Published online Jul 26, 2020. doi: 10.12998/wjcc.v8.i14.2930
Peer-review started: March 6, 2020
First decision: April 12, 2020
Revised: June 3, 2020
Accepted: July 4, 2020
Article in press: July 4, 2020
Published online: July 26, 2020
Processing time: 139 Days and 23.5 Hours
Budd-Chiari syndrome is defined as hepatic venous outflow tract obstruction. For Asian Budd-Chiari syndrome patients, the major treatment modality is recanalization (percutaneous transluminal angioplasty with or without stent implantation). The cumulative 1-, 5-, and 10-year primary patency rates and survival rates are reported to be excellent or satisfactory, but the long-term outcome of patients with restenosis (the most common complication after recanalization) is unknown.
To explore the treatment strategy for restenosis in patients with Budd-Chiari syndrome after interventional therapy and to evaluate the long-term follow-up results.
The clinical data and follow-up results of 60 patients with restenosis after interventional therapy from November 1983 to December 2013 were retrospectively analyzed.
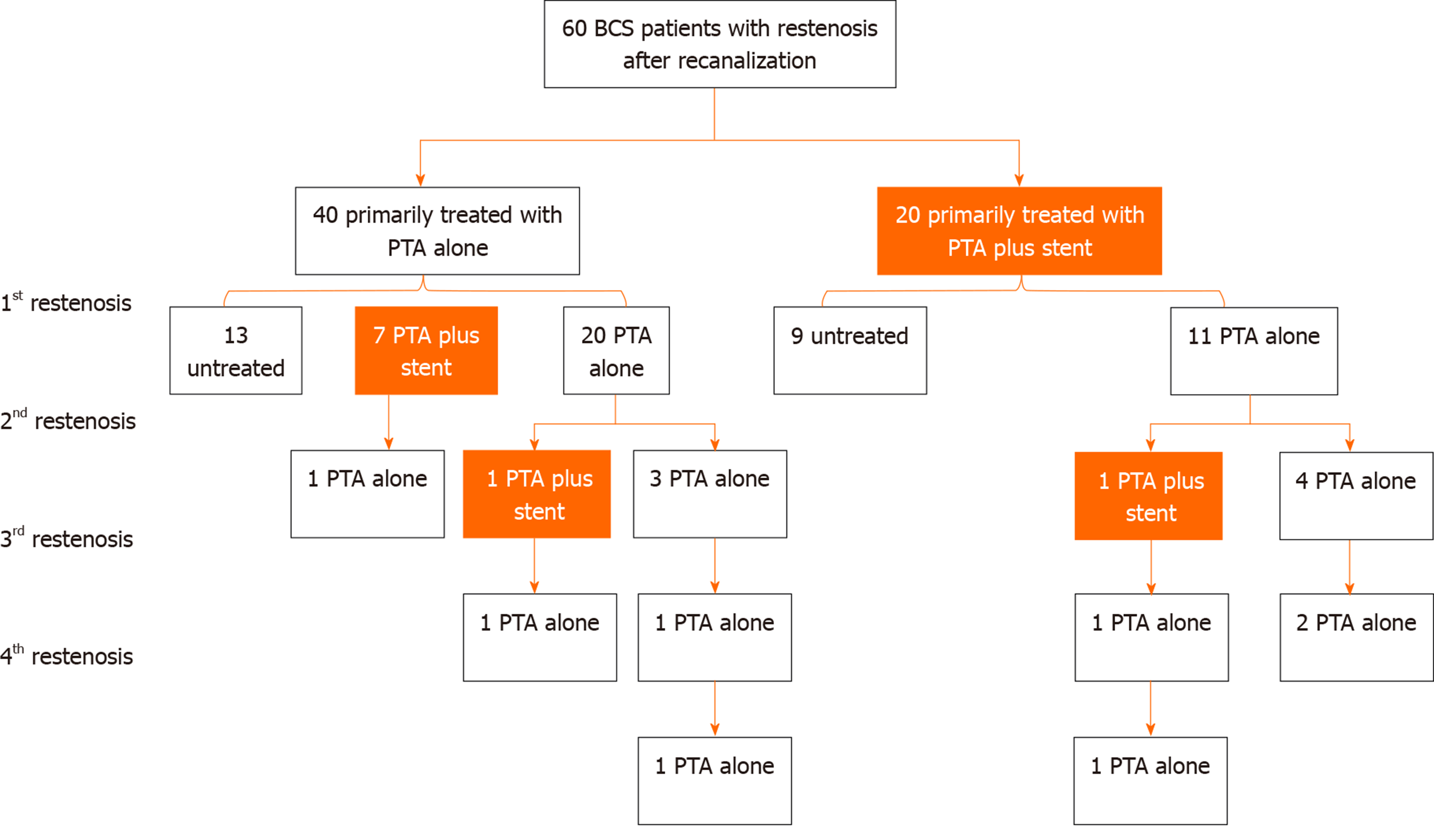
Sixty patients with restenosis were retrospectively divided into a percutaneous transluminal angioplasty (PTA) group (40 patients) and a PTA + stent group (20 patients) according to the primary recanalization method. For the patients with restenosis in the PTA group, 13 refused treatment, and 27 received further treatment; among these patients, five had a second restenosis, two had a third restenosis, and one had a fourth restenosis. For the patients with restenosis in the PTA + stent group, nine refused treatment, ten received PTA alone, and the other received PTA + stent implantation. Among the patients who received further treatment, five had a second restenosis, three had a third restenosis, and one had a fourth restenosis. The 1-, 5-, 10-, 20-, and 25-year cumulative survival rates of the 38 patients who received further treatment after restenosis were 100%, 78.3%, 78.3%, 70.5%, and 70.5%, respectively; however, for the 22 patients who refused treatment, the survival rates were 72.7%, 45.9%, 30.6%, 10.2%, and unavailable, respectively (P < 0.001).
Long-term follow-up after interventional therapy is very important. Active treatment for patients with restenosis can improve prognosis, and minimally invasive treatment strategies for restenosis allows to obtain satisfactory results.
Core tip: This is the first study to explore the treatment strategy for restenosis in Chinese patients with Budd-Chiari syndrome after interventional therapy and evaluate the outcomes with more than 20-year follow-up. The 1-, 5-, 10-, 20-, and 25-year cumulative survival rates of the 38 patients who received further treatment after restenosis were 100%, 78.3%, 78.3%, 70.5%, and 70.5%, respectively; however, for the 22 patients who refused treatment, the survival rates were 72.7%, 45.9%, 30.6%, 10.2%, and unavailable, respectively (P < 0.001). Regular follow-up and active treatment can result in satisfactory prognosis in Budd-Chiari syndrome patients with restenosis.
- Citation: Zhang W, Tian YL, Wang QZ, Chen XW, Li QY, Han JH, Chen XD, Xu K. Restenosis after recanalization for Budd-Chiari syndrome: Management and long-term results of 60 patients. World J Clin Cases 2020; 8(14): 2930-2941
- URL: https://www.wjgnet.com/2307-8960/full/v8/i14/2930.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i14.2930
Budd-Chiari syndrome (BCS) is defined as hepatic venous outflow tract obstruction at any level from the small hepatic veins (HVs) to the junction of the inferior vena cava (IVC) and the right atrium in the absence of right heart failure or constrictive pericarditis[1]. BCS is a rare disease, and the prevalence is estimated to be 2 per million inhabitants in Western countries[2], which is dramatically lower than that estimated for Chinese patients inhabiting the downstream areas of the Yellow River and the whole Huai River basin (approximately 7 to 39 per million inhabitants)[3]. In addition to the variation in epidemiological characteristics, the etiology, pattern of obstruction, and therapeutic options are also different between Western and Asian countries[4].
In Western countries, due to the high prevalence of HV thrombosis, a stepwise therapeutic strategy aimed at minimal invasiveness has been adopted, and the use of anticoagulation, a transjugular intrahepatic portosystemic shunt (TIPS), and orthotopic liver transplantation are the major treatment modalities[5-7]. For Asian BCS patients, especially Chinese patients, the most common obstructive pattern is membranous or segmental obstruction of the supra- and/or retrohepatic portion of the IVC[8-10]; therefore, the major treatment option is recanalization[11]. Recanalization is commonly referred to as percutaneous transluminal angioplasty (PTA) with or without stent implantation[12]. The outcomes of recanalization are reported to be excellent or satisfactory, with 1-, 5-, and 10-year cumulative survival rates of approximately 90%, 80%, and 70%, respectively. Furthermore, our previous study retrospectively investigated the 30-year outcomes of BCS at a tertiary hospital and found that the cumulative 10- and 20-year survival rates of patients treated with interventional radiology were 80.2% and 69.5%, respectively, which are optimistic[13].
However, in terms of restenosis, which is the most common complication after recanalization, the outcome regarding the long-term primary patency rate (more than 10 years) is unknown. Moreover, studies concerning the management of restenosis are rare, and the long-term survival of BCS patients with restenosis is still unclear. The purpose of this study was to retrospectively analyze a case series of BCS patients with restenosis and to evaluate long-term cumulative survival.
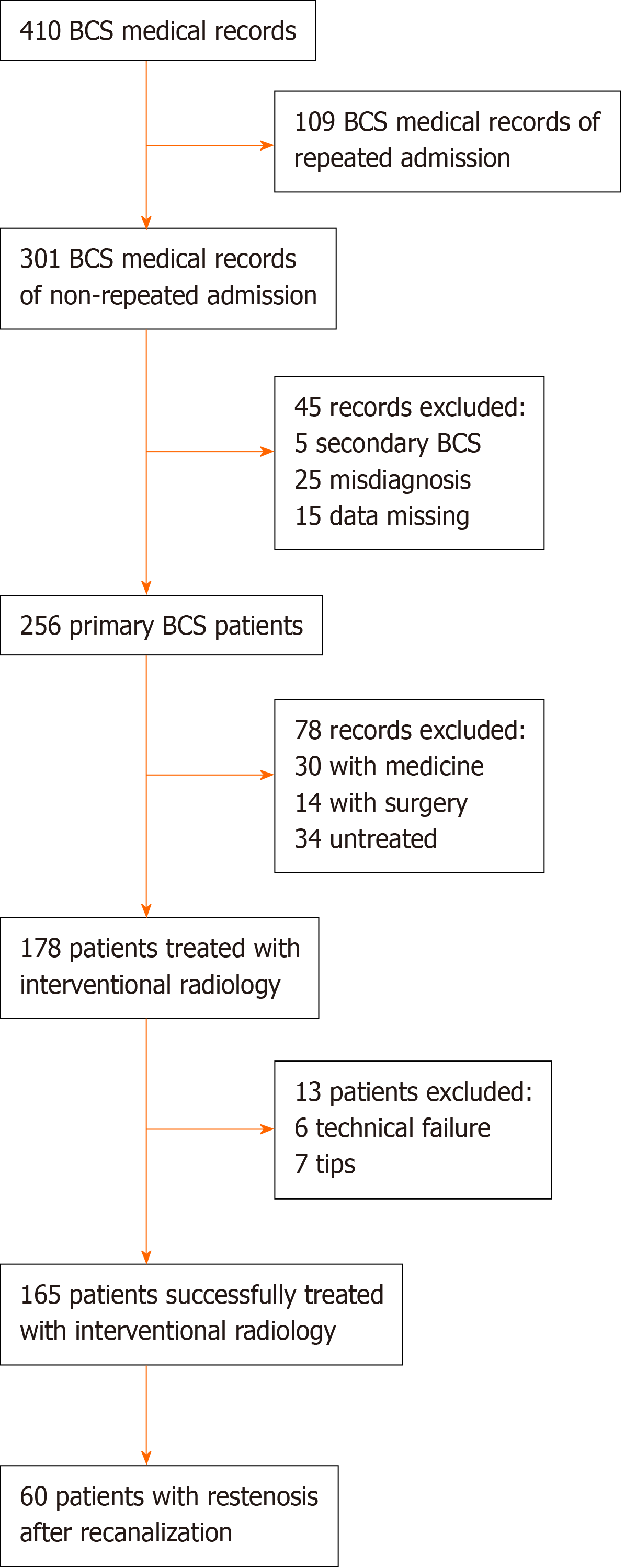
This study was approved by the ethics committee of the First Affiliated Hospital of China Medical University. All patients were informed about the benefits and related risks before treatment and provided written informed consent. Between November 1983 and September 2013, 410 medical records with an admission diagnosis of BCS were found in our hospitalization register system. There were 172 records for 63 patients who were hospitalized repeatedly, and only the primary hospitalization medical records were enrolled. Forty-five records were excluded: 5 due to secondary BCS, 25 due to a misdiagnosis of BCS, and 15 due to complete missing data regarding the laboratory and imaging investigations. For the remaining 256 primary BCS patients, 178 were treated with interventional radiology, among whom 165 were successfully treated by recanalization (for more information, see our previous study[13]). Of these patients who underwent successful recanalization, 60 patients had restenosis after recanalization and were eligible for our study. The flow chart of the case selection is shown in Figure 1.
BCS was diagnosed by color Doppler ultrasonography (CDUS), computed tomography (CT), magnetic resonance imaging (MRI), and/or venography of the HVs and IVC. According to the obstruction site of the hepatic venous outflow tract, BCS patients were classified into three types: (1) IVC type, manifesting as obstruction of the IVC with at least one patent main hepatic vein; (2) HV type, manifesting as obstruction of the three main HVs; and (3) Combined type, manifesting as obstruction of both the IVC and the three main HVs. Regarding the length of obstructive lesions, BCS patients were divided into membranous type (no more than 1 cm), segmental type (more than 1 cm), and long segmental type (more than 5 cm). Restenosis was defined as the recurrence of symptoms after recanalization (PTA with or without stent implantation) due to the re-obstruction of primary recanalized vessels or the newly formed obstruction of the hepatic venous outflow tract, which was confirmed by imaging examinations (CDUS, CT, or MRI). Patients were considered symptomatic when they had any one of the following manifestations: Abdominal pain, abdominal distention, abdominal wall varicosis, lower-extremity edema, ascites, esophageal and gastric varicosis bleeding, or encephalopathy.
Restenosis commonly occurs due to thrombosis of the IVC, membranous obstructive lesion regeneration, or elastic recoiling of segmental obstructive lesions. For restenosis that occurred with thrombosis, thrombolysis was given first if the thrombus was considered to be newly formed. Then, the effectiveness was evaluated by IVC venography every other day for 7-10 d. If the symptoms were not relieved, then PTA plus stenting was employed to compress the thrombus to the vascular wall of the IVC and to unblock the lumen. On the other hand, for restenosis caused by obsolete thrombus, stent implantation rather than thrombolysis or PTA alone was used. For restenosis without thrombosis, a stepwise treatment strategy was adopted in our center. First, for all the patients with restenosis, whether the primary operation was PTA or PTA plus stenting, PTA alone was recommended. Then, a “wait and see” attitude was employed to observe the effectiveness. If the symptoms were relieved, no further treatment was given, and the patient was followed closely after discharge; otherwise, further intervention was conducted. In cases where the obstructive lesion of restenosis was membranous, PTA was employed again with a larger diameter balloon. In cases where the obstructive lesion was segmental or long-segmental, stent implantation was considered. Notably, stent implantation was also performed on the condition that repeat balloon dilation or large balloon dilation was insufficient. When recanalization failed, TIPS and surgery were recommended. All patients received intravenous heparin for 5-7 d during hospitalization and were advised to continue with warfarin for 6-12 mo after discharge to maintain an international normalized ratio of 2-3.
Baseline data were extracted from the medical records before treatment, including demographic data, clinical presentations, laboratory test results, and imaging investigation data. Patients were followed until death, the end of this study (December 31, 2014), or the date of the last outpatient visit if the patient was lost to follow-up. Symptom remission was defined as the complete or substantial remission of the symptom of which patients mainly complained. Follow-up data were obtained from the medical records or by telephone interview of the patients themselves or their family members.
Categorical variables are expressed as absolute numbers (or frequencies if indicated) and were compared by using the chi-square test or Fisher’s exact test. Continuous variables are summarized as medians and ranges and were compared by using the independent sample t test or one-way analysis of variance. Cumulative survival rates were analyzed by using Kaplan-Meier curves. Two-tailed P values less than 0.05 were considered statistically significant. All statistical calculations were performed using the SPSS 21.0 package (SPSS Inc, Chicago, IL, United States).
Sixty primary BCS patients with restenosis after recanalization were retrospectively divided into two groups: 40 patients who were primarily treated by PTA alone (PTA group) and 20 patients who were primarily treated by PTA plus stenting (PTA + stent group). The baseline characteristics of these two groups are shown in Table 1. Of the 40 patients primarily treated by PTA alone, 15 (37.5%) cases of restenosis occurred in the first year after discharge, 34 (85%) occurred in five years, and 38 (95%) occurred in ten years, and the median time of primary patency was 19 (range, 1-136) mo. Of the 20 patients primarily treated by PTA plus stenting, 10 (50%) cases of restenosis occurred in the first year after discharge, 18 (90%) occurred in five years, and 19 (95%) occurred in ten years, and the median time of primary patency was 14.5 (range, 0.5-196) mo.
| PTA alone (n = 40) | PTA + stent (n = 20) | |
| Demographic data | ||
| Male | 22 (55%) | 18 (90%) |
| Female | 18 (45%) | 2 (10%) |
| Age (yr)1 | 36 (14-68) | 37 (15-80) |
| Duration of symptoms | ||
| ≤ 1 mo | 7 (17.5%) | 4 (20%) |
| 1-6 mo | 6 (15%) | 6 (30%) |
| ≥ 6 mo | 27 (67.5%) | 10 (50%) |
| Clinical manifestations | ||
| Abdominal distention | 18 (45%) | 9 (45%) |
| Abdominal wall varicosis | 21 (52.5%) | 11 (55%) |
| Lower-extremity edema | 22 (55%) | 13 (65%) |
| Gastroesophageal variceal bleeding | 3 (7.5%) | 6 (30%) |
| Laboratory tests1 | ||
| Hemoglobin level (g/L) | 130 (65-180) | 134 (80-168) |
| Platelet count (× 109/L) | 105 (46-306) | 130 (33-209) |
| Alanine transaminase level (× ULN) | 0.6 (0.2-28) | 0.6 (0.2-13) |
| Albumin level (g/L) | 38 (22-50) | 36 (26-58) |
| Total bilirubin level (μmol/L) | 29 (11-132) | 29 (8-148) |
| International normalized ratio | 1.3 (1.0-1.9) | 1.4 (0.9-2.9) |
| Creatinine level (μmol/L) | 66 (41-254) | 79 (33-157) |
| Imaging features | ||
| Type of obstruction | ||
| HV | 8 (20%) | 1 (5%) |
| IVC | 5 (12.5%) | 6 (30%) |
| Com | 27 (67.5%) | 13 (65%) |
| Pattern of IVC obstruction | ||
| No obstruction | 8 (20%) | 1 (5%) |
| Membranous | 30 (75%) | 9 (45%) |
| Segmental | 2 (5%) | 2 (10%) |
| Long segmental | 0 (2.5%) | 8 (40%) |
| Ascites | 17 (42.5%) | 11 (55%) |
| AHV compensatory | 7 (17.5%) | 5 (25%) |
| IVC thrombosis | 11 (27.5%) | 11 (55%) |
| Prognostic index | ||
| Child-Pugh score1 | 7 (5-11) | 7 (5-11) |
| Child-Pugh class | ||
| A | 18 (45%) | 4 (20%) |
| B | 20 (50%) | 12 (60%) |
| C | 2 (5%) | 4 (20%) |
For the 40 patients in the PTA group, 19 restenosis patients were re-treated by PTA alone, 8 were treated by PTA plus stenting, and 13 refused further treatment because of financial aspects. Among the 27 treated patients, 5 experienced a second restenosis, 2 had a third restenosis, and one encountered a fourth restenosis. In total, 27 cases of PTA alone and 8 cases of PTA plus stenting were performed. For the 20 patients in the PTA + stent group, 10 restenosis patients were treated by PTA alone, 1 underwent implantation of another stent after PTA, and 9 refused further treatment due to financial aspects. Among the 11 treated patients, 5 experienced a second restenosis, 3 had a third restenosis, and one encountered a fourth restenosis. In total, 21 cases of PTA alone and 1 case of PTA plus stenting were performed. Detailed information on the occurrence of restenosis and the corresponding treatment selection is presented in Figure 2.
In the PTA group, the median time of follow-up was 61.5 (range, 1-313) mo. Thirteen patients died during a median survival time of 36 (range, 1-123) mo; 7 died of liver or multiple organ failure, 3 died of hepatocellular carcinoma (HCC), and 3 died of variceal bleeding. All these deaths were considered to be related to BCS. The other 3 patients who died of intracranial hemorrhage induced by hypertension, disseminated intravascular coagulation, and traffic accident were considered to be cases not related to BCS. Notably, among the 13 patients who refused active treatment, 7 died (median of 55 mo, range of 1 mo to 123 mo). In the PTA + stent group, the median time of follow-up was 52.5 (range, 2-276) mo. Ten patients died, with a median time of 30 (range, 2-239) mo; 5 died of liver or multiple organ failure, 2 died of variceal bleeding, 2 died of hepatic encephalopathy, and 1 died of HCC. Notably, all 9 patients who refused further treatment after restenosis died. Detailed information on the follow-up outcomes is shown in Table 2.
| Primary recanalization | Management | Death | Remission | Non-remission |
| PTA alone 40 | PTA alone 19 (47.5%) | HCC 2 (5%), variceal bleeding 2 (5%), liver or multiple organ failure 1 (2.5%) | 14 (35%) | 0 |
| PTA + stent 8 (20%) | HCC 1 (2.5%), liver or multiple organ failure 1 (2.5%), intracranial hemorrhage induced by hypertension 1 (2.5%), accidental death 1 (2.5%) | 4 (10%) | 0 | |
| Untreated 13 (32.5%) | Liver or multiple organ failure 5 (12.5%), variceal bleeding 1 (2.5%), DIC 1 (2.5%) | 2 (5%) | Abdominal distension 3 (7.5%), lower-extremity edema 1 (2.5%) | |
| PTA + stent 20 | PTA alone 10 (50%) | Liver or multiple organ failure 1 (5%) | 9 (45%) | 0 |
| PTA + stent 1 (5%) | 0 | 1 (5%) | 0 | |
| Untreated 9 (45%) | HCC 1 (5%), variceal bleeding 2 (10%), liver or multiple organ failure 4 (20%), hepatic encephalopathy 2 (10%) | 0 | 0 |
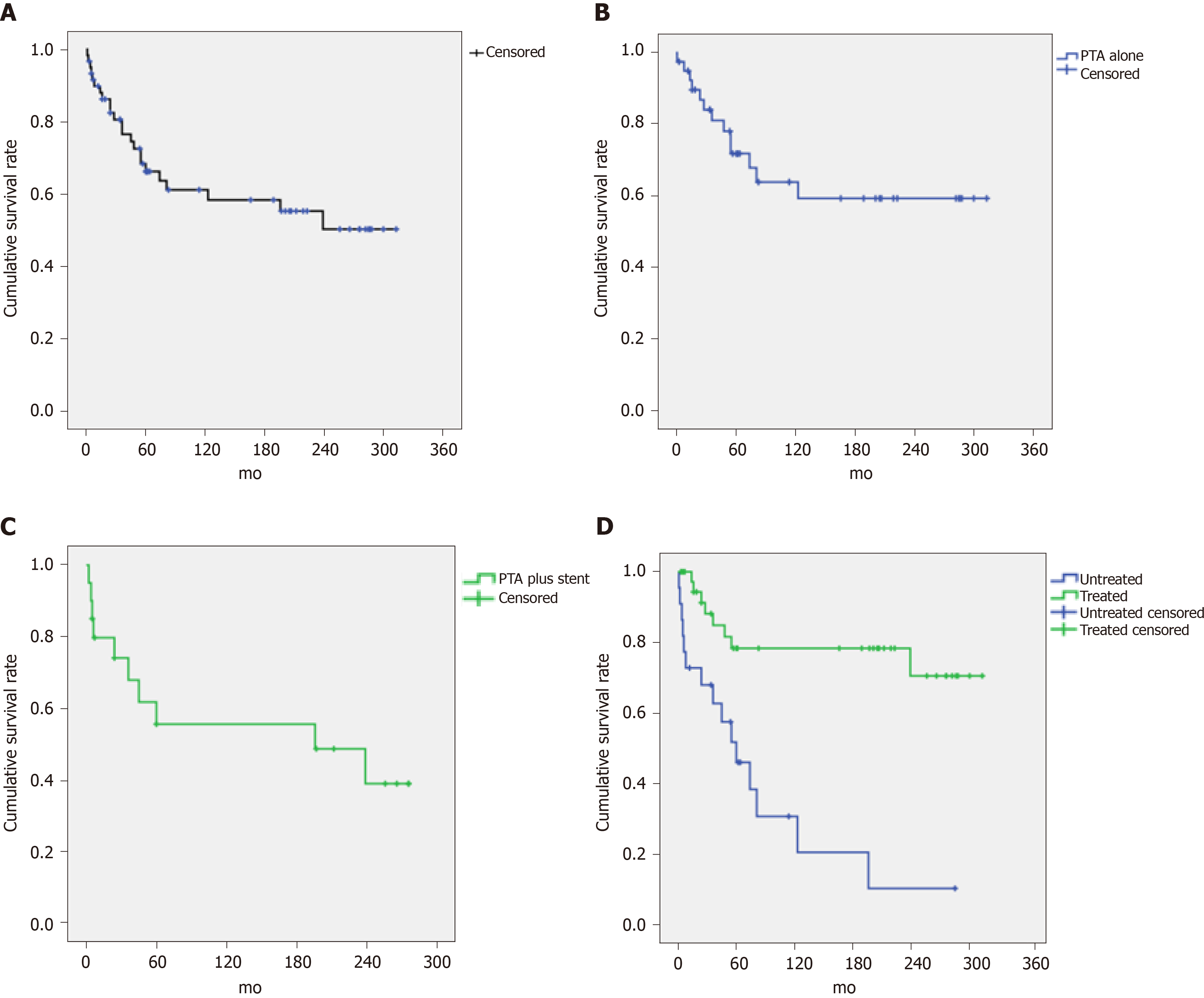
The cumulative 1-, 5-, 10-, 20-, and 25-year survival rates for the 60 patients with restenosis were 89.8%, 66.2%, 61.1%, 50.2%, and 50.2%, respectively (Figure 3A); for the 40 patients in the PTA group, the survival rates were 94.9%, 71.8%, 63.8%, 59.3%, and 59.3%, respectively (Figure 3B); and for the 20 patients in the PTA + stent group, the survival rates were 79.7%, 55.5%, 55.5%, 48.6%, and unavailable, respectively (Figure 3C). In addition, for the 38 patients who received further treatment after restenosis, the cumulative 1-, 5-, 10-, 20-, and 25-year survival rates were 100%, 78.3%, 78.3%, 70.5%, and 70.5%, respectively; for the 22 untreated patients, the survival rates were 72.7%, 45.9%, 30.6%, 10.2%, and unavailable, respectively. The difference in cumulative survival rates between these two groups was statistically significant (P < 0.001) (Figure 3D) (the baseline characteristics of these two groups were not significantly different).
This study retrospectively analyzed the treatment strategy and long-term follow-up results of a group of 60 BCS patients with restenosis treated after recanalization. The follow-up time exceeded 300 mo. To the best of our knowledge, for Chinese BCS patients with restenosis, our follow-up period of more than 25 years is by far the longest. In our study, restenosis was defined as the recurrence of symptoms after recanalization (PTA with or without stent implantation) due to the re-obstruction of primary recanalized vessels or the newly formed obstruction of the hepatic venous outflow tract, which was confirmed by imaging examinations (CDUS, CT, or MRI). A previous study showed that the cumulative 1-, 5-, and 10-year primary patency rates were 87%, 77%, and 58%, respectively[12]. Recently, a meta-analysis reported that the rate of vascular restenosis at 1 year after initial recanalization was 5.5% (4.3%-6.7%)[14]. In our study, nearly half of the cases of restenosis occurred in the first year, 37.5% (15/40) in the PTA group and 50% (10/20) in the PTA + stent group, suggesting that the first year after recanalization is a high incidence period of restenosis, and it is necessary to strengthen follow-up observations and prevent the occurrence of restenosis. For the PTA group, the patient condition was relatively mild, while for the PTA + stent group, the condition was more serious (higher Child-Turcotte-Pugh score). The 1-year and 5-year restenosis incidence rates of these two groups were approximately the same (1-year PTA 37.5% vs PTA + stent 50%, 5-year PTA 85% vs PTA + stent 90%). It should be noted that the patients in this study were retrospectively divided into the PTA group and PTA + stent group according to the initial treatment modality, but the baseline characteristics of these two groups were heterogeneous; thus, a comparison cannot be made.
In this study, we focused on the treatment strategy for BCS restenosis. For these patients, we advocated regular follow-up and active treatment by gradually increasing the invasiveness, starting from PTA (including the use of large-diameter balloons). If PTA was invalid, then stent implantation was performed. Among this series of 60 cases, 2 patients experienced restenosis 4 times. One was in the PTA group, and after 3 balloon dilatation procedures (including the use of large-diameter balloons), a stent was implanted; the other was in the PTA + stent group, and one additional stent was implanted after 2 balloon dilatation procedures. We believe that stent implantation is an appropriate choice for patients with repeated occurrence of restenosis after PTA. However, for patients initially treated by PTA + stenting, the use of PTA only is recommended in most cases. The following issues need to be considered if the stent is to be implanted: (1) The cause of restenosis and whether stent implantation can solve the problem[10]; (2) Whether stent implantation will aggravate intravascular stenosis (especially for restenosis after stenting of the hepatic vein) and whether the stent will obstruct the orifice of the hepatic vein or accessory hepatic vein[12]; and (3) Whether stent implantation will possibly affect the subsequent treatment (such as TIPS)[9,13]. In addition, the effects of stent placement in terms of retaining a foreign body in vivo for a long period of time are not clear. Moreover, complications of stent implantation, such as fracture, displacement, and blocking of the normal vessel orifice, have been reported[15-18]. In view of this, it is vital to comprehensively weigh the pros and cons before stent implantation, and we should pay more attention to possible follow-up issues.
Liver failure, esophageal variceal hemorrhage, and hepatocellular carcinoma were the main causes of death in the present study, a result that was consistent with previous studies[12,13,19]. Liver failure is mainly due to chronic liver disease exacerbated by acute thrombosis or is due to the slow progression of cirrhosis, which gradually leads to end-stage liver disease, and liver function cannot be compensated[20]. Esophageal variceal hemorrhage is the outcome of portal hypertension. Long-term liver congestion will lead to liver cirrhosis and sinus portal hypertension (possibly combined with viral and alcoholic liver diseases), which ultimately results in fatal bleeding[21]. HCC is a cause of death worthy of attention in BCS patients. Previous studies have found that HCC is commonly seen in patients with IVC obstruction or IVC combined with HV obstruction; however, there are also reports of HCC found in patients with pure HV involvement[22,23]. The cause may be related to the change in hepatic blood supply and the increase in the hepatic artery blood supply ratio.
Previous studies have shown that restenosis is a critical factor affecting survival[12,24,25], and our study also confirmed this result. The 5- and 10-year cumulative survival rates in our study were 66.2% and 61.1%, respectively, which were lower than the overall 5- and 10-year cumulative survival rates of 80% and 70% in patients with BCS. This indicates that the long-term prognosis of patients with restenosis is not optimistic, especially for those who refuse further treatment after restenosis (their 20-year cumulative survival rate was only 10%). In the present study, the overall survival rate of patients receiving treatment after restenosis was significantly better than that of patients refusing treatment. The long-term cumulative survival rate was 78% at 10 years and 70% at 20 years, which were satisfactory results. For the treatment group, the survival rate of patients receiving PTA alone seemed to be better than that of patients receiving PTA + stent implantation. However, it should be noted that the baseline characteristics of patients receiving stent implantation were more serious than those of patients receiving PTA alone, so these two groups cannot be directly compared and the role of stent implantation cannot be denied.
Recently, a randomized controlled trial demonstrated that for BCS patients with short-length stenosis, routine stent implantation with angioplasty was superior to angioplasty alone in terms of the treatment efficacy for preventing restenosis, and routine stenting with angioplasty was safe to recommend as part of first-line invasive treatment[9]. It should be noted that the follow-up period was relatively short (median 27 mo, interquartile range 19-41), and the long-term outcomes of stent implantation remain to be further observed. Based on our present study of retrospective follow-up results in the past 30 years, half of the cases of stent stenosis occurred one year after implantation, and we therefore argue that stent implantation requires caution.
For patients who refuse treatment after restenosis, the progression of disease can indirectly simulate the natural course of BCS. The cumulative 1-, 5-, 10-, and 20-year survival rates were approximately 70%, 50%, 30%, and 10%, respectively. At present, no patients surviving more than 25 years have been found during the follow-up. It is speculated that the possible reason for these untreated patients who survived more than 20 years is that the slow progression of BCS allowed for the formation of collateral circulation, which also compensated for liver function and reduced portal pressure[26]. For these untreated patients with a relatively good prognosis, they are worth further study in order to provide us with new ideas for treatment, suggesting that the timing of treatment selection needs to be further discussed.
There were several limitations in the present study. First, as a retrospective study, some biases may have been introduced in the case selection and data collection. Second, our study spanned 30 years, and in a few cases, the treatment strategies of the early stage were not quite consistent with those of the later stage, and the technique as well as expertise were also constantly updated. Third, the detailed information of technical aspects (such as balloon and stent specifications) were not presented because the focus of our study was treatment strategy for BCS patients with restenosis. Fourth, the patients were grouped retrospectively according to the treatment method, and the baseline results were not homogenous, which made the comparison infeasible. Finally, due to the relatively small number of cases, no further subgroup analysis was carried out. The shortcomings described above are expected to be overcome by multicenter large-sample randomized controlled trials in the future.
In conclusion, long-term follow-up is very important after interventional therapy. When restenosis occurs, active treatment can achieve a satisfactory prognosis. Treatment begins with balloon dilatation and escalates in a step-by-step, and whether stent implantation is needed is determined by the efficacy.
Budd-Chiari syndrome (BCS) is a rare disease, which is defined as hepatic venous outflow tract obstruction. For Chinese patients, the predominant obstructive lesions are membranous and segmental obstructions of the supra-hepatic or retro-hepatic portion of the inferior vena cava, and the most common treatment is percutaneous recanalization (percutaneous transluminal angioplasty (PTA) with or without stent implantation). Restenosis is the most common complication after recanalization. However, the management strategy and the long-term survival of BCS patients with restenosis are seldom reported.
For the treatment of restenosis after recanalization, there are different opinions; some researchers suggest stent implantation and others advocate repeated balloon dilatation (including the use of large-diameter balloons). Different treatments have their own advantages and disadvantages, and the reported results vary considerably. In brief, there is currently no consensus on the best treatment strategy. We are very interested in this issue and hope that we can present a stepwise treatment strategy adopted in our center.
The objectives were to report the long-term follow-up outcomes for the patients with restenosis treated by our stepwise invasiveness increasing strategy and to discuss the prognosis of different treatment options (active treatment or non-treatment).
We retrospectively analyzed the 30-year follow-up outcome of BCS patients at our center, and totally 60 patients with restenosis after recanalization were included in the analysis by case screening. According to their primary treatment methods, the patients were divided into two groups (PTA group and PTA + stent group) and were followed until the end of this study (December 31, 2014). Restenosis was defined as the recurrence of symptoms after recanalization due to the re-obstruction of primary recanalized vessels or the newly formed obstruction of the hepatic venous outflow tract, which is confirmed by imaging examinations (color Doppler ultrasonography, computed tomography, and/or magnetic resonance imaging). Cumulative survival rates were analyzed using Kaplan-Meier curves. Two-tailed P values less than 0.05 were considered statistically significant.
Among the 60 patients, 40 were primarily treated by PTA alone (PTA group) the rest were primarily treated by PTA plus stenting (PTA + stent group). In the PTA group, 19 restenosis patients were re-treated by PTA alone, 8 were re-treated by PTA plus stenting, and 13 refused further treatment; and among the 13 patients who refused active treatment, 7 died. In the PTA + stent group, 10 restenosis patients were treated by PTA alone, 1 underwent implantation of another stent after PTA, and 9 refused further treatment; the same point is that those who refused further treatment after restenosis died. There is a statistically significant difference between the two groups — the group of patients who received further treatment after restenosis and the other group of patients who did not (P < 0.001).
To the best of our knowledge, for Chinese BCS patients with restenosis, our follow-up period of more than 25 years is by far the longest. In this study, we focused on the treatment strategy for BCS restenosis. For these patients, we advocate regular follow-up and active treatment by gradually increasing the invasiveness, starting from PTA (including the use of large-diameter balloons). If PTA does not work, then stent implantation will be performed. It is vital to comprehensively weigh the pros and cons before performing stent implantation, also we should pay more attention to possible complications during the follow-up. Regular follow-up and active treatment can result in satisfactory prognosis in BCS patients with restenosis.
For patients with restenosis who refuse further treatment but have a relatively good prognosis, it is worth further investigating potential protective factors to provide us with new ideas for treatment. The shortcomings of our study are expected to be overcome by multicenter large sample randomized controlled trials in the future.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Gupta P, Lazăr DC, Suc B, Tripathi D S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Xing YX
| 1. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Vascular diseases of the liver. J Hepatol. 2016;64:179-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 532] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 2. | Valla DC. Primary Budd-Chiari syndrome. J Hepatol. 2009;50:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 3. | Zhang W, Qi X, Zhang X, Su H, Zhong H, Shi J, Xu K. Budd-Chiari Syndrome in China: A Systematic Analysis of Epidemiological Features Based on the Chinese Literature Survey. Gastroenterol Res Pract. 2015;2015:738548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 4. | Mancuso A. Budd-Chiari syndrome in the West and the East: Same syndrome, different diseases. Liver Int. 2019;39:2417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Plessier A, Sibert A, Consigny Y, Hakime A, Zappa M, Denninger MH, Condat B, Farges O, Chagneau C, de Ledinghen V, Francoz C, Sauvanet A, Vilgrain V, Belghiti J, Durand F, Valla D. Aiming at minimal invasiveness as a therapeutic strategy for Budd-Chiari syndrome. Hepatology. 2006;44:1308-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 192] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 6. | Seijo S, Plessier A, Hoekstra J, Dell'era A, Mandair D, Rifai K, Trebicka J, Morard I, Lasser L, Abraldes JG, Darwish Murad S, Heller J, Hadengue A, Primignani M, Elias E, Janssen HL, Valla DC, Garcia-Pagan JC; European Network for Vascular Disorders of the Liver. Good long-term outcome of Budd-Chiari syndrome with a step-wise management. Hepatology. 2013;57:1962-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 7. | Darwish Murad S, Plessier A, Hernandez-Guerra M, Fabris F, Eapen CE, Bahr MJ, Trebicka J, Morard I, Lasser L, Heller J, Hadengue A, Langlet P, Miranda H, Primignani M, Elias E, Leebeek FW, Rosendaal FR, Garcia-Pagan JC, Valla DC, Janssen HL; EN-Vie (European Network for Vascular Disorders of the Liver). Etiology, management, and outcome of the Budd-Chiari syndrome. Ann Intern Med. 2009;151:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 339] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 8. | Ding PX, Han XW, Liu C, Zhang Y, Cheng AL, Wu Y, Liang XX, Gao XM, Lee EW. Long-term outcomes of individualized treatment strategy in treatment of type I Budd-Chiari syndrome in 456 patients. Liver Int. 2019;39:1577-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Wang Q, Li K, He C, Yuan X, Luo B, Qi X, Guo W, Bai W, Yu T, Fan J, Wang Z, Yuan J, Li X, Zhu Y, Han N, Niu J, Lv Y, Liu L, Li J, Tang S, Guo S, Wang E, Xia D, Wang Z, Cai H, Wang J, Yin Z, Xia J, Fan D, Han G. Angioplasty with versus without routine stent placement for Budd-Chiari syndrome: a randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4:686-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Cheng DL, Zhu N, Xu H, Li CL, Lv WF, Fang WW, Li CT. Outcomes of endovascular interventional therapy for primary Budd-Chiari syndrome caused by hepatic venous obstruction. Exp Ther Med. 2018;16:4141-4149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Qi XS, Ren WR, Fan DM, Han GH. Selection of treatment modalities for Budd-Chiari Syndrome in China: a preliminary survey of published literature. World J Gastroenterol. 2014;20:10628-10636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Han G, Qi X, Zhang W, He C, Yin Z, Wang J, Xia J, Xu K, Guo W, Niu J, Wu K, Fan D. Percutaneous recanalization for Budd-Chiari syndrome: an 11-year retrospective study on patency and survival in 177 Chinese patients from a single center. Radiology. 2013;266:657-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Zhang W, Wang QZ, Chen XW, Zhong HS, Zhang XT, Chen XD, Xu K. Budd-Chiari syndrome in China: A 30-year retrospective study on survival from a single center. World J Gastroenterol. 2018;24:1134-1143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Zhang F, Wang C, Li Y. The outcomes of interventional treatment for Budd-Chiari syndrome: systematic review and meta-analysis. Abdom Imaging. 2015;40:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Zhang QQ, Xu H, Zu MH, Gu YM, Shen B, Wei N, Xu W, Liu HT, Wang WL, Gao ZK. Strategy and long-term outcomes of endovascular treatment for Budd-Chiari syndrome complicated by inferior vena caval thrombosis. Eur J Vasc Endovasc Surg. 2014;47:550-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Qiao T, Liu CJ, Liu C, Chen K, Zhang XB, Zu MH. Interventional endovascular treatment for Budd-Chiari syndrome with long-term follow-up. Swiss Med Wkly. 2005;135:318-326. [PubMed] |
| 17. | Zhang CQ, Fu LN, Xu L, Zhang GQ, Jia T, Liu JY, Qin CY, Zhu JR. Long-term effect of stent placement in 115 patients with Budd-Chiari syndrome. World J Gastroenterol. 2003;9:2587-2591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Ding PX, Li Z, Zhang SJ, Han XW, Wu Y, Wang ZG, Fu MT. Outcome of the Z-expandable metallic stent for Budd-Chiari syndrome and segmental obstruction of the inferior vena cava. Eur J Gastroenterol Hepatol. 2016;28:972-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Khan F, Armstrong MJ, Mehrzad H, Chen F, Neil D, Brown R, Cain O, Tripathi D. Review article: a multidisciplinary approach to the diagnosis and management of Budd-Chiari syndrome. Aliment Pharmacol Ther. 2019;49:840-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Menon KV, Shah V, Kamath PS. The Budd-Chiari syndrome. N Engl J Med. 2004;350:578-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 326] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 21. | Valla DC, Cazals-Hatem D. Vascular liver diseases on the clinical side: definitions and diagnosis, new concepts. Virchows Arch. 2018;473:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Paul SB, Shalimar, Sreenivas V, Gamanagatti SR, Sharma H, Dhamija E, Acharya SK. Incidence and risk factors of hepatocellular carcinoma in patients with hepatic venous outflow tract obstruction. Aliment Pharmacol Ther. 2015;41:961-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Vilgrain V, Paradis V, Van Wettere M, Valla D, Ronot M, Rautou PE. Benign and malignant hepatocellular lesions in patients with vascular liver diseases. Abdom Radiol (NY). 2018;43:1968-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Gao X, Gui E, Lu Z, Ning X, Zu M, Zhang P, Sun G. Risk factors of recurrence among 471 Chinese patients with Budd-Chiari syndrome. Clin Res Hepatol Gastroenterol. 2015;39:620-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Sun J, Zhang Q, Xu H, Huang Q, Shen B, Zu M, Gu Y. Clinical outcomes of warfarin anticoagulation after balloon dilation alone for the treatment of Budd-Chiari syndrome complicated by old inferior vena cava thrombosis. Ann Vasc Surg. 2014;28:1862-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Valla DC. Budd-Chiari syndrome/hepatic venous outflow tract obstruction. Hepatol Int. 2018;12:168-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |