S | 15 | 24 | [35] |
| 25 | 58/M | NA | II | 3.5 | SO | N | CT | S, Cmt | 20 | 11 | [36] |
| 26 | 78/M | Cecum | III | 7 | SO | 38.6 | CT, PET | Cmt, S | 37 | 9 | [37] |
| 27 | 64/F | Cecum | I | 4.9 | SO | 38 | CT | S | 16 | 6 | [2] |
| 28 | 76/F | Descending | III | 1.6 | SO | N | PET | S, Cmt | 24 | 21 | [6] |
| 29 | 46/F | Sigmoid | III | 0.4-3 | M | 206.8 | PET-CT/MRI | S, Cmt, TT | 21 (14, 7) | 7 | Our case |
No sign of tumor recurrence or metastasis was observed 7 mo after totally laparoscopic splenectomy.
The most common metastatic organ of CRC is the liver, and it has been reported that almost 50% of patients diagnosed with CRC experienced simultaneous or heterogeneous liver metastases[3,7]. Splenic metastases in patients with malignant tumors generally indicate multiple metastases[1,2]. A splenic primary tumor or metastasis a rare disease, and the most common sources of splenic metastasis are melanoma (34%), breast cancer (12%), ovarian cancer (12%), CRC (10%), and lung cancer (9%)[8,9]. However, isolated splenic metastasis is even rarer. Berge et al[1] found that the splenic metastases accounted for 4.4% in 7165 cadaver reports, of which 1.6% were from colon cancer, and isolated splenic metastases in colon cancer was not mentioned in their paper[1,10]. Another global study showed that up to 2012, less than 100 cases of isolated splenic metastases from colon cancer had been recorded[11].
Splenic metastases are clinically infrequent; thus, the mechanism of their formation is unknown and many theories have focused on anatomical and histological aspects. With regard to the anatomy of the metachronous metastasis pathway, the splenic artery and abdominal cavity are formed at acute angles and the rhythmic contraction of the spleen[12] prevents tumor cells from remaining and growing in the spleen. Other researchers have proposed that tumor cells from the inferior mesenteric vein flow back into the splenic vein and reverse flow into the spleen, resulting in intra-splenic metastasis. With regard to lymphatic metastasis, there is no direct lymphatic input to the spleen, but there are lymphatic systems in the portal vein, subcapsule and trabeculae of the spleen. Tumor cells entering the spleen through these lymphatic systems may also explain the fact that these tumor cells are limited to the subcapsular membrane and thus form isolated lesions. Histologically, the spleen is the second largest lymphatic reticuloendothelial organ in the body. It is composed of a large number of monocytes, Kupffer cells, immunoglobulins and opsonins, which have a high phagocytic activity. In addition, studies have shown that tumor cells entering the spleen will be destroyed by splenic lymphatic reticuloendothelial cells, preventing tumor implantation. Research has also determined that adenocarcinoma cells flow into the liver faster than they flow into the spleen[1,2,9]. This might indicate that the spleen may generate anti-tumor fixations and reproductive factors. Possible causes of rare metastatic tumors of the spleen are summarized in Table 2.
| Possible causes of splenic metastasis |
| Anatomical causes |
| Blood metastasis: The splenic artery and abdominal cavity are formed at acute angles; rhythmic contraction of the spleen; tumor cells from the inferior mesenteric vein flow back into the splenic vein and reverse flow into the spleen |
| Lymphatic metastasis: No direct lymphatic input to the spleen; lymphatic systems are present in the portal vein, subcapsule and trabeculae of the spleen |
| Histological causes |
| The spleen is the second largest lymphatic reticuloendothelial organ in the body; Monocytes, Kupffer cells, immunoglobulins and opsonins have a high phagocytic activity; Anti-tumor fixations and reproductive factors |
We identified 34 cases of isolated splenic metastasis after CRC surgery from the PubMed database (28 cases of metachronous metastasis and 6 cases of simultaneous metastasis, shown in Table 1). We report the 28th case of solitary metachronous splenic metastasis in this study. Our case was characterized by a solitary splenic metastasis during the second surgical procedure, followed by multiple metastases in the spleen, which was different from previous reports. It can be seen from Table 1 that the 15 male and 14 female cases were aged between 33 years and 81 years, with a median age of 63.5 years. The majority of cases presented with an increased CEA level (85.7%) and plasma CEA level ranged from 7.2 to 223 ng/mL, while the CEA level in our case reached a maximum of 206.8 ng/mL. The first disease-free interval in these cases ranged from 3 to 144 mo and the first local colon recurrence in our case was observed 14 mo after the first surgery, and the second splenic metastasis was observed 7 mo later. The diameter of the tumors ranged from 1.5 to 18 cm, all of which were a single metastasis, including 6 cases not mentioned in the reports, and our patient had multiple metastases, which ranged from 0.4 to 3.0 cm in diameter (Figure 2). Primary lesions were found in the right hemi-colon in 8 cases (3 cases in the cecum, 4 cases in the ascending colon and 1 case in the colonic liver curvature), accounting for 27.6%. The primary tumor was found in the transverse colon in 1 patient (3.4%), and in 18 cases the tumor was found in the left hemi-colon (1 case in the colonic splenic curvature, 4 cases in the descending colon, 9 cases in the sigmoid colon and 3 cases in the rectum), accounting for 62.1%. The tumor site was not mentioned in 2 cases. The high proportion originating from the left hemi-colon also supports the hypothesis that tumor cells have the trait of a counter current to the spleen via the inferior mesenteric vein.
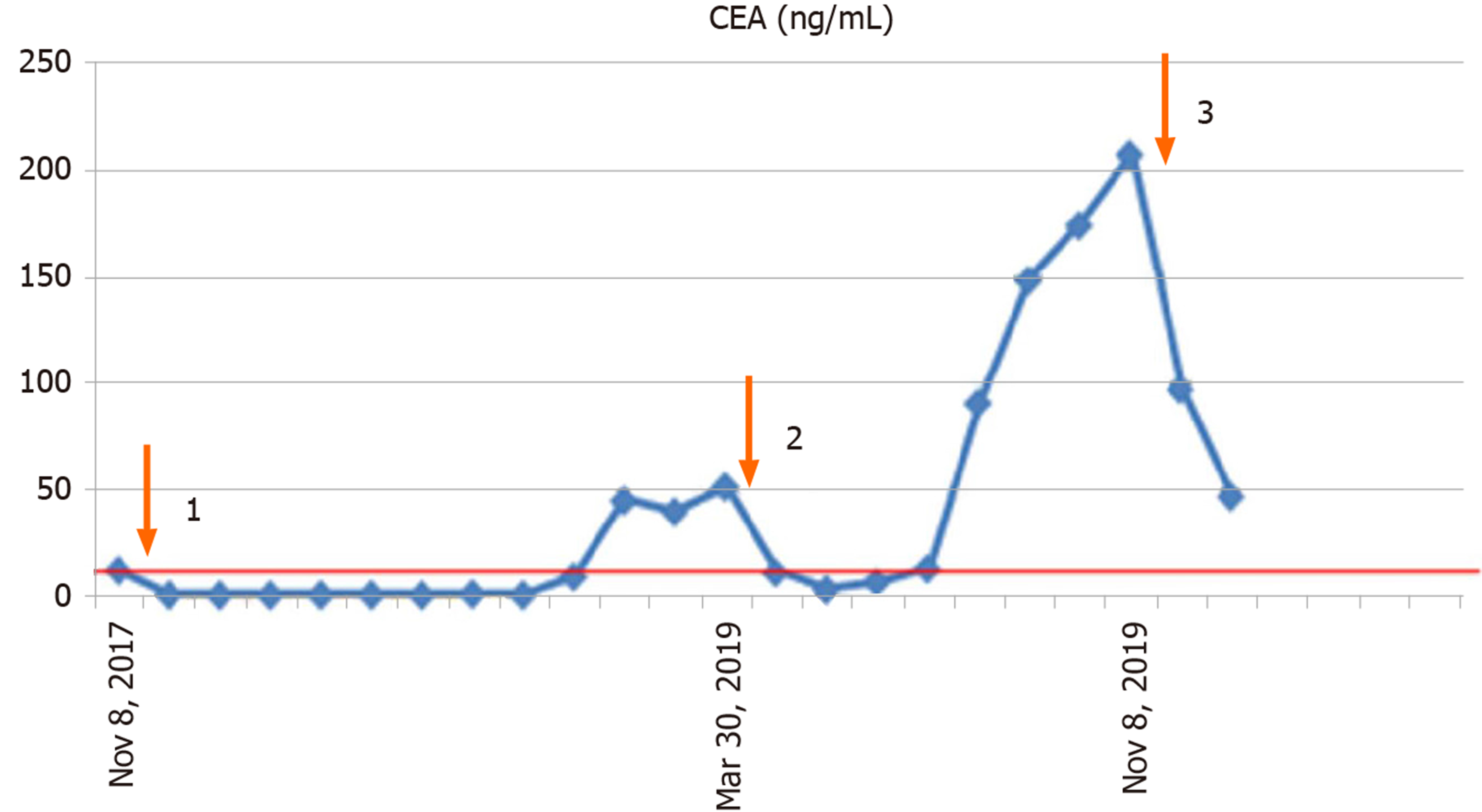
We report, for the first time, the occurrence of multiple metastases in the spleen during the second postoperative progression of colon cancer. Our findings enrich the data on metastases of the spleen and provide experience in the management of this disease. Close monitoring of serum CEA levels plays a crucial role in the postoperative follow-up of these patients (Figure 4). Otherwise, the patient may have multiple organ metastases and lose the opportunity of a second or third operation. Surgical removal of the tumor is just part of the treatment regimen, and postoperative chemotherapy, radiation therapy, targeted therapy, immune therapy and follow-up are also important. Appropriate treatment strategies based on MDT consultation is conducive to improving cancer patients’ overall survival and progression-free survival.
We thank the patient for permitting us to use her data to complete this article.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tenreiro N S-Editor: Ma YJ L-Editor: Filipodia E-Editor: Liu JH
| 1. | Berge T. Splenic metastases. Frequencies and patterns. Acta Pathol Microbiol Scand A. 1974;82:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Abdou J, Omor Y, Boutayeb S, Elkhannoussi B, Errihani H. Isolated splenic metastasis from colon cancer: Case report. World J Gastroenterol. 2016;22:4610-4614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Wang K, Xu D, Yan XL, Poston G, Xing BC. The impact of primary tumour location in patients undergoing hepatic resection for colorectal liver metastasis. Eur J Surg Oncol. 2018;44:771-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Lopez Monclova J, Targarona Soler E, Peraza Solis Y, Vidal Gonzalez P, Balague Ponz C, Rodriguez Luppi C, Trias Folch M. Laparoscopic approach for isolated splenic metastasis: comprehensive literature review and report of 6 cases. Surg Laparosc Endosc Percutan Tech. 2013;23:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Tajima T, Nagata J, Akiyama Y, Torigoe T, Fujimoto K, Sato N, Fujino Y, Shibao K, Matsuda S, Hirata K. Open colectomy vs. laparoscopic colectomy in Japan: a retrospective study using real-world data from the diagnosis procedure combination database. Surg Today. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Vega EA, Yamashita S, Shin CY, Kim M, Fleming JB, Katz MH, Raghav KP, Vauthey JN, Lee JE, Conrad C. Laparoscopic Partial Splenectomy for Unknown Primary Cancer: A Stepwise Approach. Ann Surg Oncol. 2017;24:1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Sultana A, Meng R, Piantadosi C, Brooke-Smith M, Chen J, Dolan P, Maddern G, Price T, Padbury R. Liver resection for colorectal cancer metastases: a comparison of outcomes over time in South Australia. HPB (Oxford). 2018;20:340-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Hasadia R, Kazarin O, Sofer O, Shulman K, Troitsa A, Alfici R, Ashkenazi I. Splenectomy for breast carcinoma diffusely metastatic to the spleen presenting as severe transfusion-dependent anaemia and thrombocytopaenia. BMJ Case Rep. 2018;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Rizzo F, Calamia S, Mingoia G, Fulfaro F, Grassi N, Cipolla C. Isolated Metachronous Splenic Metastasis from Colon Cancer: Possible Explanations for This Rare Entity. J Gastrointest Cancer. 2019;50:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Jain S, Munjal S, Yantiss RK, Sonoda T, Fahey TJ, Ruggiero JT, Anand A, Gersten A, Goldsmith SJ, Ocean AJ. Isolated splenic metastasis from rectal carcinoma: a rare occurrence. Case Rep Oncol. 2011;4:499-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Dias AR, Pinto RA, Ravanini JN, Lupinacci RM, Cecconello I, Ribeiro U. Isolated splenic metastasis from lung squamous cell carcinoma. World J Surg Oncol. 2012;10:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Zhao H, Zhong W, Chen D, Cheng X. Synchronous isolated splenic metastasis from cancer of hepatic flexure of colon: A case report. Medicine (Baltimore). 2019;98:e15016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Dunbar WH, Beahrs OH, Morlock CG. Solitary splenic metastasis incidental to rectal carcinoma: report of a case. Mayo Clin Proc. 1969;44:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Waller RM, Fajman WA. An unusual cause of an isolated, focal splenic defect demonstrated by liver-spleen scintigraphy. Clin Nucl Med. 1982;7:5-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Slavin JD, Mathews J, Spencer RP. Splenectomy for splenic metastasis from carcinoma of colon. Clin Nucl Med. 1986;11:491-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Capizzi PJ, Allen KB, Amerson JR, Skandalakis JE. Isolated splenic metastasis from rectal carcinoma. South Med J. 1992;85:1003-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Thomas SM, Fitzgerald JB, Pollock RE, Evans DB. Isolated splenic metastases from colon carcinoma. Eur J Surg Oncol. 1993;19:485-490. [PubMed] |
| 18. | Mainprize KS, Berry AR. Solitary splenic metastasis from colorectal carcinoma. Br J Surg. 1997;84:70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 19. | Indudhara R, Vogt D, Levin HS, Church J. Isolated splenic metastases from colon cancer. South Med J. 1997;90:633-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Ishida H, Konno K, Ishida J, Shirayama K, Naganuma H, Komatsuda T, Hamashima Y, Masamune O. Isolated splenic metastases. J Ultrasound Med. 1997;16:743-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Weathers BK, Modesto VL, Gordon D. Isolated splenic metastasis from colorectal carcinoma: report of a case and review of the literature. Dis Colon Rectum. 1999;42:1345-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Kim JC, Jeong CS, Kim HC, Yu CS, Kang GH, Lee MG. Isolated splenic metastasis from colorectal carcinoma: a case report. J Korean Med Sci. 2000;15:355-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Lee SS, Morgenstern L, Phillips EH, Hiatt JR, Margulies DR. Splenectomy for splenic metastases: a changing clinical spectrum. Am Surg. 2000;66:837-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 24. | Place RJ. Isolated colon cancer metastasis to the spleen. Am Surg. 2001;67:454-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Okuyama T, Oya M, Ishikawa H. Isolated splenic metastasis of sigmoid colon cancer: a case report. Jpn J Clin Oncol. 2001;31:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Cavallaro A, Modugno P, Specchia M, Pontenza AE, Loschiavo V, Colli R, Lauriola L, Barone C. Isolated splenic metastasis from colon cancer. J Exp Clin Cancer Res. 2004;23:143-146. [PubMed] |
| 27. | Avninder S, Bhatnagar A, Agrawal U, Saxena S. Isolated splenic metastasis from colorectal mucinous carcinoma. Int J Gastrointest Cancer. 2006;37:98-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Gencosmanoglu R, Aker F, Kir G, Tozun N. Isolated metachronous splenic metastasis from synchronous colon cancer. World J Surg Oncol. 2006;4:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Popović M, Barisić G, Krivokapić Z. Isolated splenic metastases of colorectal carcinoma--case report and review of literature. Acta Chir Iugosl. 2008;55:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Bigot P, Goodman C, Hamy A, Teyssedou C, Arnaud JP. Isolated splenic metastasis from colorectal cancer: report of a case. J Gastrointest Surg. 2008;12:981-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Gasent Blesa JM, de la Morena E, Laforga Canales JB, Vilaseca Martínez D, Vázquez C. Clinical case report and literature review: metachronous colorectal splenic metastases. Clin Transl Oncol. 2008;10:445-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Montemurro S, Maselli E, Ruggieri E, Caliandro C, Rucci A, Zito AF, Sciscio V. Isolated splenic metastasis from colon cancer. Report of a case. Tumori. 2008;94:422-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Sileri P, D'Ugo S, Benavoli D, Stolfi VM, Palmieri G, Mele A, Gaspari AL. Metachronous splenic metastasis from colonic carcinoma five years after surgery: a case report and literature review. South Med J. 2009;102:733-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Busić Z, Cupurdija K, Kolovrat M, Cavka V, Cavka M, Patrlj L, Servis D, Kvesić A. Isolated splenic metastasis from colon cancer--case report and literature review. Coll Antropol. 2010;34 Suppl 1:287-290. [PubMed] |
| 35. | Genç V, Akbari M, Karaca AS, Çakmak A, Ekıncı C, Gürel M. Why is isolated spleen metastasis a rare entity? Turk J Gastroenterol. 2010;21:452-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Dogan M, Ozal G, Ekinci C, Utkan G, Urun Y, Yalcin B, Icli F. Two cases with atypical metastasis in colorectal cancer: splenic and renal metastasis. Exp Oncol. 2010;32:277-279. [PubMed] |
| 37. | Pavlović M, Separović R, Vukelić-Marković M, Patrlj L, Kolovrat M, Kopljar M, Babić N, Kosuta D, Babić Z. Isolated splenic metastasis from colorectal carcinoma in a high risk patient: a case report. Coll Antropol. 2011;35:1307-1310. [PubMed] |