Published online Jul 26, 2020. doi: 10.12998/wjcc.v8.i14.3090
Peer-review started: February 19, 2020
First decision: May 26, 2020
Revised: June 5, 2020
Accepted: June 25, 2020
Article in press: June 25, 2020
Published online: July 26, 2020
Processing time: 156 Days and 5.4 Hours
Mucoepidermoid carcinoma is the most common primary epithelial salivary gland malignancy. It mostly occurs in the major or intraoral minor salivary glands but rarely in the infratemporal fossa. Here, we present a case of aggressive mucoepidermoid carcinoma in the infratemporal fossa with neck lymph node metastasis and also discuss diagnostic and treatment strategies.
A 39-year-old woman with a mass located in the right submandibular area presented to our department. Physical examination revealed lymphadenopathy on the right submandibular side measuring 2.5 cm × 3 cm that was hard and had poor mobility. Results of nasal endoscopy were unremarkable. Ultrasound examination revealed an enlarged lymph node at level II of the right side. Fine needle aspiration cytology of the metastatic lymph node revealed malignant cells with infection. Contrast-enhanced computed tomography revealed an enhancing ill-defined soft tissue mass in the right infratemporal region. Positron emission tomography/computed tomography revealed hyperintensity in the right infratemporal fossa along with lymphadenopathy at level II of the right-side lymph node. The patient underwent extended resection of the primary tumor, and ipsilateral radical neck dissection was also completed. Hematoxylin-eosin staining and immunohistochemistry revealed a high-grade mucoepidermoid carcinoma. No signs and symptoms of recurrence of the neoplasm were present after 20 mo of follow-up.
Positron emission tomography/computed tomography play a key role in primary tumor localization. Furthermore, histopathology and immunohistochemistry play pivotal roles in disease diagnosis.
Core tip: Here, we report a patient with mucoepidermoid carcinoma in the infratemporal fossa. Because of the particular location and atypical oral presentation, we also review corresponding literature to discuss the diagnostic and treatment strategies for mucoepidermoid carcinoma.
- Citation: Zhang HY, Yang HY. Mucoepidermoid carcinoma in the infratemporal fossa: A case report. World J Clin Cases 2020; 8(14): 3090-3096
- URL: https://www.wjgnet.com/2307-8960/full/v8/i14/3090.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i14.3090
Mucoepidermoid carcinoma (MEC) is one of the most common malignant salivary gland tumors and usually affects the parotid and minor salivary glands[1]. MEC accounts for < 5% of all head and neck malignancies[2]. Histologically, MEC comprises a variable percentage of epidermoid, mucous, and intermediate cells. According to the proportion of the three types of cells and cell differentiation degree, MEC has been classified as low-, intermediate-, and high-grade. High-grade tumors are highly aggressive, and regional lymph node spread is common. Furthermore, the low-grade variant is defined by a lack of aggressive invasion pattern and usually has a more benign nature.
MEC was first reported in 1945 by Stewart et al[3]. MECs also occur in other organs such as the larynx, mandible, breast, and thymus[4-7], and they tend to occur in the fourth and fifth decades of life and have a female preponderance[8,9]. MEC typically presents as a major salivary gland or intraoral mass. However, the effect of tumors in the infratemporal region on the oral cavity was not obvious. Comprehensive head and neck examinations and advanced imaging techniques such as positron emission tomography/computed tomography (CT) can facilitate the identification of the primary site. Thus, we present an unusual case of high-grade MEC in the infratemporal fossa of a woman and also discuss diagnostic and treatment strategies for MEC.
A 39-year-old woman presented with a 3-mo history of a mass located in the right submandibular area.
The mass in the right submandibular lymph node had rapidly grown over the past 2 wk. Furthermore, it was accompanied by radiating pain in the right maxillary sinus region. The symptoms of the patient were not relieved despite taking anti-inflammatory drugs for 10 d. The results of previous examinations were as follows: Ultrasound examination revealed an enlarged lymph node at level II of the right-side lymph node; nasal endoscopy results were unremarkable; and fine needle aspiration cytology of the metastatic lymph node demonstrated malignant cells with infection but with no obvious structure to determine the tissue source.
She had a history of chronic hepatitis for 12 years.
The patient’s medical history and family history were unremarkable.
Physical examination revealed a 2.5 cm × 3 cm-sized lymphadenopathy of the right submandibular lymph nodes, and the mass was tender, hard, and had poor mobility.
Laboratory tests showed the following results: Urine analysis of blood: BLD (+-); Percentage of monocytes: 10.8%; liver and kidney function normal; hepatitis B surface antigen and hepatitis B core antibody were positive.
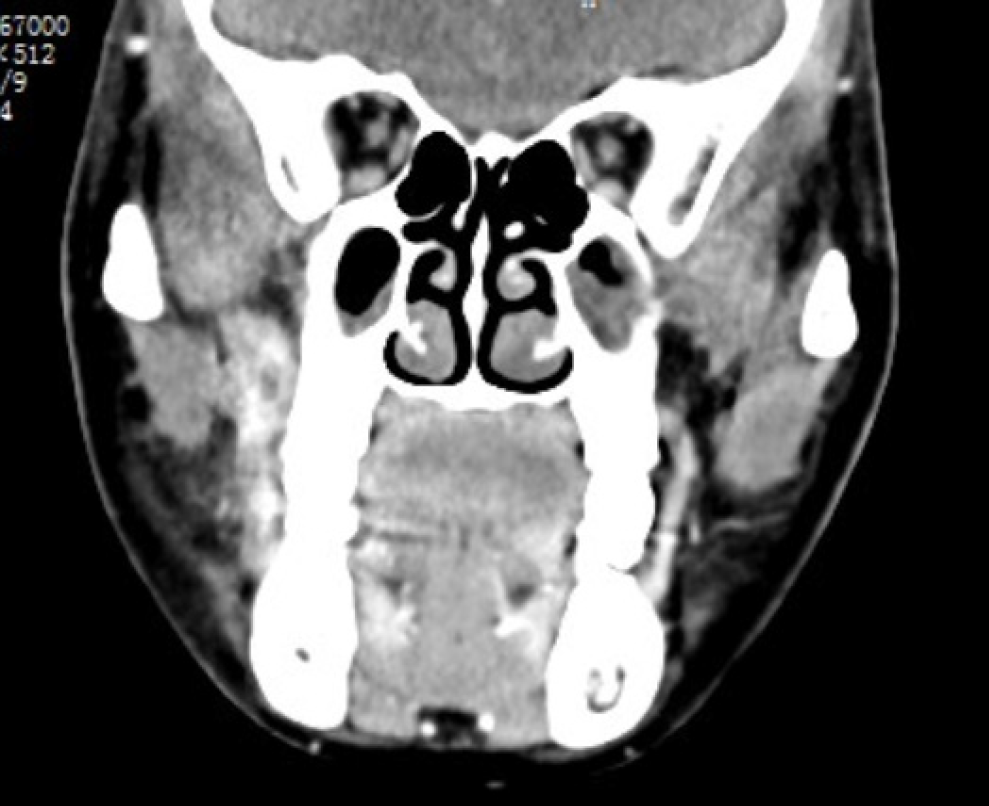
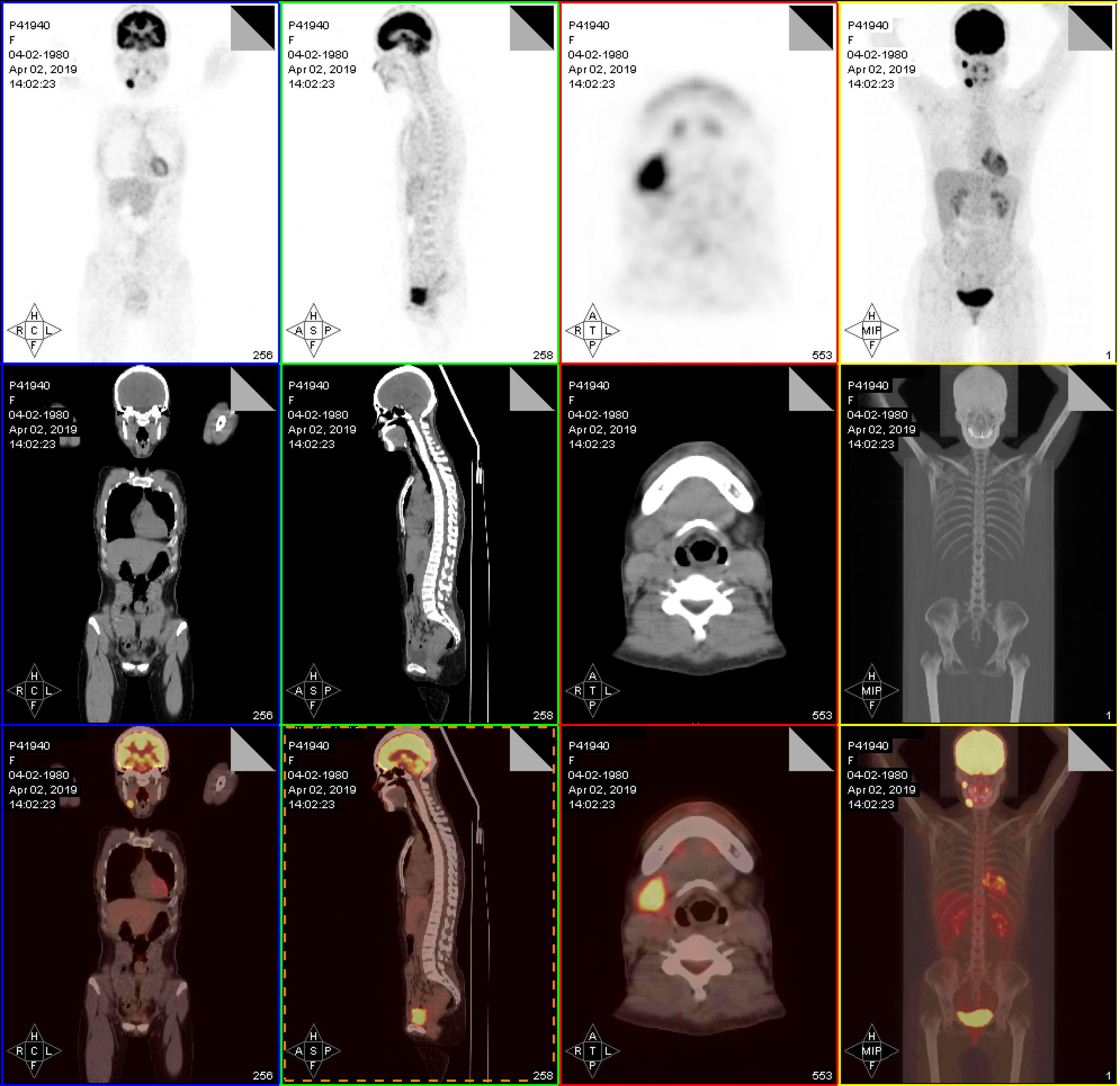
Contrast-enhanced CT revealed an enhancing ill-defined soft tissue mass in the right infratemporal region (Figure 1). Positron emission tomography/CT revealed an increased uptake of fluorodeoxyglucose in the right infratemporal fossa and at level II of the right-side lymph node (Figure 2).
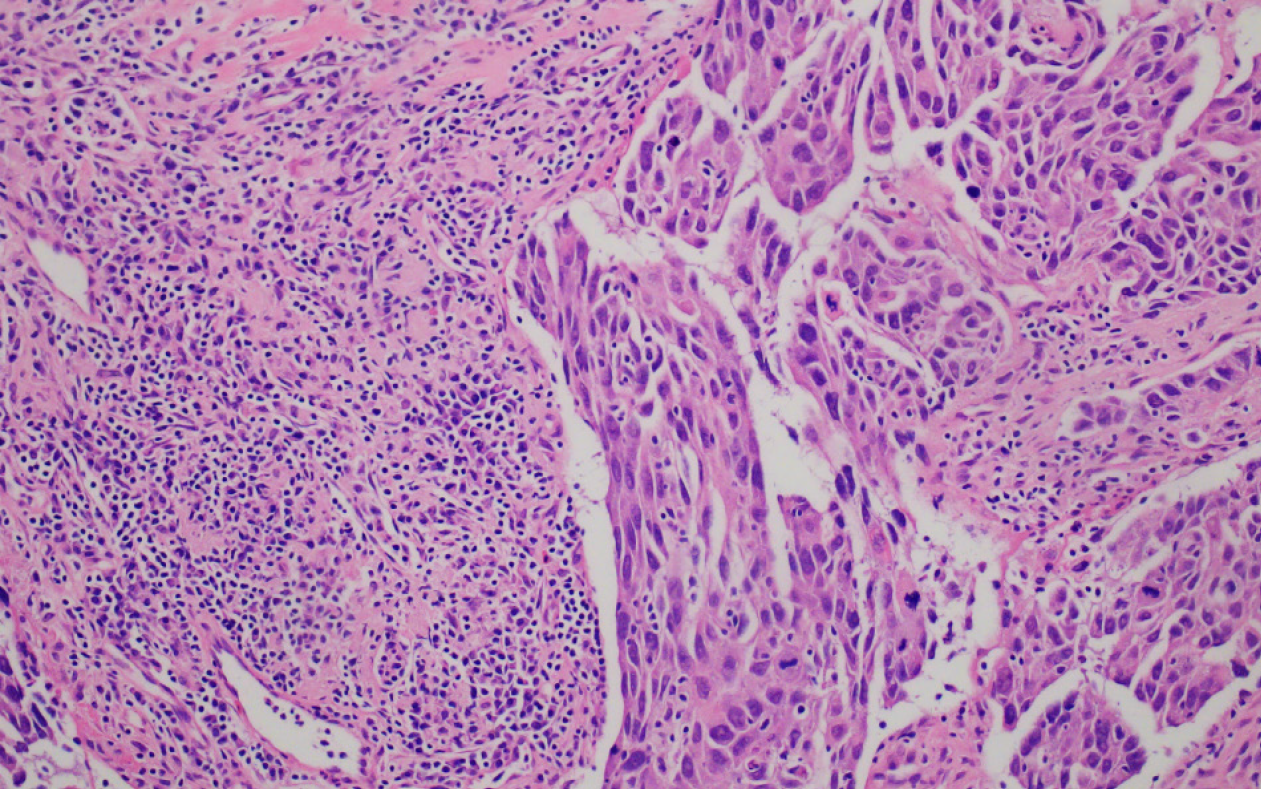
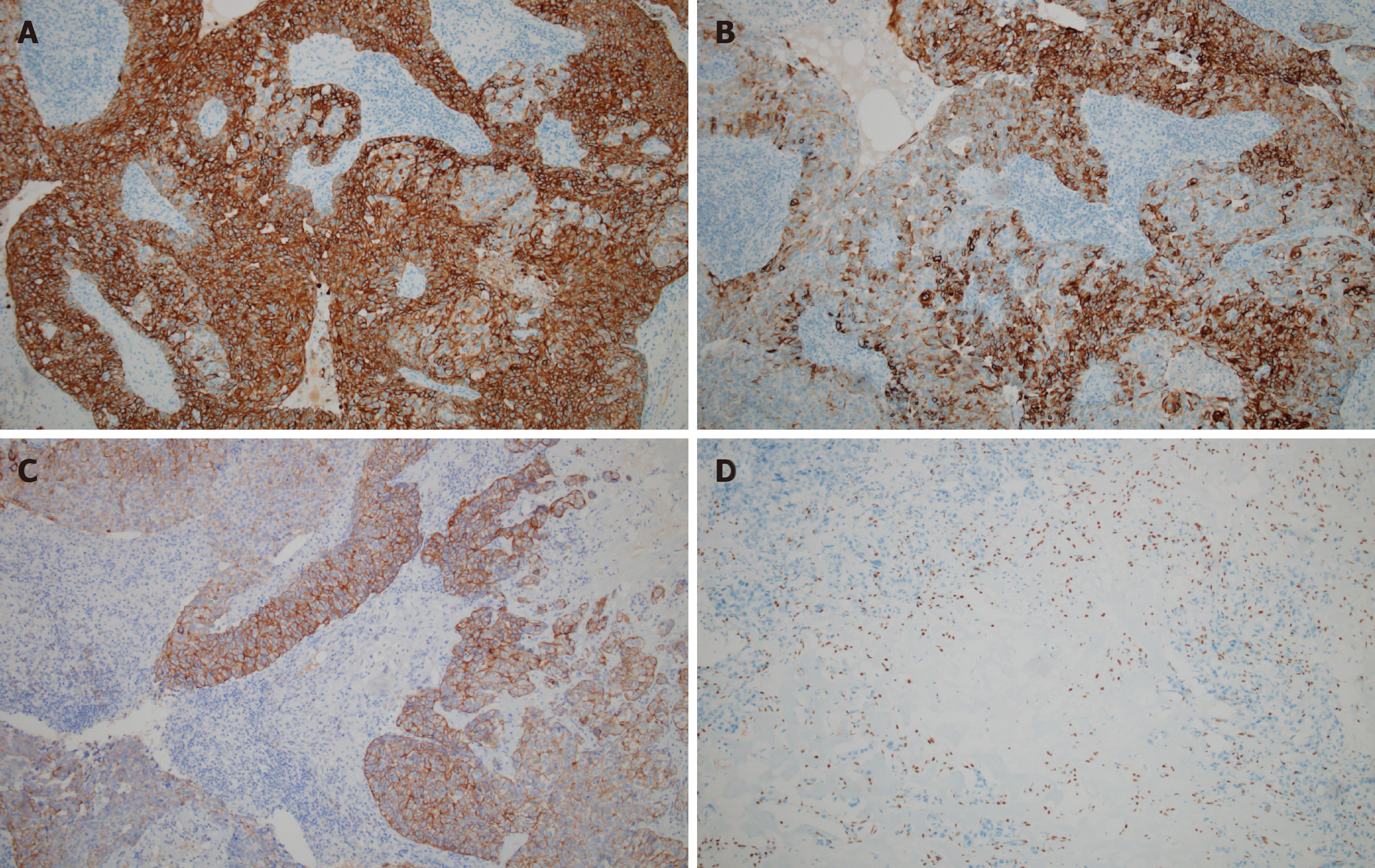
Hematoxylin-eosin staining of the specimen revealed that the tumor majorly comprised two types of lesional cells: Epidermoid and intermediate cells (Figure 3). In the tumor stroma, nests of squamous cell infiltration, cell heteromorphism, and mitotic figures were obvious, and focal necrosis was also observed, which was indicative of a high-grade MEC. Immunohistochemistry was performed for further pathological diagnosis, and the result revealed cytokeratin 7 (+), cytokeratin 5/6 (partial, +), cytokeratin 18 (partial, +), P40 (partial, +), and negative for estrogen and androgen receptors and human epidermal growth factor receptor 2/neu protein (Figure 4).
Based on radiographic results and histopathology, we finally confirmed the diagnosis of a high-grade MEC with lymph node metastasis. The final clinicopathologic findings were those of a high stage (T2N2M0).
Under general anesthesia, the primary tumor was extensively resected via approach from the infratemporal fossa, and then ipsilateral radical neck dissection, facial nerve dissection, and arbitrary flap formation were performed. The tumor was completely resected. Intraoperative frozen pathology suggested that the tumor originated from the epithelium. A drainage tube was placed in the mouth and neck area. No apparent surgical complications occurred after surgery, and the patient was discharged 15 d after surgery.
The patient underwent radiotherapy and regular follow up. There were no signs and symptoms of recurrence of neoplasm from the past 20 mo since the surgery.
The infratemporal fossa is an irregular space in the skull base, with the anterior boundary on the posterior surface of the maxilla, posteriorly by deep lobe of the parotid gland, laterally by ascending ramus of mandible and descending lamina sphenoid bone, and superiorly by external rhytidectomy infratemporal surface of greater wing of sphenoid and squamous part of temporal bone. Common primary tumors in the infratemporal fossa are fibrosarcoma, hemangioma, pleomorphic adenoma from ectopic salivary tissue, or neurogenic tumors[10]. The incidence of MEC in this location is extremely rare.
MEC accounts for approximately 30% of all salivary gland malignancies, and it is the most common malignant tumor of the parotid gland[11,12]. The histologic grade of MEC has prognostic value and directs adjuvant therapy[13]. The grade of MEC is determined based on the relative proportion of three types of cells and grades of differentiation. The low-grade type is characterized by > 50% mucinous cells and epidermoid cells, whereas the high-grade type is characterized by a predominance of epidermoid and intermediate cells with < 10% mucinous cells[14]. Intermediate-grade type has characteristics that are between the above two types. Because of the existence of epidermoid cells, MEC is often confused with squamous cell carcinoma, and mucicarmine staining is used to differentiate between these two types of tumors.
Intermediate- and high-grade tumors are associated with high potential risks of metastasis. Neck node metastases usually indicate a worse prognosis[15]. In this case, fine needle aspiration cytology from the neck node determined the nature of the malignancy. Localization of the primary site and accurate pathological diagnosis are particularly important for treating patients with MEC. However, because of the multiple structures that are present within the infratemporal fossa and concealed location, early diagnosis is difficult owing to the lack of atypical symptoms. Furthermore, the diagnosis of a tumor in the infratemporal fossa can be complicated by similar clinical features such as trigeminal neuralgia and temporomandibular arthropathy. In our case, because of atypical oral manifestations, it was necessary to perform a complete oncologic workup to exclude the possibility of secondary metastasis before treating the lesion as MEC in the infratemporal fossa. Positron emission tomography/CT helped determine the location of the primary tumor, and hematoxylin-eosin staining and immunohistochemical analysis confirmed the final diagnosis.
MEC is a malignancy in which histological grading and clinical behavior correlate well[16]. Ozawa et al[17] analyzed 43 patients with head and neck MECs and concluded that T and N stages are significant prognostic factors for MECs. Treatment is largely based on histological tumor grading, and surgical resection is the main treatment for all grades of MEC. Neck dissection is indicated when clinical evidence of regional metastasis, high TNM stage, or high histological grade is noted[18,19]. Moreover, surgical tumor resection is considered sufficient treatment for low-grade MEC. High-grade tumors are generally treated with surgical excision with wide margins or neck dissection followed by postoperative radiotherapy[13]. Furthermore, Wu et al[20] reported that postoperative adjuvant 125I seed brachytherapy appears to be an effective and safe treatment option for MEC of the parotid gland with a clinically node-negative neck, especially when the tumor is of low or intermediate grade.
Low-grade tumors have a more benign course with a 5-year overall survival rate of approximately 90%. In contrast, high-grade tumors are much more likely to recur and have a 5-year overall survival rate of approximately 50%[21]. In our case, the patient developed neck lymph node metastasis before surgery. At 6 wk after surgery, the patient received 30 courses of radiotherapy with a total radiation dose of 60 Gy.
The incidence of MEC in the infratemporal fossa is extremely low, and the clinical symptoms are atypical. Our case findings emphasize the importance of oncologic workup to determine the primary tumor location and ensure accurate histopathology and postoperative radiotherapy. For such rare tumor sites, it is important for oral and maxillofacial surgeons to review the clinical presentation, histology, and management of MECs.
The authors thank Ms. Liu ML for her support of the study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dereci O S-Editor: Gong ZM L-Editor: Filipodia E-Editor: Wang LL
| 1. | Pires FR, Chen SY, da Cruz Perez DE, de Almeida OP, Kowalski LP. Cytokeratin expression in central mucoepidermoid carcinoma and glandular odontogenic cyst. Oral Oncol. 2004;40:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Devaraju R, Gantala R, Aitha H, Gotoor SG. Mucoepidermoid carcinoma. BMJ Case Rep. 2014;2014:bcr-2013-202776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Stewart FW, Foote FW, Becker WF. Muco-Epidermoid Tumors of Salivary Glands. Ann Surg. 1945;122:820-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 339] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 4. | Prgomet D, Bilić M, Bumber Z, Manojlović S, Katić V. Mucoepidermoid carcinoma of the larynx: report of three cases. J Laryngol Otol. 2003;117:998-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Li X, Wang F, Wang Y, Sun S, Yang H. An unusual case of intraosseous mucoepidermoid carcinoma of the mandible: A case report and literature review. Medicine (Baltimore). 2018;97:e13691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Cheng M, Geng C, Tang T, Song Z. Mucoepidermoid carcinoma of the breast: Four case reports and review of the literature. Medicine (Baltimore). 2017;96:e9385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Woo WL, Panagiotopoulos N, Gvinianidze L, Proctor I, Lawrence D. Primary mucoepidermoid carcinoma of the thymus presenting with myasthenia gravis. J Thorac Dis. 2014;6:E223-E225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Jarvis SJ, Giangrande V, Brennan PA. Mucoepidermoid carcinoma of the tonsil: a very rare presentation. Acta Otorhinolaryngol Ital. 2013;33:286-288. [PubMed] |
| 9. | Noda T, Higashiyama M, Oda K, Higaki N, Takami K, Okami J, Kodama K, Kuriyama K, Tsukamoto Y, Kobayashi H. Mucoepidermoid carcinoma of the thymus treated by multimodality therapy: a case report. Ann Thorac Cardiovasc Surg. 2006;12:273-278. [PubMed] |
| 10. | Casale J, Bordoni B. Anatomy, Head and Neck, Infratemporal Fossa. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. [PubMed] |
| 11. | Bhattacharyya N, Fried MP. Determinants of survival in parotid gland carcinoma: a population-based study. Am J Otolaryngol. 2005;26:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Johns ME, Goldsmith MM. Incidence, diagnosis, and classification of salivary gland tumors. Part 1. Oncology (Williston Park). 1989;3:47-56; discussion 56, 58, 62. [PubMed] |
| 13. | Nance MA, Seethala RR, Wang Y, Chiosea SI, Myers EN, Johnson JT, Lai SY. Treatment and survival outcomes based on histologic grading in patients with head and neck mucoepidermoid carcinoma. Cancer. 2008;113:2082-2089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Dossani RH, Akbarian-Tefaghi H, Lemonnier L, Mehta V, Jacobsohn JA, Guthikonda B. Mucoepidermoid Carcinoma of Palatal Minor Salivary Glands with Intracranial Extension: A Case Report and Literature Review. J Neurol Surg Rep. 2016;77:e156-e159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Chen AM, Lau VH, Farwell DG, Luu Q, Donald PJ. Mucoepidermoid carcinoma of the parotid gland treated by surgery and postoperative radiation therapy: clinicopathologic correlates of outcome. Laryngoscope. 2013;123:3049-3055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Magliulo G, Fusconi M, Pulice G. Mucoepidermoid carcinoma of the external auditory canal: case report. Am J Otolaryngol. 2003;24:274-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Ozawa H, Tomita T, Sakamoto K, Tagawa T, Fujii R, Kanzaki S, Ogawa K, Kameyama K, Fujii M. Mucoepidermoid carcinoma of the head and neck: clinical analysis of 43 patients. Jpn J Clin Oncol. 2008;38:414-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Brandwein MS, Ivanov K, Wallace DI, Hille JJ, Wang B, Fahmy A, Bodian C, Urken ML, Gnepp DR, Huvos A, Lumerman H, Mills SE. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol. 2001;25:835-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 323] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 19. | Pires FR, de Almeida OP, de Araújo VC, Kowalski LP. Prognostic factors in head and neck mucoepidermoid carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Wu ZY, Wu WJ, Zheng L, Huang MW, Shi Y, Lv XM, Liu SM, Zhang JG, Zhang J. Efficacy of combined surgery and 125I seed brachytherapy for treatment of primary mucoepidermoid carcinoma of the parotid gland. Head Neck. 2019;41:3219-3225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Ghosh-Laskar S, Murthy V, Wadasadawala T, Agarwal J, Budrukkar A, Patil N, Kane S, Chaukar D, Pai P, Chaturvedi P, D'Cruz A. Mucoepidermoid carcinoma of the parotid gland: factors affecting outcome. Head Neck. 2011;33:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |