Published online Aug 6, 2020. doi: 10.12998/wjcc.v8.i15.3240
Peer-review started: March 25, 2020
First decision: April 22, 2020
Revised: May 1, 2020
Accepted: July 4, 2020
Article in press: July 4, 2020
Published online: August 6, 2020
Processing time: 133 Days and 21.6 Hours
Augmentation cystoplasty is indispensable in many pediatric diseases, especially neurogenic bladder. Various methods and materials are used to augment the bladder, and these methods are associated with different shortcomings and complications.
The present study reported the mid-term outcomes of patients undergoing various bladder augmentation procedures in a single institution, and assessed whether seromuscular cystoplasty lined with urothelium (SCLU) provided better urodynamic results than auto-augmentation (AA).
A retrospective review of 96 patients undergoing various augmentation methods between 2003 and 2018 was performed. The patients were divided into three groups according to the type of augmentation, and their outcomes were compared. All patients developed neurogenic bladder due to myelomeningocele or sacrococcygeal teratoma. The clinical data of all patients were collected.
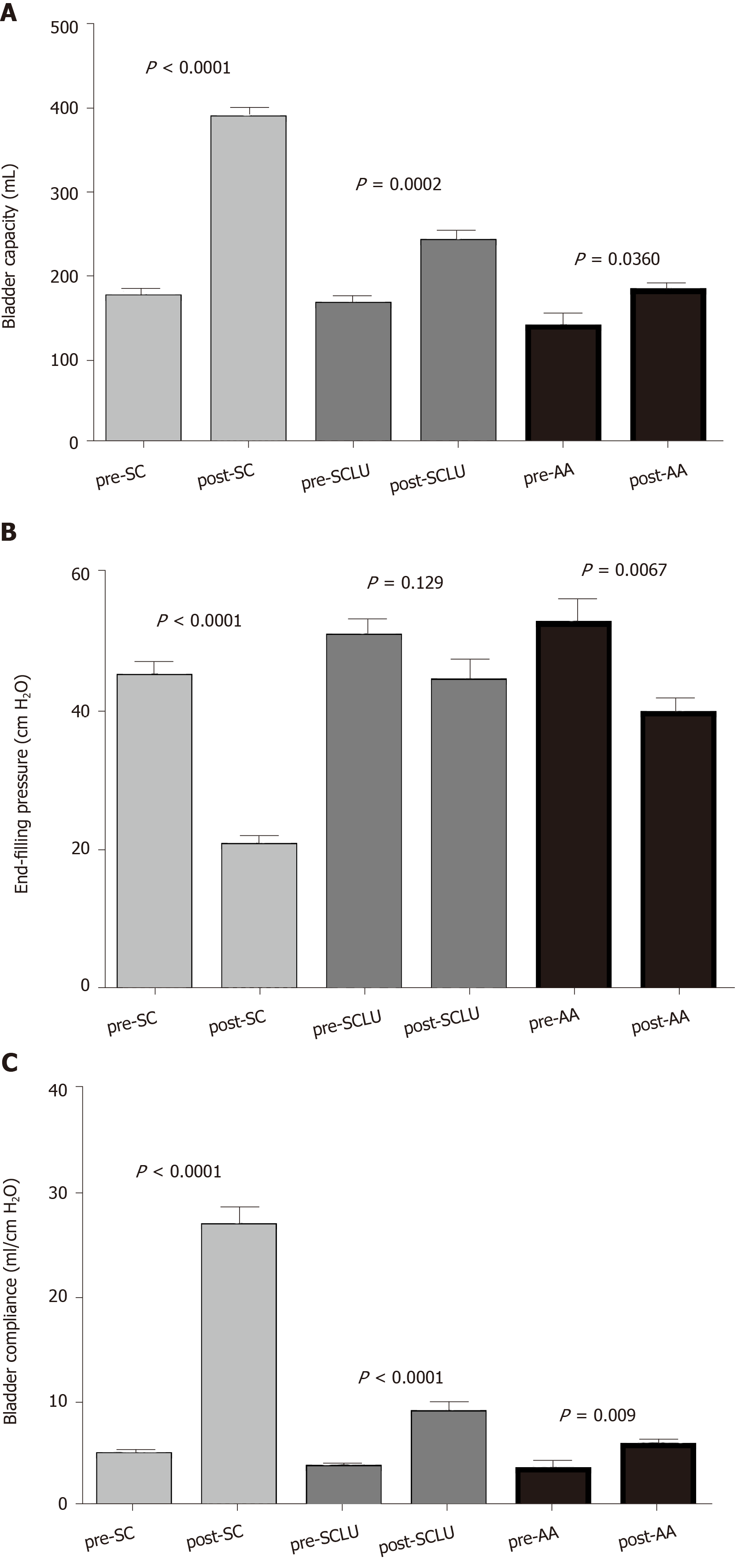
The mean ages at surgery in the three groups (standard cystoplasty [SC], SCLU, AA) were 10.8, 7.5, and 4.8 years, respectively, with mean follow-ups of 36, 61, and 36 mo, respectively. The mean preoperative and postoperative bladder capacities of the SC, SCLU, and AA groups were 174 ± 11.7 vs. 387 ± 13.7 (P < 0.0001), 165 ± 12.2 vs. 240 ± 14.7 (P = 0.0002), and 138 ± 16.7 vs. 181 ± 9.9 (P = 0.0360), respectively. Compared with the AA group, the SCLU procedure did not have better postoperative urodynamic parameters. Incontinence was reduced in most patients. The mean times of clean intermittent catheterization per day in the SC, SCLU, and AA groups were 5.6, 7.8, and 8.2, respectively. The main complications of the SC group were recurrent urinary tract infections (8%) and bladder calculi (6%). Re-augmentation was done in patients in the SCLU (8) and AA (3) groups.
SC provided sufficient bladder capacity and improved compliance with acceptable complications. After AA and SCLU, the patients acquired limited increases in bladder capacity and compliance with a high rate of re-augmentation. Compared with AA, SCLU did not yield better postoperative urodynamic parameters.
Core tip: We performed a retrospective review of the mid-term outcomes of patients undergoing different bladder augmentation procedures for neurogenic bladder in a single institution. We studied a total of 96 patients who underwent one of the following augmentation cystoplasty procedures: Standard cystoplasty (SC), auto-augmentation (AA), and seromuscular cystoplasty lined with urothelium (SCLU). We found that SC provided sufficient bladder capacity and improved compliance with acceptable complications. Bladder capacity and compliance improved with a high rate of re-augmentation after AA and SCLU. Compared with AA, SCLU did not have better postoperative urodynamic parameters.
- Citation: Sun XG, Wang RY, Xu JL, Li DG, Chen WX, Li JL, Wang J, Li AW. Surgical outcomes of bladder augmentation: A comparison of three different augmentation procedures. World J Clin Cases 2020; 8(15): 3240-3248
- URL: https://www.wjgnet.com/2307-8960/full/v8/i15/3240.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i15.3240
Augmentation cystoplasty (AC) increases bladder capacity, decreases bladder pressure, protects the upper urinary tract and enables the patient to acquire continence. Despite its complex procedure and numerous complications, AC is indispensable in many pediatric diseases, especially neurogenic bladder (NB). The primary indications for AC are low bladder capacity and poor compliance that are refractory to conservative treatment methods such as anticholinergic medication, botulinum toxin A injection, and clean intermittent catheterization (CIC).
Various methods and materials are used to augment the bladder. Ileocystoplasty and colocystoplasty are the current standard cystoplasty (SC) procedures because the ileum and colon are sufficiently long and provide satisfactory capacity. Other segments of the gastrointestinal tract are used, but these segments are associated with mucosa-related complications including metabolic complications, stone, infection, mucus production, cancer, growth retardation, and osteomalacia. To preclude the contact of urine with the gastrointestinal mucosa, alternative methods were investigated including urothelial augmentation, seromuscular bladder augmentation, auto-augmentation (AA), and seromuscular cystoplasty lined with urothelium (SCLU). The principal procedure of AA is detrusorectomy, which resects part of the detrusor muscle to create a bladder diverticulum. Detrusorectomy is performed first in SCLU, after which the bulging urothelium is covered with a de-epithelialized patch of bowel. Several reports[1,2] have demonstrated promising results with fewer complications after AA and SCLU. During SCLU, the bladder epithelium is covered with the intestinal seromuscular layer, which is the primary difference from AA. The present study assessed the outcomes of patients undergoing AA and SCLU and examined whether SCLU provided better urodynamic results than AA. We performed a retrospective analysis of patients undergoing SC, SCLU, and AA. We compared the outcomes of patients who received SCLU and AA with patients who received SC to evaluate the augmenting effect and compared the results of SCLU with AA to ascertain whether it was necessary to cover the seromuscular layer after detrusorectomy.
Informed consent was obtained from the patients, and the Institutional Review Board granted ethical approval. Overall, 96 patients underwent augmentation by multiple surgeons in a single institution between August 2003 and May 2018. The surgery indications were small bladder capacity, poor compliance, incontinence, and vesicoureteric reflux (VUR). SCLU was performed in the first 6 years between August 2003 and December 2008, and SC was performed between January 2009 and May 2018. Patients with a bladder capacity greater than 75% of the expected capacity underwent AA between July 2008 and July 2017. The patients’ clinical data including sex, age at surgery, primary diagnosis, follow-up duration, type of bladder augmentation, additional procedures, preoperative and postoperative urodynamic studies, urinary continence, and late complications were collected. The primary diagnosis was NB from myelomeningocele (90) or sacrococcygeal teratoma (6). The patients were divided into three groups according to the type of augmentation (SC, SCLU, and AA), and their medical records were compared.
The following surgical procedure was used for SC. The bladder was incised, and 20-cm intestinal (ileum or colon) segments were isolated and reconfigured. A whole layer patch was sutured with the bladder edge. For AA, the bladder detrusor was partially excised above the trigone, and the mucosal layer was bulged, as described by Hansen et al[1]. For SCLU, the bladder detrusor was removed, as in AA, and 30-cm intestinal (ileum or colon) segments were isolated. After de-epithelialization, the double intestine seromuscular layer was reconfigured to form a cup-patch, which was sutured with the bladder detrusor incisal edge and covered the bladder epithelium. Concomitant ureteral reimplantation was performed for patients with high grade (III-V) VUR, which was confirmed using a voiding cystourethrogram. A pyramidalis myofascial sling was performed for patients with a detrusor leak point pressure lower than 60 cm H2O and incontinence. A continent catheterizable channel (Mitrofanoff’s procedure) was created using the appendix if it was not convenient to perform CIC.
After surgery, a transurethral Foley catheter was left indwelling for 14 d, after which CIC was initiated. The catheter remained clamped in the SCLU and AA groups, and it was opened at intervals. A cutaneous suprapubic catheter also remained indwelling in the SC group for continuous drainage, and both of catheters were kept open. Catheterization was prolonged if urine extravasation occurred. Anticholinergic medications, including tolterodine and solifenacin, were prescribed to patients with postoperative end filling pressures higher than 20 cm H2O.
Few patients achieved complete continence in our study. Relative continence or dryness was defined as occasional leakage with one pad per day at most, and life quality was not disturbed. Most patients acquired relative dryness after surgery, and the primary influencing factor was the frequency and interval of CIC. We recorded and compared the mean times of CIC per day without micturition to determine continence in the three groups. The real routine times of CIC per day were smaller because numerous patients relied on micturition if the residual urine was minimal or CIC was not convenient. Generally, fewer CIC times indicated bigger bladder capacity. Recurrent urinary tract infections (UTIs) were defined as more than three incidents of symptomatic bacteriuria. Expected bladder capacity for age in mL = (age in years + 1) × 30.
Statistical analyses were performed using the t-test with statistical significance considered at P < 0.05.
The characteristics and additional procedures of all three groups are shown in Table 1. Patients in the SCLU and AA groups underwent extravesical ureteric reimplantation because their bladders were not incised, and patients in the SC group underwent intravesical ureteric reimplantation.
| Patients, n | Sex, male/female | Age at surgery in yr | Follow-up duration in mo | Ureteral reimplantation | Pyramidalis myofascial sling | Continent catheterizable channel | |
| SC | 46 | 21/25 | 10.8 | 36 | 32 | 16 | 19 |
| SCLU | 37 | 22/15 | 7.5 | 61 | 14 | 35 | 0 |
| AA | 13 | 5/8 | 4.8 | 36 | 11 | 0 | 0 |
The urodynamic parameters of all three groups are shown in Table 2. Comparisons of parameters before and after surgery in the three groups are shown in Figure 1A-C. There were no differences in the mean preoperative bladder capacity, compliance, or end-filling intravesical pressure between the SCLU and SC groups. Significant differences were found in the mean postoperative bladder capacity, compliance, and end-filling intravesical pressure. A significant difference (P = 0.027) was seen in the postoperative capacity between the SCLU and AA groups. The other parameters, including postoperative compliance and end filling pressure, were similar (P > 0.05).
| SC | SCLU | AA | |
| Preoperative capacity in mL | 174 ± 11.7 | 165 ± 12.2 | 138 ± 16.7 |
| Postoperative capacity in mL | 387 ± 13.7a | 240 ± 14.7 | 181 ± 9.9a |
| Preoperative compliance in mL/cm H2O | 4.8 ± 0.6 | 3.6 ± 0.2 | 3.4 ± 0.7 |
| Postoperative compliance in mL/cm H2O | 26.7 ± 1.9a | 8.8 ± 1.1 | 5.7 ± 0.5 |
| Preoperative end-filling pressure in cm H2O | 44.9 ± 2.4 | 50.6 ± 2.6 | 52.5 ± 3.7 |
| Postoperative end-filling pressure in cm H2O | 20.7 ± 1.6a | 44.2 ± 3.2 | 39.6 ± 2.3 |
Incontinence was reduced in most patients after surgery. The mean times of CIC per day in the SC, SCLU, and AA groups were 5.6, 7.8, and 8.2, respectively, and a significant difference was seen between the SC and SCLU groups. The numbers of patients receiving anticholinergic therapy were 8, 25, and 10 in the SC, AA, and SCLU groups, respectively.
The primary late complications were recurrent UTIs and bladder calculi, as shown in Table 3. The numbers of patients with recurrent UTIs in the SC and SCLU groups were 4 and 2, respectively. The UTIs resolved with continuous drainage and antibiotics. Bladder calculi occurred in 3 patients from the SC group. The calculi were removed using holmium laser lithotrity, and 1 patient had a repeat treatment. Re-augmentation was performed in 22% (8/37) and 23% (3/13) of patients in the SCLU and AA groups, respectively. The procedures were performed due to low bladder capacity, frequent CIC, or upper tract deterioration. All patients selected the SC procedure. Shrinkage of the epithelium and fibrosis tissue were found during the repeat surgery.
| Total patients, n | Recurrent UTIs | Bladder calculi | Re-do augmentation | |
| SC | 46 | 4 (8) | 3 (6) | 0 |
| SCLU | 37 | 2 (5) | 0 | 8 (22) |
| AC | 13 | 0 | 0 | 3 (23) |
Cystoplasty is a complicated surgery associated with various complications. However, cystoplasty may be the only choice when conservative treatment is ineffective. Ileocystoplasty and colocystoplasty are the standard and most frequently performed surgeries. These procedures produce mucosa-related complications. Therefore, replacement procedures were actively pursued. AA provides acceptable bladder capacity and compliance with few complications[1]. SCLU, which is the most widely performed surgery after SC, effectively augments bladder capacity and has minimal complications[2].
A low bladder capacity is the primary indication for augmentation. Therefore, bladder capacity is the most important parameter to evaluate the effectiveness of the procedure. A low-capacity bladder is rapidly filled with urine, which leads to frequent CIC and high pressure. Patients find it difficult to regularly comply with undergoing CIC, which often leads to incontinence and upper urinary tract injury. Bladder capacity in the SC group in our study increased greatly after surgery, but a continuous mucus discharge in the urine was detected, which may lead to bacteria in urine. This discharge perplexed patients and physicians, and regular irrigation was suggested. Compliance, end filling pressure, and continence also improved substantially. These results are consistent with the findings of other studies[3,4].
The AA procedure was performed extraperitoneally in our study. Therefore, it was simpler than SC and did not result in the complications associated with bowel integration. Following AA, the bladder muscle did not contract toward the center, and bladder compliance and pressure were improved. However, bladder capacity may not increase effectively. Hansen et al[1] reported that bladder capacity and compliance after AA exhibited a transient decrease followed by a long-term gradual increase. They highlighted the vital role of an extensive incision of the detrusor and early active bladder cycling. Gurocak et al[5] reported that improvement in compliance after AA was not obvious and durable and suggested that patient selection was the crucial step of successful AA. Cartwright suggested AA for patients with greater than 75% of the predicted capacity[6]. Our data showed that bladder capacity, compliance, and pressure in the AA and SCLU groups improved to some degree, but these improvements were less significant than the changes in the SC group. Because the blood supply of the left mucosa may be damaged, the detrusor was not excised as extensively in our series as reported by Hansen et al[1] Compared to the SC and SCLU groups, patients in the AA group were younger, had larger preoperative capacity relative to their age, and were not resistant to major trauma. Also, the simple procedures did not preclude future SC or other surgeries.
The postoperative capacity in the SCLU group was larger than the AA group, but the follow-up duration in the SCLU group was double the AA group. Because the capacity increases with time, the difference in the bladder capacity of the SCLU and AA groups is not very significant. The capacity of the SCLU and AA groups did not increase to the expected capacity for age, and they were both much lower than the SC group. The other urodynamic parameters were similar between the SCLU and AA groups. Compared with AA, the SCLU procedure did not improve the urodynamic parameters. The bladder seromuscular layer was partially resected, but the compliance of the remaining mucosa remained lower compared to the whole layer intestine, which may gradually dilate greatly with low pressure. After SCLU, the seromuscular layer with limited elasticity may restrict the bulging of the mucosa, especially when the intestinal seromuscular layer shrinks for various reasons, such as ischemia.
Several reports of SCLU have shown that satisfactory bladder capacity is acquired[7-10], but patients with SCLU are at higher risk of repeat augmentation compared to SC[11]. The bladder pressure in these reports was maintained at 20 to 30 cm H2O for 5 to 10 d by placing the drainage bag above the level of the symphysis pubis, and the bladder was kept distended to facilitate coaptation of the urothelium and the seromuscular layer. Gonzalez et al[11] suggested that early postoperative bladder distention was critical, and failure to achieve bladder distention led to unsuccessful augmentation. Late postoperative bladder distention, which meant high outlet resistance, was also necessary, and patients with a leak point pressure less than 40 cm H2O required an outlet procedure. Jung et al[12] considered that constant high bladder outlet pressure was a prerequisite for successful surgical outcome and recommended simultaneous anti-incontinence surgery for patients with a leak point pressure less than 60 cmH2O. Immediate postoperative high bladder pressure may not be acquired in our series with the intermittent occlusion of the catheter in the SCLU and AA groups, but no urine accumulated between the urothelium and the seromuscular layer. A pyramidalis myofascial sling to increase the bladder outlet pressure was performed in most patients undergoing SCLU, which was much more often than patients with SC, but the capacity was smaller. The reason for failing to achieve sufficient capacity was not confirmed.
As a subjective parameter, incontinence is affected by many factors and it is hard to define. With the help of augmentation, bladder outlet procedure, pharmacotherapy, CIC, and regular management, most patients acquired relative continence. We introduced the frequency of CIC to evaluate the effect of surgery. The frequency of CIC and number of patients receiving pharmacotherapy in the AA and SCLU groups were much greater than the SC group, and these results were consistent with their bladder capacity and compliance. Frequent CIC and the side effects of pharmacotherapy made it difficult for patients to comply and may disturb their normal life quality, which led to repeat surgery.
Most patients experienced asymptomatic bacteriuria during CIC, but recurrent UTIs occurred only in 8% (4/46) of patients in the SC group and 5% (2/37) of patients in the SCLU group. Bladder irrigation one time per week was requested, and antibiotic prophylaxis was not suggested. Bladder calculi developed in 6% of patients in the SC group, and no patients had calculi in the SCLU or AA group. Re-do augmentation was performed in 8 (22%) and 3 (23%) patients from the SCLU and AA groups, respectively. Marte et al[13] reported that 5 of 11 patients with AA underwent repeat augmentation for high-grade VUR or incontinence and did not suggest the routine use of AA. Gonzalez et al[11] reported 3 of 20 cases with SCLU who required re-augmentation, which was close to the rate with SC. Another report[9] showed a similar rate of repeat augmentation. The higher rate of repeat augmentation in our series was consistent with the results of unsatisfactory postoperative bladder capacity in the SCLU and AA groups. Most patients in the SCLU group underwent a bladder outlet procedure to increase postoperative bladder pressure. The only difference from other series was the manner of increasing bladder pressure 2 wk after surgery. No definite reason was found, and the unfavorable results impeded our use of SCLU.
Our study has some limitations. This study was a retrospective research study, and multiple surgeons performed the surgeries during different periods. Concomitant urological surgeries performed in the three groups were different, which may influence their outcomes. The number and age of patients in the AA group was lower than the SC and SCLU groups, but the difference in preoperative bladder capacity between the three groups was not significant. The SCLU procedure was similar to AA, but no reports comparing their outcomes were found. Our series showed that the differences in their urodynamic parameters were not very significant. Further research is needed to confirm the role of the seromuscular layer that covered the bladder epithelium.
The material for bladder augmentation should be elastic tissue with a sufficient blood supply, and its surface should be able to resist the erosive effects of urine. Cervellione observed a significant reduction in microcirculation after mucosectomy, which may result in contraction[14]. The blood supply for the bladder urothelium without detrusor and the de-epithelialized intestinal segment was damaged, and these tissues were less elastic and at a higher risk of ischemic contraction. Separation of the muscular and epithelial layers is a complicated process, and ensuring a sufficient blood supply is extremely difficult,especially when the detrusor was extensively resected. Various biomaterials and tissue-engineered bladders were investigated with non-satisfactory outcomes[15]. Autologous tissue remains the best option for bladder augmentation.
In conclusion, our study compared the mid-term outcomes of patients who underwent different augmentation procedures. SC provided sufficient bladder capacity and improved compliance with an acceptable level of complications. After AA and SCLU, patients acquired limited increases in bladder capacity and compliance with a high rate of re-augmentation. AA may be temporally used in select patients to alleviate deterioration of the upper urinary tract. Compared with AA, SCLU did not yield better postoperative urodynamic parameters, which means that SCLU provided little benefit from the seromuscular layer covering the epithelium. The surgical techniques and postoperative management may require further adjustment, and longer follow-ups are needed.
Augmentation cystoplasty increases bladder capacity, decreases bladder pressure, protects the upper urinary tract and enables the patient to acquire continence. Various segments of the gastrointestinal tract are used, but these segments are associated with mucosa-related complications. To preclude the contact of urine with the gastrointestinal mucosa, alternative methods were investigated, including urothelial augmentation, seromuscular bladder augmentation, auto-augmentation (AA), and seromuscular cystoplasty lined with urothelium (SCLU). The present study compared the outcomes of three different augmentation procedures. More data of the characteristics and indications of these procedures were reported, which may assist the selection of a particular procedure.
The principal procedure of AA is detrusorectomy, which resects part of the detrusor muscle to create a bladder diverticulum. For SCLU, detrusorectomy is performed first, after which the bulging urothelium is covered with a de-epithelialized patch of bowel. The two procedures were similar, but no reports compared their outcomes. Details about surgical techniques, outcomes, and complications are discussed.
The present study assessed the outcomes of patients undergoing AA and SCLU and determined whether SCLU provided better urodynamic results than AA. We compared the outcomes of patients who received SCLU and AA with patients who received standard cystoplasty (SC) to evaluate any augmenting effects and compared the results of SCLU with AA to ascertain the necessity of covering the seromuscular layer after detrusorectomy.
We performed a retrospective analysis of patients undergoing SC, SCLU, and AA.
SC provided sufficient bladder capacity and improved compliance with an acceptable level of complications. After AA and SCLU, patients acquired limited increases in bladder capacity and compliance with a high rate of re-augmentation. AA may be temporally used in select patients to alleviate deterioration of the upper urinary tract. Compared with AA, SCLU did not yield better postoperative urodynamic parameters, which means that SCLU provided little benefit from the seromuscular layer covering the epithelium. The surgical techniques and postoperative management may require further adjustment, and longer follow-ups are needed.
The bladder capacity and compliance of patients after AA and SCLU were not satisfactory. Compared with AA, SCLU is a more complicated procedure and did not yield better postoperative urodynamic parameters. Cautious selection of the two procedures is suggested.
Autologous tissue remains the best option for bladder augmentation. All of the methods and materials have shortcomings. The surgical techniques and postoperative management require further adjustment. Biomaterials and tissue-engineered bladders are promising prospects.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Caca K, Hashash JG, Kawai HF S-Editor: Wang JL L-Editor: Filipodia E-Editor: Wang LL
| 1. | Hansen EL, Hvistendahl GM, Rawashdeh YF, Olsen LH. Promising long-term outcome of bladder autoaugmentation in children with neurogenic bladder dysfunction. J Urol. 2013;190:1869-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Jednak R, Schimke CM, Barroso U, Barthold JS, González R. Further experience with seromuscular colocystoplasty lined with urothelium. J Urol. 2000;164:2045-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Zaragoza Torres RI, Galarza-Flores ME, Gómez-Castellanos JC, Barrera-de León JC. [Urodynamic changes after bladder augmentation surgery in paediatric patients with myelomeningocele due to neurogenic bladder]. Cir Cir. 2016;84:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Hayashi Y, Yamataka A, Kaneyama K, Kato Y, Lane GJ, Miyano T. Review of 86 patients with myelodysplasia and neurogenic bladder who underwent sigmoidocolocystoplasty and were followed more than 10 years. J Urol. 2006;176:1806-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Gurocak S, De Gier RP, Feitz W. Bladder augmentation without integration of intact bowel segments: critical review and future perspectives. J Urol. 2007;177:839-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Cartwright PC. Bladder autoaugmentation (partial detrusor myectomy)--where does it stand after 2 decades? J Urol. 2013;190:1643-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Gonzalez R, Buson H, Reid C, Reinberg Y. Seromuscular colocystoplasty lined with urothelium: experience with 16 patients. Urology. 1995;45:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Bandi G, Al-Omar O, McLorie GA. Comparison of traditional enterocystoplasty and seromuscular colocystoplasty lined with urothelium. J Pediatr Urol. 2007;3:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Shekarriz B, Upadhyay J, Demirbilek S, Barthold JS, González R. Surgical complications of bladder augmentation: comparison between various enterocystoplasties in 133 patients. Urology. 2000;55:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Dayanç M, Kilciler M, Tan O, Gökalp A, Göktaş S, Peker AF. A new approach to bladder augmentation in children: seromuscular enterocystoplasty. BJU Int. 1999;84:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | González R, Ludwikowski B, Horst M. Determinants of success and failure of seromuscular colocystoplasty lined with urothelium. J Urol. 2009;182:1781-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Jung HJ, Lee H, Im YJ, Lee YS, Hong CH, Han SW. Prerequisite for successful surgical outcome in urothelium lined seromuscular colocystoplasty. J Urol. 2012;187:1416-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Marte A, Di Meglio D, Cotrufo AM, Di Iorio G, De Pasquale M, Vessella A. A long-term follow-up of autoaugmentation in myelodysplastic children. BJU Int. 2002;89:928-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Cervellione RM, Hajnal D, Varga G, Rakoczy G, Kaszaki J, Keene D, Goyal A, Dickson A, Cserni T. Mucosectomy impairs ileal microcirculation and results in flap contraction after experimental ileocystoplasty. J Pediatr Urol. 2017;13:81.e1-81.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Chua ME, Farhat WA, Ming JM, McCammon KA. Review of clinical experience on biomaterials and tissue engineering of urinary bladder. World J Urol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |