Published online Jul 6, 2020. doi: 10.12998/wjcc.v8.i13.2833
Peer-review started: February 14, 2020
First decision: May 26, 2020
Revised: May 29, 2020
Accepted: June 17, 2020
Article in press: June 17, 2020
Published online: July 6, 2020
Processing time: 143 Days and 11.6 Hours
Pembrolizumab is a highly selective IgG4 kappa isotype monoclonal antibody against the programmed cell death-1 (PD-1) molecule. In the treatment of non-small cell lung cancer (NSCLC), pembrolizumab has demonstrated significant efficacy, significant survival outcomes, long-lasting responses, and a good safety profile compared with cytotoxic chemotherapy.
A 79-year-old Korean male presented with a left side palpable neck mass. An ultrasound-guided core-needle biopsy of the largest neck mass was performed, and squamous cell carcinoma was confirmed based on the histological and immunohistochemical findings. He was diagnosed with squamous cell carcinoma of the lung with multiple lymph nodes and rib metastases (T1N3M1b, Stage IVA) using enhanced chest computed tomography and 18F-fluorodeoxyglucose positron emission/computed tomography. After 4 cycles of gemcitabine and carboplatin, we clinically judged the disease as progressive. Owing to the high PD-1 expression demonstrated by the patient, pembrolizumab was initiated (200 mg every 3 wk). After 3 cycles of pembrolizumab, a complete response was achieved. At the 4th cycle of pembrolizumab, the white blood cell count was markedly elevated. Peripheral blood smear analysis and bone marrow biopsy were performed. The patient was diagnosed with acute myelomonocytic leukemia.
We present the first report of acute myelomonocytic leukemia during pembrolizumab treatment in an NSCLC patient; the mechanism remains unknown.
Core tip: In the treatment of non-small cell lung cancer (NSCLC), pembrolizumab has demonstrated significant efficacy, significant survival outcomes, long-lasting responses, and good safety profile. To the best of our knowledge, this is the first report of acute myelomonocytic leukemia during pembrolizumab in a patient with NSCLC. However, the precise underlying mechanism remains unknown.
- Citation: Kim HB, Park SG, Hong R, Kang SH, Na YS. Acute myelomonocytic leukemia during pembrolizumab treatment for non-small cell lung cancer: A case report. World J Clin Cases 2020; 8(13): 2833-2840
- URL: https://www.wjgnet.com/2307-8960/full/v8/i13/2833.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i13.2833
Lung cancer is the leading cause of cancer-related deaths worldwide. The treatment for non-small cell lung cancer (NSCLC) has changed in the last 10 years with the development of new and multiple treatments. Notably, immune checkpoint inhibitors are considered important treatment options for patients with NSCLC[1,2].
Pembrolizumab is a highly selective IgG4 kappa isotype monoclonal antibody against the programmed cell death-1 (PD-1). In NSCLC, pembrolizumab has demonstrated significant efficacy, significant survival outcomes, long-lasting responses, and good safety profile when compared to cytotoxic chemotherapy[1]. Among the adverse events observed with pembrolizumab treatment, acute myeloid leukemia (AML) has not been reported.
Herein, we report the first case of acute myelomonocytic leukemia during pembrolizumab therapy in a patient with NSCLC.
A 79-year-old Korean male presented with a left side palpable neck mass for 4 wk.
An ultrasound was performed at another institution and showed multiple various sized lymphadenopathies on both sides of the neck. He was referred to our hospital for evaluation of the neck mass
He was a non-smoker. He had no history of alcohol abuse. No previous medical history was available, and the patient presented no family history of malignant disease.
On examination of the neck, he had multiple nontender firm neck masses on both sides of the neck. The largest neck mass was 3.5 cm at level V of the left neck.
Based on the laboratory findings, the white blood cell (WBC) count was 3870/µL (normal range: 4000-8000/µL), hemoglobin was 8.3 g/dL (normal range: 12-16 g/dL), platelet count was 149 × 103/µL (normal range: 150-400 × 103/µL), and the C-reactive protein level was 0.84 mg/dL (normal range: 0.0-0.3 mg/dL), with the WBC differential including 43.8% neutrophils, 46.8% lymphocytes, 9.2% monocytes, and 0.2% eosinophils. The peripheral blood smear showed normocytic normochromic anemia.
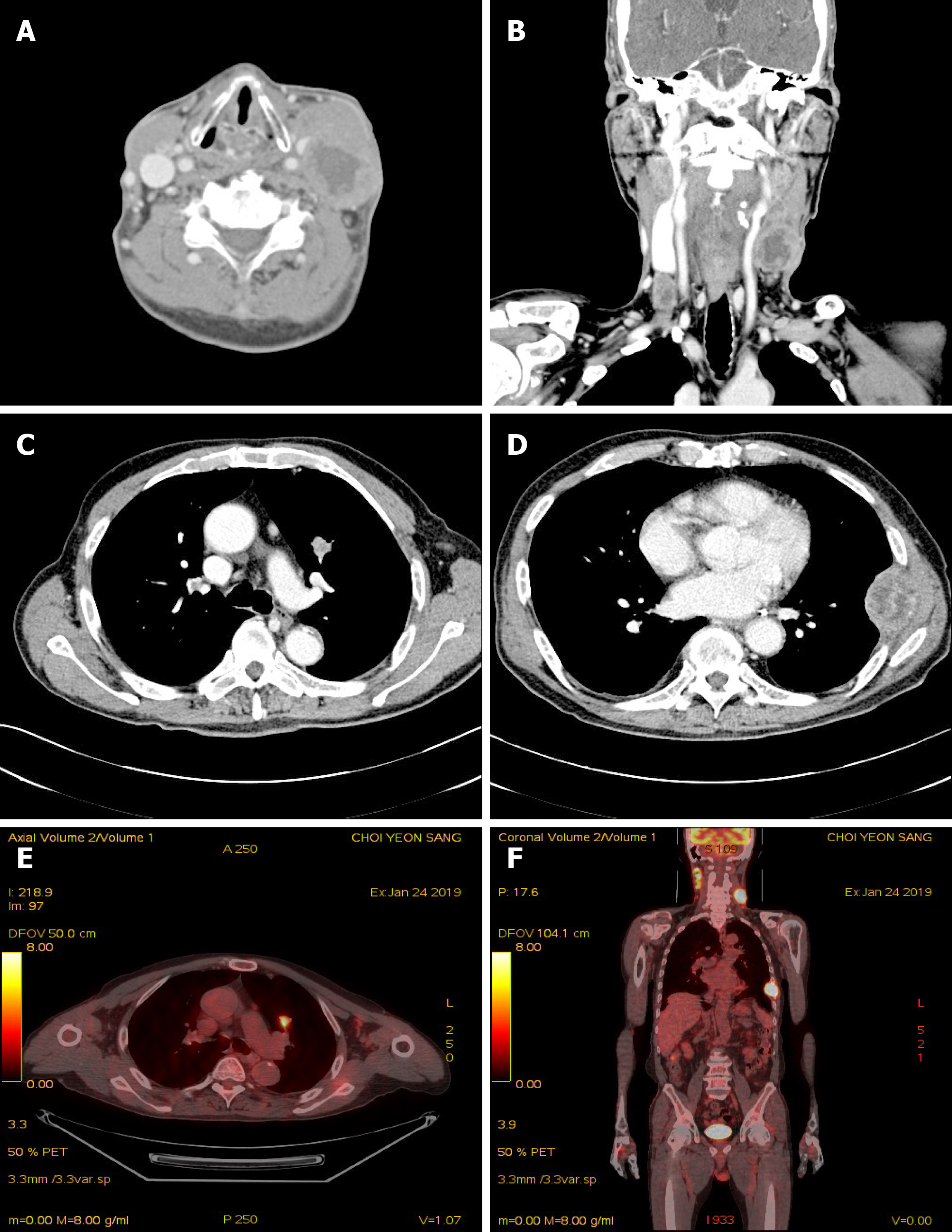
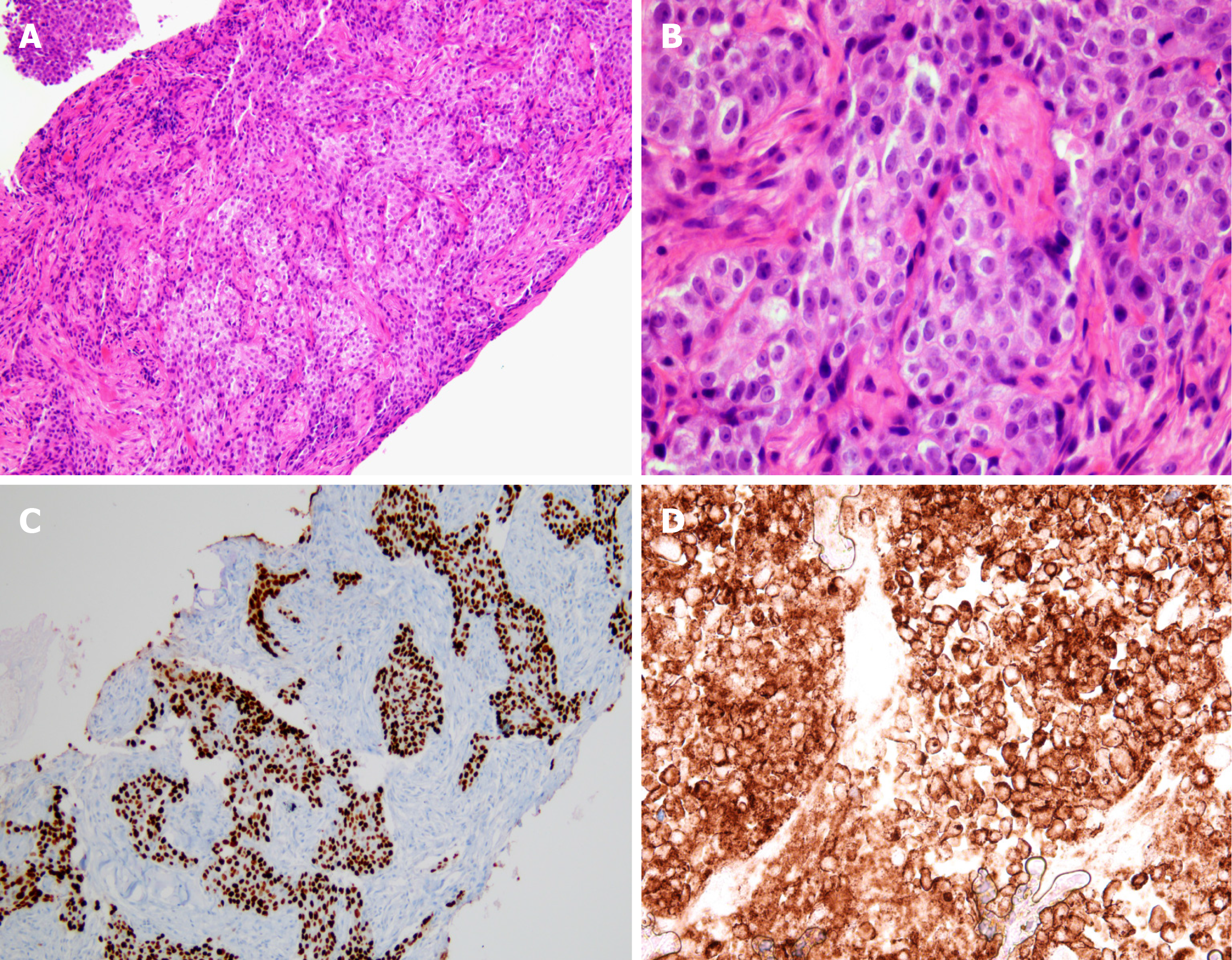
Contrast-enhanced computed tomography (CT) of the neck demonstrated multiple various sized necrotizing lymphadenopathies, from both cervical level II to V (Figure 1). Ultrasound-guided core-needle biopsy of the largest neck mass was performed. Histological findings on hematoxylin and eosin staining demonstrated keratinized malignant cells. Immunohistochemistry showed neoplastic cells positive for p63, and negative for TTF-1. The programmed death-ligand 1 (PD-L1) tumor proportion score was ≥ 50% (PD-L1 IHC 22C3 pharmDx™ Kit, DAKO, Denmark) (Figure 2). Squamous cell carcinoma was confirmed based on the histological and immunohistochemistry findings.
For evaluating the origin of lymph node metastasis, contrast-enhanced chest and abdominopelvic CT and 18F-FDG PET-CT were performed. The contrast-enhanced chest CT showed 2 cm sized heterogeneously enhanced nodules in the anterior segment of left upper lobe (LUL) and large periosteal mass formation involving the lateral arc of the 7th left rib. 18F-fluorodeoxyglucose positron emission/CT showed multiple enlarged hypermetabolic masses on both cervical level II to IV, a hypermetabolic nodule in LUL, and hypermetabolic mass on the 7th left rib (Figure 1). On head and neck examination, he demonstrated no remarkable findings.
The patient was diagnosed with squamous cell carcinoma of the lung with multiple lymph nodes and rib metastases. According to the 8th edition of the TNM classification of lung cancer, it was classified as clinical T1N3M1b, Stage IVA.
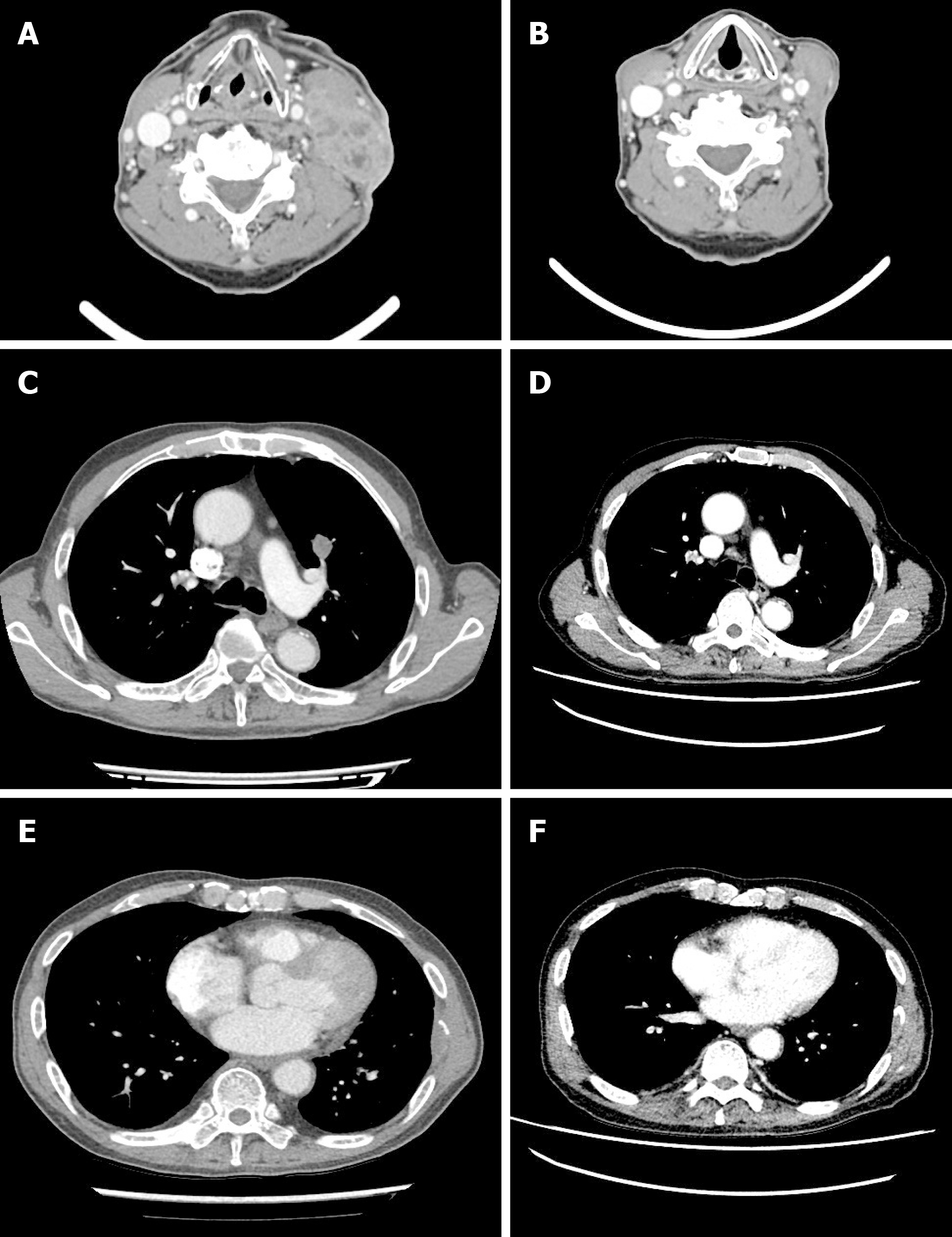
Beginning in February 2019, the patient was administered gemcitabine and carboplatin. After 2 cycles, the summed-up diameters of both target lesions (lymph node in both cervical level IV, a nodule in LUL, and mass on the 7th left rib) decreased from 156 mm to 97 mm, i.e., a 37.82% reduction of the baseline diameter. Partial response was achieved according to the Response Evaluation Criteria in Solid Tumors version 1.1. However, after 4 cycles, the summed-up diameters of both target lesions increased from 97 mm to 112 mm i.e., a 15.5% increase of nadir diameter. A comprehensive evaluation of the curative effect assessed the disease as stable. However, a lymph node at left cervical level IV was growing rapidly (42 mm to 56 mm). We clinically judged the disease as progressive. Hence, in April 2019, pembrolizumab was initiated with 200 mg every 3 wk since the patient demonstrated high PD-L1 expression. After 3 cycles, a complete response was achieved (Figure 3). However, during the visit for the 4th cycle of pembrolizumab, he presented symptoms of upper respiratory tract infection and a markedly elevated WBC count. Hence, we discontinued treatment.
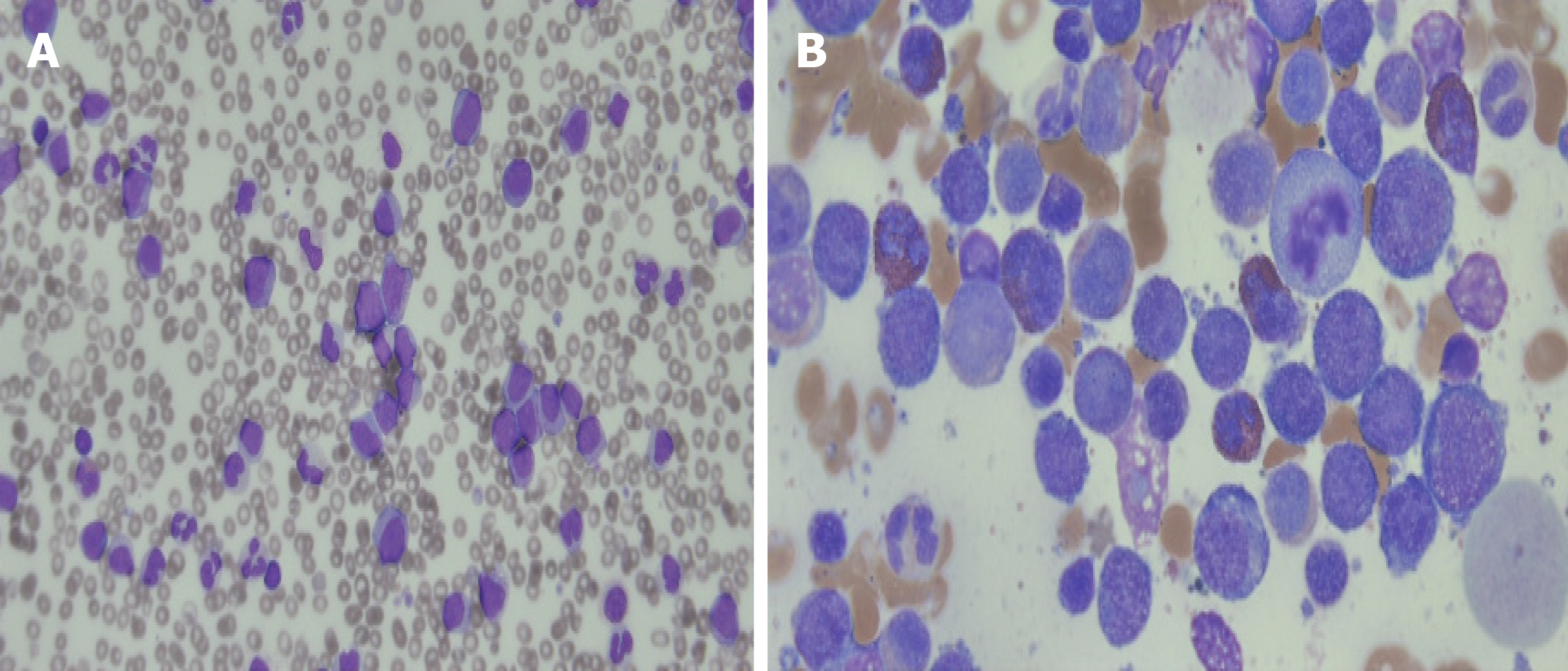
A week after discontinuation of pembrolizumab therapy, the elevated WBC count persisted (Table 1) and a peripheral blood smear analysis was performed. The blood smear showed 15% blast cells and hypogranulation with basophilia with monocytosis. Therefore, a bone marrow biopsy was performed which demonstrated an increased number of myeloblasts, with 40.92% of nucleated cells (Figure 4). The myeloblasts were positive for CD11c, CD13, CD33, and MPO expression. Routine cytogenetic analysis revealed a normal male karyotype [46, XY (20)]. A multiplex, nested reverse transcription PCR assay for BCR/ABL, AML1/ETO, and PML/RARA gene rearrangement associated with acute leukemia did not detect any abnormalities. He was diagnosed with acute myelomonocytic leukemia. With a WBC count exceeding 100000/µL, he was started on hydroxyurea to control the leukocytosis. During the preparation of induction chemotherapy for acute myelomonocytic leukemia, he demonstrated diffuse bilateral coalescent opacities on chest CT. He went int acute respiratory distress syndrome and died.
| CBC (reference values) | Pem-1 | Pem-2 | Pem-3 | HD-0 (3 wk after Pem-3) | HD-7 | HD-17 | HD-20 |
| WBC (/µL) (4000-8000/µL) | 2290 | 6030 | 7410 | 24630 | 40720 | 163740 | 273920 |
| Monocyte (%) (2%-8%) | 7.4 | 5.0 | 13.6 | 12.1 | 18.3 | 14.9 | 16.6 |
| Hgb (g/dL) (12-16 g/dL) | 7.7 | 8.8 | 8.3 | 8.0 | 7.4 | 7.5 | 5.7 |
| Plt (× 103/µL) (150-400 × 103/µL) | 63 | 228 | 87 | 33 | 52 | 62 | 84 |
The treatment-related adverse events associated with pembrolizumab include diarrhea, pruritus, and immune-related events[3]. Notably, as an activated immune system reacts against both tumor antigens and antigens on healthy tissues, immune checkpoint blockade can present inflammatory side effects, termed as immune-related adverse events. However, the precise mechanism remains unknown. Immune-related adverse events are most common within 12 wk post-therapy, and could occur up to 6 mo after the discontinuation of immune checkpoint inhibitors[4]. In our patient, leukocytosis was observed 9 wk after the start of pembrolizumab.
Immune-related adverse events can affect any organ. Immune checkpoint blockade most commonly affects the gastrointestinal tract, endocrine glands, skin, and the liver[5,6]. In advanced NSCLC patients, the most common immune-related adverse events reported during pembrolizumab therapy include hypothyroidism, hyperthyroidism, and pneumonitis[7]. Hematological toxicities observed with immune checkpoint inhibitors are anemia, thrombocytopenia, and platelet factor-related acquired bleeding disorder[4]. However, an association between immune-related adverse events and AML has not been reported. Our patient was diagnosed with acute myelomonocytic leukemia after 3 cycles of pembrolizumab. The relationship between AML and immune-related adverse events associated with pembrolizumab remains controversial. Owing to the activation of endogenous T cell responses, current clinical trials using immune checkpoint inhibitors for the treatment of AML are ongoing. Furthermore, immune checkpoint inhibitors for immunotherapy in AML are yet to demonstrate clinical effectiveness[8].
In the case of our patient, therapy-related myeloid neoplasms could be considered. Therapy-related myeloid neoplasms are a distinct subgroup in the AML classification and generally develop in patients who have received alkylating agents, topoisomerase II inhibitors, and/or radiation therapy for a primary malignancy or autoimmune disease[9]. In a report from the Swedish acute leukemia registry, in patients with therapy-related AML, the most commonly reported primary malignancies were breast cancer (21%) and non-Hodgkin lymphomas (19%)[10]. The latency periods may vary from several months up to 10 years between exposure to anticancer drugs and the development of therapy-related myeloid neoplasms. Alkylating agents can induce therapy-related myeloid neoplasms after a median latency of 4-7 years[11,12]. Our patient was administered a chemotherapy regimen consisting of carboplatin and gemcitabine for 4 cycles. The latency interval for the development of therapy-related AML was 4 mo that the latency was shorter than in other case reports. No chromosomal abnormalities were observed in the case of our patient.
As an additional hypothesis, hyperprogression after immunotherapy may be considered. An acceleration of tumor growth in patients treated with PD-1/PD-L1 inhibitors was reported in 9% of advanced cancers and was associated with a high metastatic burden and poor prognosis[13,14]. However, there is no consensual definition for hyperprogression. Recently, Matos et al[15] proposed a new definition that was based on RECIST 1.1 as time to treatment failure < 2 mo and minimum increase in measurable lesions of 10 mm plus: (1) Increase of ≥ 40% in target tumor burden compared to baseline; or (2) Increase of ≥ 20% plus the appearance of multiple new lesions[15]. Ratner et al[16] reported that PD-1 inhibitor, nivolumab, led to rapid progression of adult T-cell leukemia/lymphoma after treatment[16,17]. Although this case report was not for AML, it was shown that hyperprogression may occur after immunotherapy in the hematologic malignancy. If we assumed that our patient presented a double primary cancer with advanced lung cancer and pre-clinical AML, the advanced lung cancer showed complete response after pembrolizumab, but pre-clinical AML might have progressed to clinical AML due to hyperprogression. This is could be explained by the enhanced rate of leukocyte increase following 3 cycles of pembrolizumab, and the infiltration of the lungs with the elevated leukocytes, unlike the progression of AML.
In summary, to the best of our knowledge, this is the first report of acute myelomonocytic leukemia during pembrolizumab in a patient with NSCLC. However, the precise underlying mechanism remains unknown. Further research is required to understand the mechanism and evaluate the prevalence of this adverse event.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liang Y S-Editor: Yan JP L-Editor: A E-Editor: Xing YX
| 1. | Corrales L, Scilla K, Caglevic C, Miller K, Oliveira J, Rolfo C. Immunotherapy in Lung Cancer: A New Age in Cancer Treatment. In: Naing A, Hajjar J. Immunotherapy. Cham: Springer International Publishing 2018; 65-95. |
| 2. | Kim HC, Choi CM. Current Status of Immunotherapy for Lung Cancer and Future Perspectives. Tuberc Respir Dis (Seoul). 2020;83:14-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A; KEYNOTE-006 investigators. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4026] [Cited by in RCA: 4487] [Article Influence: 448.7] [Reference Citation Analysis (1)] |
| 4. | Johnson DB, Chandra S, Sosman JA. Immune Checkpoint Inhibitor Toxicity in 2018. JAMA. 2018;320:1702-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 198] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 5. | Esfahani K, Meti N, Miller WH, Hudson M. Adverse events associated with immune checkpoint inhibitor treatment for cancer. CMAJ. 2019;191:E40-E46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 3157] [Article Influence: 451.0] [Reference Citation Analysis (0)] |
| 7. | Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4116] [Cited by in RCA: 5052] [Article Influence: 561.3] [Reference Citation Analysis (0)] |
| 8. | Lichtenegger FS, Krupka C, Haubner S, Köhnke T, Subklewe M. Recent developments in immunotherapy of acute myeloid leukemia. J Hematol Oncol. 2017;10:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 9. | Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2981] [Cited by in RCA: 3182] [Article Influence: 198.9] [Reference Citation Analysis (0)] |
| 10. | Hulegårdh E, Nilsson C, Lazarevic V, Garelius H, Antunovic P, Rangert Derolf Å, Möllgård L, Uggla B, Wennström L, Wahlin A, Höglund M, Juliusson G, Stockelberg D, Lehmann S. Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: a report from the Swedish Acute Leukemia Registry. Am J Hematol. 2015;90:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 199] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 11. | Fianchi L, Criscuolo M, Fabiani E, Falconi G, Maraglino AME, Voso MT, Pagano L. Therapy-related myeloid neoplasms: clinical perspectives. Onco Targets Ther. 2018;11:5909-5915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Fianchi L, Pagano L, Piciocchi A, Candoni A, Gaidano G, Breccia M, Criscuolo M, Specchia G, Maria Pogliani E, Maurillo L, Aloe-Spiriti MA, Mecucci C, Niscola P, Rossetti E, Mansueto G, Rondoni M, Fozza C, Invernizzi R, Spadea A, Fenu S, Buda G, Gobbi M, Fabiani E, Sica S, Hohaus S, Leone G, Voso MT. Characteristics and outcome of therapy-related myeloid neoplasms: Report from the Italian network on secondary leukemias. Am J Hematol. 2015;90:E80-E85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 13. | Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, Chaput N, Eggermont A, Marabelle A, Soria JC, Ferté C. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin Cancer Res. 2017;23:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 703] [Cited by in RCA: 905] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 14. | Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, Mazieres J, Zalcman G, Brosseau S, Le Moulec S, Leroy L, Duchemann B, Lefebvre C, Veillon R, Westeel V, Koscielny S, Champiat S, Ferté C, Planchard D, Remon J, Boucher ME, Gazzah A, Adam J, Bria E, Tortora G, Soria JC, Besse B, Caramella C. Hyperprogressive Disease in Patients With Advanced Non-Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA Oncol. 2018;4:1543-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 557] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 15. | Matos I, Martin-Liberal J, Hierro C, Ochoa De Olza M, Viaplana C, Costa M, Felip-Falg’s E, Mur-Bonet G, Vieito M, Brana I, Azaro A, Perez-Gago C, Rodriguez-Freixinos V, Argiles G, Oliveira M, Felip E, Muñoz-Couselo E, Tabernero J, Dienstmann R, Garralda E. Incidence and clinical implications of a new definition of hyperprogression (HPD) with immune checkpoint inhibitors (ICIs) in patients treated in phase 1 (Ph1) trials. J Clin Oncol. 2018;36:3032-3032. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Ratner L, Waldmann TA, Janakiram M, Brammer JE. Rapid Progression of Adult T-Cell Leukemia-Lymphoma after PD-1 Inhibitor Therapy. N Engl J Med. 2018;378:1947-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 17. | Rauch DA, Conlon KC, Janakiram M, Brammer JE, Harding JC, Ye BH, Zang X, Ren X, Olson S, Cheng X, Miljkovic MD, Sundaramoorthi H, Joseph A, Skidmore ZL, Griffith O, Griffith M, Waldmann TA, Ratner L. Rapid progression of adult T-cell leukemia/lymphoma as tumor-infiltrating Tregs after PD-1 blockade. Blood. 2019;134:1406-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |