Published online Jul 6, 2020. doi: 10.12998/wjcc.v8.i13.2841
Peer-review started: December 23, 2019
First decision: March 5, 2020
Revised: May 12, 2020
Accepted: June 7, 2020
Article in press: June 7, 2020
Published online: July 6, 2020
Processing time: 196 Days and 19.6 Hours
Retroperitoneal fibrosis is an exceptionally rare disease characterized by proliferation of fibrous tissue and inflammation in the retroperitoneum. It features many symptoms in the kidneys and in other organs and usually leads to ureteral obstruction.
Here we present 9 consecutive cases of idiopathic retroperitoneal fibrosis (IRPF) in patients who presented to the Department of Nephrology or Department of Rheumatology, Xuanwu Hospital, Capital Medical University, Beijing, China, between January 2012 and June 2017 with ureteral obstruction due to external compression of the ureter that led to hydronephrosis and kidney dysfunction. Computed tomography imaging was used to identify hydronephrosis and ureteral obstruction and to evaluate kidney function. Each patient was diagnosed with IRPF based on clinical observation and computed tomography examination results. To restore kidney function, a retrograde metallic stent was placed in the ureter under X-ray guidance 2 d after each patient’s admission. No perioperative complications occurred in any patient, but postoperative complications occurred in two patients as follows: Patient 2 had stent migration and repeated metallic stent infections that resolved with treatment; and patient 4 had postoperative hematuria because he resumed normal activities too soon after stent placement (contrary to instruction). Placement of the metallic ureteral stents provided relief from ureteral obstruction and restored kidney function in all patients.
Our 9-case series underscores the utility and efficacy of applying the Resonance® metallic ureteral stent to treat ureteral obstruction in patients with IRPF. For all retroperitoneal fibrosis cases in our series, ureteral stents provided effective relief and were shown to reduce the incidence rate of perioperative and postoperative complications.
Core tip: Nine patients with idiopathic retroperitoneal fibrosis admitted to our institution within a 5-year period received Resonance® metallic stents to restore kidney function. Ureteral obstruction was relieved in all 9 cases, and only 2 patients experienced adverse effects (stent migration and repeated metallic stent infection in one patient and postoperative hematuria in another). Overall, these stents demonstrated effective relief and resulted in fewer complications in the treatment of ureteral stenosis in patients with idiopathic retroperitoneal fibrosis.
- Citation: Gao W, Ou TW, Cui X, Wu JT, Cui B. Metallic ureteral stent in restoring kidney function: Nine case reports. World J Clin Cases 2020; 8(13): 2841-2848
- URL: https://www.wjgnet.com/2307-8960/full/v8/i13/2841.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i13.2841
Retroperitoneal fibrosis (RPF) is a disease featuring the proliferation of fibrous tissue in the retroperitoneum. It is a rare disease characterized by extensive fibrosis and inflammation in the retroperitoneal region and causes many symptoms in the kidneys and other organs[1]. Clinicians have classified the disease into two forms: Idiopathic RPF (IRPF) and RPF secondary to a malignancy[2]. The pathology of RPF secondary to a malignancy is similar to that of IRPF, except for the invasive growth of cancer cells in muscle and bone tissues. IRPF may be considered an autoimmune disease due to its association with various immune-related conditions and response to immunosuppression[3]. Patients with either of these two forms of RPF may experience compression of the ureter, resulting in ureteral obstruction with traction displacement[4]. Ureterolysis is a standard treatment, but it is time-consuming, risky and may not be tolerated by some patients[5,6].
A ureteral stent can be used to drain the kidney and restore kidney function, thereby providing time for further treatment and improving the patient’s quality of life. However, the conventional plastic or polymeric stents may become occluded after several months, so frequent monitoring is needed. Also, the plastic stent must be replaced immediately once the hydronephrosis has occurred. Moreover, a typical plastic or polymeric ureteral stent may provide insufficient resistance to the traction displacement of the ureter[4].
Metallic ureteral stents are widely used for the treatment of ureteral obstructions secondary to malignant retroperitoneal metastases[7]. In these RPF patients, the retroperitoneal metastatic foci are characterized by the infiltration of fibroblasts and cancer cells[7]. The Resonance® metallic stent has no magnetic metal but has potent tensile strength and provides good drainage. In addition, it is resistant to external compression secondary to RPF and also is resistant to occlusion. These properties allow longer dwelling time and less frequent stent exchanges. Thus, this stent provides a long-term renoprotective effect and might be more cost-effective[8]. However, the stent should still be refreshed once a year, and it may be more difficult to exchange a metallic stent than a plastic or a polymeric stent. Herein, we explored the outcomes of using a metallic ureteral stent for treatment of 9 cases of IRPF.
This case series includes 9 consecutive patients with IRPF who were admitted to the Department of Nephrology or Division of Rheumatology of Xuanwu Hospital, Capital Medical University, Beijing, China, where they received comprehensive examinations from January 2012 to June 2017 (Table 1). Patients’ mean age was 61-years-old, and 8 patients were male. Each patient had ureteral obstruction due to external compression, which led to hydronephrosis and kidney dysfunction. After consultation with urologists, they were transferred to the Department of Urology and then were scheduled to receive Resonance® metallic ureteral stents.
| Patient No. | Age in yr, sex | Initial admission | Metallic stent properties | Stent placement, n | Reason for stent failure/removal | Follow-up in mo |
| 11 | 53, M | January, 2012 | Left: 6 Fr, 26 cm | 6 | NA | 66 |
| 22 | 55, F | July, 2012 | Bilateral: 6 Fr, 26 cm (first); Right: 26 cm (second); Right: 24 cm (third) | 3 | Recurrent obstruction after removal | 60 |
| 3 | 64, M | August, 2012 | Bilateral: 6 Fr, 26 cm | 1 | NA | 6 |
| 43 | 74, M | December, 2012 | Right: 6 Fr, 28 cm (patient height > 180 cm) | 1 | Removal at 7 mo following patient's request | 37 |
| 5 | 64, M | January, 2013 | Right: 6 Fr, 26 cm | 4 | Obstruction after refreshing with a general stent | 54 |
| 6 | 63, M | February, 2014 | Right: 6 Fr, 26 cm | 4 | NA | 41 |
| 7 | 61, M | March, 2015 | Bilateral: 6 Fr, 26 cm | 2 | NA | 28 |
| 8 | 44, M | May, 2015 | Left: 6 Fr, 28 cm | 1 | NA | 6 |
| 9 | 71, M | October, 2012 | Left: 6 Fr, 26 cm | 4 | NA | 51 |
Case 1: A 53-year-old male was admitted on 19 January 2012. He was the first to receive a metallic stent for treatment of ureteral obstruction due to IRPF.
Case 2: A 55-year-old female was admitted to the Department of Nephrology due to acute renal failure on 8 July 2012. She received a diagnosis of IRPF and bilateral hydronephrosis based on computed tomography (CT) results.
Case 3: A 64-year-old male was admitted in August 2012 and was diagnosed with IRPF, bilateral hydronephrosis and chronic renal failure.
Case 4: A 74-year-old male was admitted and diagnosed with IRPF, right hydronephrosis and left renal atrophy. He had received bilateral ureterolysis more than 10 years previously.
Case 5: A 64-year-old male was admitted in 2013 and diagnosed with IRPF and right hydronephrosis.
Case 6: A 63-year-old male diagnosed with IRPF and right hydronephrosis was suspected to have immunoglobulin G (IgG)4-related sclerotic disease. On February 14, 2014, he was admitted to the Division of Rheumatology, and IgG4-related sclerotic disease was confirmed.
Case 7: A 61-year-old male was admitted in 2014 and diagnosed with IRPF, bilateral hydronephrosis and chronic renal failure.
Case 8: A 44-year-old male was admitted in 2014 and diagnosed with IRPF, left hydronephrosis, right renal atrophy and acute renal failure.
Case 9: This 71-year-old patient was admitted into the Department of Urology on 11 April 2013, and ultrasonography indicated left hydronephrosis.
Case 1: This patient had left hydronephrosis because he received a left ureteral stent that was refreshed on 9 January 2013. In January 2014, the clinician in the Division of Rheumatology believed a metallic stent was unnecessary for treatment of ureteral obstruction due to IRPF and recommended stent removal due to the patient’s stable condition.
Cases 2, 3 and 5-8: Patient had a free previous medical history.
Case 4: The patient had received bilateral ureterolysis more than 10 years previously.
Case 9: At a prior admission on 17 October 2012, this patient complained of dull pain in the left waist for more than 4 mo and left hydronephrosis. CT results indicated a left-middle ureteral obstruction (inflammation-related adhesion) and left hydronephrosis. Exploratory ureteroscopy of the left ureter was performed, with dilation of the stenotic ureter, biopsy and 7 French scale (Fr) double pigtail stent placement. Stenosis was observed after the ureteroscope was inserted 15 cm, and a 5 Fr ureteral catheter was inserted through the stenotic site. The ureteral wall was smooth, pelvic urine was collected for anti-acid staining and bacterial culture, and ureteral mucosa was collected at the stenotic site. The ureter dilator was used for progressive dilation of the stenotic segment until 12 Fr. Then, we attempted, but failed, to place 5 Fr and 7 Fr double pigtail stents and instead placed a 7 Fr double pigtail stent. There was no evidence of infection.
Case 2: On admission, this patient’s creatinine clearance rate (CCr) was 708 mmol/L.
Case 4: At admission, this patient’s glomerular filtration rate (GFR) was 1.4 and 46.3 mL/min in the left and right kidney, respectively.
Case 6: Prior to surgery, this patient’s serum CCr was 266 mmol/L.
Case 9: This patient’s initial serum creatinine (SCr) was 108 μmol/L, carcinoembryonic antigen was 6.06 ng/mL, and CYFRA 21-1 was 5.59 ng/mL. The GFR was 15.7 and 44.6 in the left kidney and right kidney, respectively. At subsequent admission, his SCr was 95 μmol/L and the GFR was 9.56 and 44.7 in the left and right kidney, respectively
Kidney function was dynamically monitored for all patients at baseline and postoperatively. CT scans of the kidney, ureter and bladder were performed, which allowed the identification of hydronephrosis and ureteral obstruction and the evaluation of kidney function based on findings in the renal arterial and venous phases.
Final diagnosis in all cases was ureteral obstruction due to external compression, which led to hydronephrosis and kidney dysfunction. All patients were indicated for metallic stent placement.
For all 9 patients, on the 2nd d after admission, a retrograde metallic stent was placed in the ureter under X-ray guidance, while the patients were under general anesthesia. This procedure involved the application of a cystoscope (Olympus 24 Fr, Tokyo, Japan) and a Resonance® nickel/chrome/cobalt/molybdenum alloy stent (6 Fr) (Cook Medical, Bloomington, IN, United States). The stent was 24 cm for patients shorter than 165 cm, 26 cm for patients who were 165-175 cm, and 28 cm for patients taller than 175 cm. The retrograde insertion of a guide wire was performed to the renal pelvis, then a ureteral catheter (5, 6 or 8 Fr) was used to dilate the ureter. A 10 Fr double-chamber ureteral catheter was then inserted for further dilation of the ureter, while preserving the guide wire. An 8.3 Fr metallic stent sheath was then inserted, followed by withdrawal of the guide wire. The stent was then inserted retrogradely along the sheath, and the sheath was then removed. All procedures were performed in accordance with X-ray guidance, and the metallic stent was directly observed using the cystoscope.
The 9 patients received a mean number of 2.5 metallic stent placements. The mean follow-up time was 39 mo. No perioperative complications occurred in any patient, and stent placement provided relief from ureteral obstruction for all patients. Postoperative complications occurred in two patients: patient 2 had stent migration and repeated metallic stent infections between July 2016 and October 2016; and patient 4 had postoperative hematuria because he resumed normal activities after stent placement, contrary to instructions. The final location of the stent was confirmed by X-ray imaging after surgery. The examinations described above were performed postoperatively to evaluate therapeutic efficacy.
Because stent removal was recommended for this patient, a general double pigtail tube was placed (4.7 Fr Bard INLAY®). In July 2014, ultrasonography showed severe left hydronephrosis, so the patient was readmitted to the Department of Urology. A metallic stent was placed again and was refreshed. CT scans of the kidney, ureter and bladder in another hospital had shown that the renal cortical thickness was identical in the left and right kidneys. Renal angiography findings were favorable, and there was no left hydronephrosis.
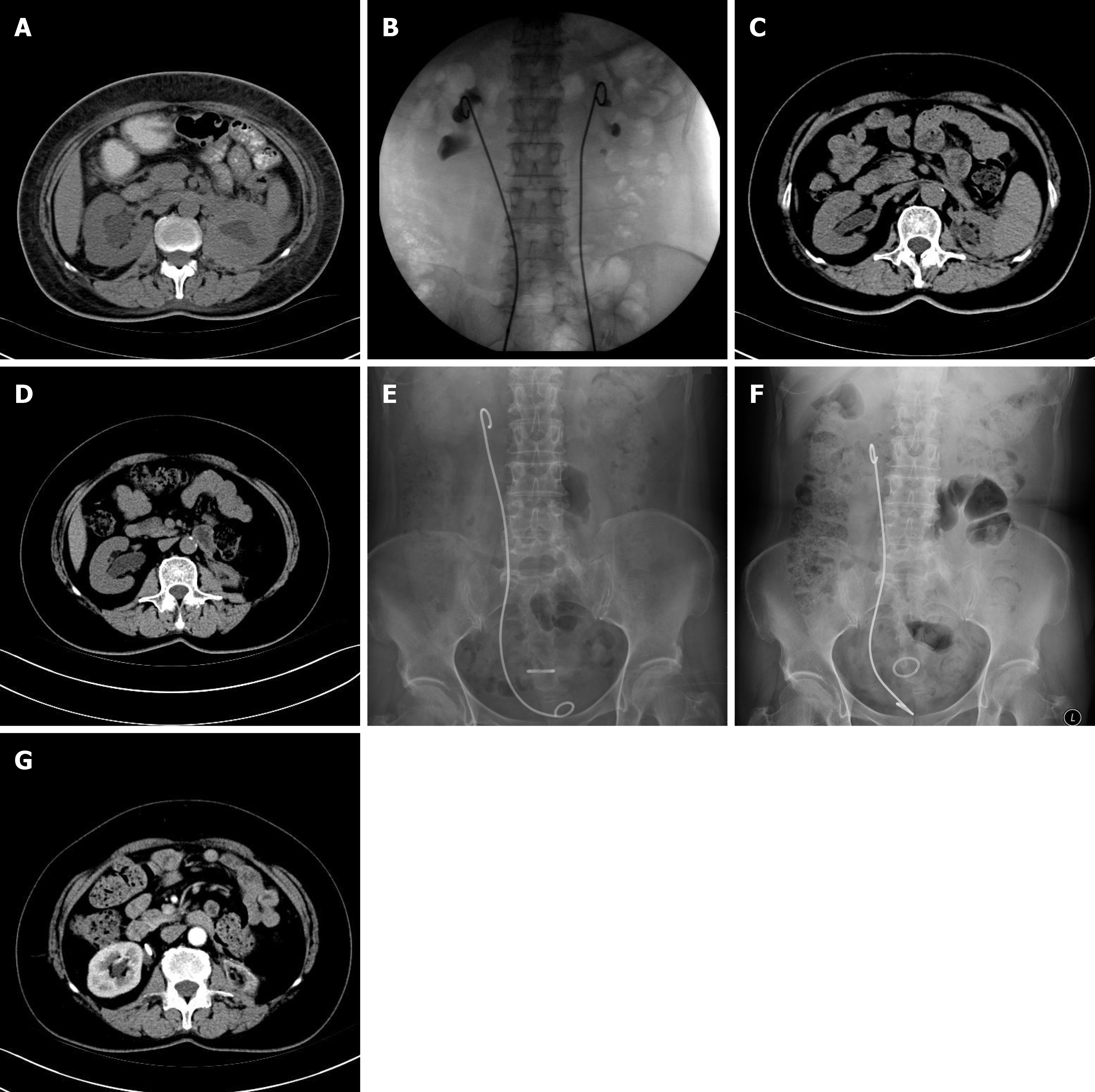
Obstruction was relieved for this patient after she received bilateral metallic stents. At 1 year later, the stent was removed in our department by another surgeon because a rheumatologist in another hospital refused further treatment if the stent was not removed. On 27 November 2014, a CT scan indicated complete left renal atrophy. Because this stent had migrated, the patient developed a urinary tract infection, with frequent urination, urinary urgency and dysuria; thus, she was admitted again on 24 October 24 2016. The stent was subsequently replaced with a shorter one (26 cm to 24 cm) because of her long legs and compact trunk. Her symptoms resolved after the operation. She received follow-up visits every 3 mo, including ultrasonography, and currently remains symptom-free (Figure 1).
After a bilateral metallic stent was placed, this patient’s kidney function improved soon after surgery. His GFR was 18.9 and 11.1 in the right and left kidney, respectively. However, this patient was lost to follow-up.
A right metallic stent was placed successfully for this patient. However, the patient remained physically active, which presumably led to repeated hematuria. A clinician from another hospital who did not agree with placement of the metallic stent recommended removal, but removal failed due to lack of cystoscopic equipment. A laparotomy was then performed for stent removal in the other institution. In January 2016, this patient was reported to have kidney dysfunction and leg edema. We recommended metallic stent placement, but he refused.
This patient received a right metallic stent in January 2013. On 18 July 2014, the stent was replaced with a polymeric stent (7 Fr Bard, INLAY®). Although he received follow-up ultrasonography every 3 mo, he developed moderate right hydronephrosis. On 29 June 2015, the polymeric stent was replaced with a metallic stent. Thereafter, the stent was refreshed yearly, and the last placement was in July 2016. The indwelling metallic stent existed for 13–39 mo.
A metallic stent was placed in the right ureter of this patient and relieved obstruction successfully. The stent was refreshed yearly thereafter.
In March 2015, a bilateral metallic stent was placed for this patient, and ureteral obstruction was relieved. The stent was refreshed in May 2016.
This patient received a left metallic stent placement in May 2015. His serum CCr had been 266 mmol/L before surgery, and 185 mmol/L 1 d after surgery. The GFR was 3.8 and 37.4 mL/min in the right and left kidney, respectively. On 22 May 2015, he was transferred into the Department of General Surgery because of T12-L5 fusion and a mass, suspected of being a tumor. Retroperitoneal exploration was conducted by laparoscopy, and laparotomy was performed. Postoperative pathological examination confirmed the diagnosis of IRPF, but this patient was not re-examined and the stent was also not replaced. A general stent had been placed at the initial treatment, but as hydronephrosis and kidney dysfunction worsened, it was replaced by a metallic stent after his kidney function stabilized.
After this patient received a 26 cm metallic stent in April 2013, he was readmitted into the Division of Rheumatology on 7 May 2013, where he received a diagnosis of IRPF and was treated with prednisone and tamoxifen, with dose reduction on 24 February 2014. Ultrasonography showed that both kidneys were normal. However, a CT scan showed the metallic stent in the collection system of the left kidney, the left kidney was smaller than the right kidney, the left renal cortex was thinned, soft tissues in the left ureteral area and around the left common iliac artery were reduced, and there was no retroperitoneal lymphadenopathy, leading to a suspected diagnosis of IgG4-related sclerotic disease. The patient was again admitted into the Department of Urology on 23 September 2014, and the metallic stent was refreshed. On admission, he received oral tamoxifen, but the steroid treatment was discontinued. His SCr was 106 μmol/L, and the GFR was 10.5 and 44.2 in the left and right kidney, respectively. He was readmitted to the Department of Urology on 30 May 2016, and the metallic stent was refreshed. On admission, he received oral tamoxifen. His SCr was 99 μmol/L, and the GFR was 10.46 and 44.6 mL/min in the left kidney and right kidney, respectively. The patient was followed regularly. On 1 June 2017, the contrast-enhanced CT still showed that the anterior and the bilateral sides of the aorta were surrounded by fibrotic tissues of peri-aortic inflammation caused by IRPF. The left ureter was involved in these tissues. However, contrast revealed that drainage of the left ureteral lumen was ensured by the metallic stent without obstruction.
Our institution admitted 9 patients with IRPF over a 5-year period, all of whom received Resonance® metallic stents to restore kidney function. We found that these stents relieved ureteral obstruction in all 9 cases, and that only 2 patients (patients 2 and 4) experienced adverse effects (stent migration and repeated metallic stent infections in patient 2, and postoperative hematuria in patient 4). Thus, these stents demonstrated effective relief and resulted in fewer complications for the treatment of ureteral stenosis in patients with IRPF.
In this case series, the Resonance® metallic ureteral stent had a mean indwelling time of 12 mo. The stent was longer than plastic or polymeric stents, which was echoed in the results reported by Christman et al[4] showing that the critical pressure was 4 lbs for plastic stents and 32 lbs for the Resonance® metallic stent. The authors of that study also reported that, after removal of the pressure, the plastic stent did not recover its original shape, but the metallic stent recovered completely and continued to provide good drainage. This parallels our experience with insertion of metallic stents.
The pathogenesis of IRPF is similar to that of other autoimmune diseases, in that it causes chronic and non-specific inflammation. The progressive fibrous hyperplasia around the retroperitoneal major arteries and compression of major tissues, especially the ureter, can cause obstruction[1]. In addition to pharmacological treatments for IRPF[9,10], surgery is also needed to provide relief from the inevitable ureteral obstruction. A ureteral stent is usually successful in restoring renal flow, but laparotomy /laparoscopy may be needed for ureterolysis if the disease continues to progress or the ureteroscopy fails[5]. In the present case series, we found that stent placement was successful in all 9 patients, and none of them required ureterolysis. Ultrasonography provides reliable evidence of hydronephrosis, aortic dilatation, aneurysm and masses besides the aorta[11-13]. CT, magnetic resonance imaging, Doppler-weighted ultrasound and fluorodeoxyglucose-positron emission tomography may also be used for diagnosis and the assessment of disease progression and treatment efficacy, as reported previously[14].
Detailed review of all 9 cases in this series shows that ureteral stents provide effective relief and reduce the incidence rate of perioperative and postoperative complications in patients with IRPF. IRPF is a rare and chronic disease, which made it very difficult for us to increase case numbers of IRPF. However, we conclude that our experience with these 9 cases underscores the utility and efficacy of applying the Resonance® metallic ureteral stent to treat ureteral obstruction in this patient population.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Moralioglu S S-Editor: Zhang L L-Editor: Filipodia E-Editor: Xing YX
| 1. | Vaglio A, Salvarani C, Buzio C. Retroperitoneal fibrosis. Lancet. 2006;367:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 472] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 2. | Urban ML, Palmisano A, Nicastro M, Corradi D, Buzio C, Vaglio A. Idiopathic and secondary forms of retroperitoneal fibrosis: a diagnostic approach. Rev Med Interne. 2015;36:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Oshiro H, Ebihara Y, Serizawa H, Shimizu T, Teshima S, Kuroda M, Kudo M. Idiopathic retroperitoneal fibrosis associated with immunohematological abnormalities. Am J Med. 2005;118:782-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Christman MS, L'esperance JO, Choe CH, Stroup SP, Auge BK. Analysis of ureteral stent compression force and its role in malignant obstruction. J Urol. 2009;181:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Duchene DA, Winfield HN, Cadeddu JA, Clayman RV, Gomella LG, Kavoussi LR, Mikhail AA, Park S, Permpongkosol S, Shalhav AL. Multi-institutional survey of laparoscopic ureterolysis for retroperitoneal fibrosis. Urology. 2007;69:1017-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Stifelman MD, Shah O, Mufarrij P, Lipkin M. Minimally invasive management of retroperitoneal fibrosis. Urology. 2008;71:201-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Benson AD, Taylor ER, Schwartz BF. Metal ureteral stent for benign and malignant ureteral obstruction. J Urol. 2011;185:2217-2222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Polcari AJ, Hugen CM, López-Huertas HL, Turk TM. Cost analysis and clinical applicability of the Resonance metallic ureteral stent. Expert Rev Pharmacoecon Outcomes Res. 2010;10:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Khosroshahi A, Stone JH. A clinical overview of IgG4-related systemic disease. Curr Opin Rheumatol. 2011;23:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 211] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 10. | Pelkmans LG, Hendriksz TR, Westenend PJ, Vermeer HJ, van Bommel EFH. Elevated serum IgG4 levels in diagnosis and treatment response in patients with idiopathic retroperitoneal fibrosis. Clin Rheumatol. 2017;36:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Borin JF, Melamud O, Clayman RV. Initial experience with full-length metal stent to relieve malignant ureteral obstruction. J Endourol. 2006;20:300-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Mishra K, Zargar H, Tarplin S, Ramanathan R, Stein RJ. Idiopathic retroperitoneal fibrosis. J Urol. 2015;193:1657-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Wah TM, Irving HC, Cartledge J. Initial experience with the resonance metallic stent for antegrade ureteric stenting. Cardiovasc Intervent Radiol. 2007;30:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Jansen I, Hendriksz TR, Han SH, Huiskes AW, van Bommel EF. (18)F-fluorodeoxyglucose position emission tomography (FDG-PET) for monitoring disease activity and treatment response in idiopathic retroperitoneal fibrosis. Eur J Intern Med. 2010;21:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |