Published online Jul 6, 2020. doi: 10.12998/wjcc.v8.i13.2738
Peer-review started: February 27, 2020
First decision: April 21, 2020
Revised: May 25, 2020
Accepted: June 9, 2020
Article in press: June 9, 2020
Published online: July 6, 2020
Processing time: 131 Days and 0.1 Hours
The effects of prostaglandin E (PGE) combined with continuous renal replacement therapy (CRRT) on renal function and inflammatory responses in patients with septic acute kidney injury (SAKI) remain unclear.
To investigate the effects of PGE combined with CRRT on urinary augmenter of liver regeneration (ALR), urinary Na+/H+ exchanger 3 (NHE3), and serum inflammatory cytokines in patients with SAKI.
The clinical data of 114 patients with SAKI admitted to Yichang Second People's Hospital from May 2017 to January 2019 were collected. Fifty-three cases treated by CRRT alone were included in a control group, while the other 61 cases treated with PGE combined with CRRT were included in an experimental group. Their urinary ALR, urinary NHE3, serum inflammatory cytokines, renal function indices, and immune function indices were detected. Changes in disease recovery and the incidence of adverse reactions were observed. The 28-d survival curve was plotted.
Before treatment, urinary ALR, urinary NHE3, blood urea nitrogen (BUN), serum creatinine (SCr), CD3+ T lymphocytes, CD4+ T lymphocytes, and CD4+/CD8+ T lymphocyte ratio in the control and experimental groups were approximately the same. After treatment, urinary ALR and NHE3 decreased, while BUN, SCr, CD3+ T lymphocytes, CD4+ T lymphocytes, and CD4+/CD8+ T lymphocyte ratio increased in all subjects. Urinary ALR, urinary NHE3, BUN, and SCr in the experimental group were significantly lower than those in the control group, while CD3+ T lymphocytes, CD4+ T lymphocytes, and CD4+/CD8+ T lymphocyte ratio were significantly higher than those in the control group (P < 0.05). After treatment, the levels of tumor necrosis factor-α, interleukin-18, and high sensitivity C-reactive protein in the experimental group were significantly lower than those in the control group (P < 0.05). The time for urine volume recovery and intensive care unit treatment in the experimental group was significantly shorter than that in the control group (P < 0.05), although there was no statistically significant difference in hospital stays between the two groups. The total incidence of adverse reactions did not differ statistically between the two groups. The 28-d survival rate in the experimental group (80.33%) was significantly higher than that in the control group (66.04%).
PGE combined with CRRT is clinically effective for treating SAKI, and the combination therapy can significantly improve renal function and reduce inflammatory responses.
Core tip: Prostaglandin E combined with continuous renal replacement therapy is clinically effective for treating septic acute kidney injury, and the combination therapy can significantly improve renal function and reduce inflammatory responses. However, there are still unsolved problems in this study. For instance, the patient survival has been observed for 28 d, and causes of patient death are various, but no detailed analysis has been made on changes in the survival rate to improve the selection accuracy of therapeutic schemes. In addition, more reference directions for adverse reactions can be provided to improve the safety of drugs. These deficiencies will be our further research direction, so as to provide effective measures for the early treatment of patients with septic acute kidney injury.
- Citation: Lei L, Wang MJ, Zhang S, Hu DJ. Effects of prostaglandin E combined with continuous renal replacement therapy on septic acute kidney injury. World J Clin Cases 2020; 8(13): 2738-2748
- URL: https://www.wjgnet.com/2307-8960/full/v8/i13/2738.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i13.2738
As a common disease in the intensive care unit (ICU), sepsis refers to a condition that immune function is affected by inflammatory cells and then leads to related multiple organ dysfunction. The disease is uncontrollable and persistent, and there are more new cases and deaths worldwide every year, which have been increasing year by year[1,2]. Acute kidney injury (AKI) is the most common complication of sepsis, and sepsis combined with AKI has a mortality rate about 2 times higher than that of sepsis alone[3]. Sepsis is usually caused by severe trauma, suppuration, and other infectious conditions. Severe conditions cause toxins and pathogenic bacteria to rapidly spread from the infected site to blood circulation, finally resulting in systemic infection[4]. Since inflammatory responses are a critical factor during sepsis progression, the clearance of inflammatory mediators in vivo is an important direction for the prevention and treatment of septic AKI (SAKI).
Continuous renal replacement therapy (CRRT) is a continuous and slow blood purification treatment that removes retained water and solute in an adsorptive, convective, and dispersive blood purification mode with extracorporeal circulation. This therapy removes serum pro- and anti-inflammatory mediators and helps the recovery of body immune function in patients, thus greatly restoring the physiological functions of damaged kidneys[5,6]. Prostaglandin E (PGE) pharmacologically affects vasodilation and platelet aggregation, so as to protect vascular endothelium and improve microcirculation[7]. Studies have shown that the apoptosis or necrosis of proximal tubular epithelial cells changes when AKI occurs. According to histology, the brush border of renal tubular epithelial cells (RTECs) falls off, inflammatory cells infiltrate, and secretory protein casts form[8,9]. Augmenter of liver regeneration (ALR) inhibits local inflammatory pathological changes in the renal tissue and promotes the regeneration and repair of RTECs[10,11]. Urinary Na+/H+ exchanger 3 (NHE3), which can be seen in proximal convoluted tubules, is a transmembrane glycoprotein that regulates transmembrane transport, renal water and salt reabsorption, and intracellular pH balance[12]. As there are few related reports on PGE in SAKI, the effects of PGE combined with CRRT on the improvement of SAKI prognosis are doubtful. Therefore, the specific effects of the combination therapy on the urinary ALR, urinary NHE3, and inflammatory responses in patients with SAKI were investigated in the present study.
The clinical data of 114 patients with SAKI admitted to Yichang Second People's Hospital from May 2017 to January 2019 were collected. Fifty-three patients treated with CRRT alone were included in a control group, while the other 61 patients treated with PGE combined with CRRT were included in an experimental group. The patients consisted of 80 males and 34 females, with an average age of 52.99 ± 12.86 years and an average course of disease of 2.62 ± 0.59 years. Based on pathogenic bacteria, there were 65 cases of Gram-negative bacteria, 40 cases of Gram-positive bacteria, and 9 cases of mixed infection. Based on causes, there were 30 cases of severe pancreatitis, 46 cases of pneumonia, 25 cases of non-suppurative cholangitis, and 13 cases of unknown causes. The inclusion criteria were: (1) Patients with SAKI conforming to the Diagnostic Criteria for Sepsis[13] and Diagnostic Criteria Guidelines of AKI[14]; (2) Patients complicated with glomerulonephritis, interstitial nephritis, or urinary tract obstruction; (3) Patients who did not receive immunosuppression-related treatment within 3 mo; and (4) Patients without severe active hemorrhage. The exclusion criteria were: (1) Patients with previous kidney transplantation; (2) Patients who had been treated by dialysis for a long time; and (3) Patients with dysgnosia and those who could not participate in the experiment.
Patients in both groups were treated with comprehensive medical treatment such as fluid resuscitation, anti-infective shock, and nutritional support. A bedside hemofiltration apparatus (FX80, Fresenius, German) was used for CRRT, adjusted to the continuous veno-venous hemofiltration mode. The flow range was between 20-50 mL/(kg·h) for treatment over 12-24 h each time, and the blood flow was controlled to 200-220 mL/min. The patients were anticoagulated with low molecular heparin, and treated for 5 consecutive days. Patients in the experimental group were additionally treated with PGE (Shanghai YuanYe Biotechnology Co., Ltd., Batch No.: S27091), and the mixed solution (20 μg of PGE + 100 mL of normal saline) of standard dose was slowly and intravenously dripped twice per day. They were treated for 7 consecutive days.
Urine and serum samples were collected before and after treatment. Enzyme-linked immunosorbent assay (ELASA) was used to determine the expression levels of urinary ALR, urinary NHE3, tumor necrosis factor-α (TNF-α), interleukin (IL)-18, and high sensitivity C-reactive protein (hs-CRP), with the operating steps strictly carried out according to the instructions of the ELASA kit (BRAHMS, German). Levels of blood urea nitrogen (BUN) and serum creatinine (SCr) were detected with a fully automated biochemical analyzer (Shenzhen iCubio Biomedical Technology Co., Ltd.). The percentages of CD3+, CD4+, and CD8+ T lymphocytes were detected by flow cytometry (BD, United States).
The levels of urinary ALR and urinary NHE3 before and after treatment, serum inflammatory cytokines after treatment, and renal and immune functions before and after treatment were compared between the control and experimental groups. Disease recovery indices (time for urine volume recovery, time for ICU treatment, and hospital stays) and adverse reactions in the two groups were recorded. Kaplan-Meier (K-M) survival curve was plotted to analyze the survival of patients with SAKI within 28 d after treatment.
SPSS 19.0 was used to statistically analyze the experimental data. Measurement data are expressed as the mean ± SD, and were analyzed by t test. Count data are expressed as percentages (%) and were analyzed by χ2 test, and their comparison between two groups was analyzed by t test. The K-M survival curve was plotted to show the patient survival within 28 d after treatment, and log-rank test was used for analysis. Graphpad Prism8 was used to plot figures. P < 0.05 indicated a statistically significant difference.
There were no significant differences between the control and experimental groups in terms of basic data, pathogenic bacteria, disease conditions (course of disease and causes), or basic diseases (Table 1).
| Control group (n = 53) | Experimental group (n = 61) | t/χ2 | P value | |
| Gender (cases) | 0.216 | 0.642 | ||
| Male | 38 (71.70) | 42 (68.85) | ||
| Female | 15 (28.30) | 19 (31.15) | ||
| Age (yr) | 52.64 ± 12.85 | 53.14 ± 12.93 | 0.207 | 0.837 |
| BMI (kg/m2) | 26.84 ± 3.42 | 26.94 ± 3.36 | 0.157 | 0.875 |
| Course of disease (yr) | 2.56 ± 0.58 | 2.67 ± 0.59 | 1.001 | 0.319 |
| Time for intensive care unit treatment before enrollment (d) | 2.13 ± 1.03 | 2.16 ± 1.05 | 0.154 | 0.878 |
| Pathogenic bacteria (cases) | 1.092 | 0.579 | ||
| Gram-negative bacteria | 31 (58.49) | 34 (55.74) | ||
| Gram-positive bacteria | 19 (35.85) | 21 (34.43) | ||
| Mixed infection | 3 (5.66) | 6 (9.84) | ||
| Causes (cases) | 0.147 | 0.986 | ||
| Severe pancreatitis | 14 (26.42) | 16 (26.23) | ||
| Pneumonia | 21 (39.62) | 25 (40.98) | ||
| Non-suppurative cholangitis | 12 (22.64) | 13 (21.31) | ||
| Unknown causes | 6 (11.32) | 7 (11.48) | ||
| SOFA score (points) | 10.38 ± 1.03 | 10.36 ± 1.06 | 0.102 | 0.919 |
| APACHE II score (points) | 23.91 ± 2.17 | 23.67 ± 2.11 | 0.598 | 0.551 |
| RIFLE classification (cases) | 0.040 | 0.980 | ||
| Risk period | 11 (20.75) | 12 (19.67) | ||
| Injury period | 17 (32.08) | 20 (32.79) | ||
| Failure period | 25 (47.17) | 29 (47.54) | ||
| Diabetes (cases) | 0.185 | 0.667 | ||
| Yes | 21 (39.62) | 26 (42.62) | ||
| No | 32 (60.38) | 35 (57.38) | ||
| Hypertension (cases) | 0.020 | 0.887 | ||
| Yes | 23 (43.40) | 27 (44.26) | ||
| No | 30 (56.60) | 34 (55.74) |
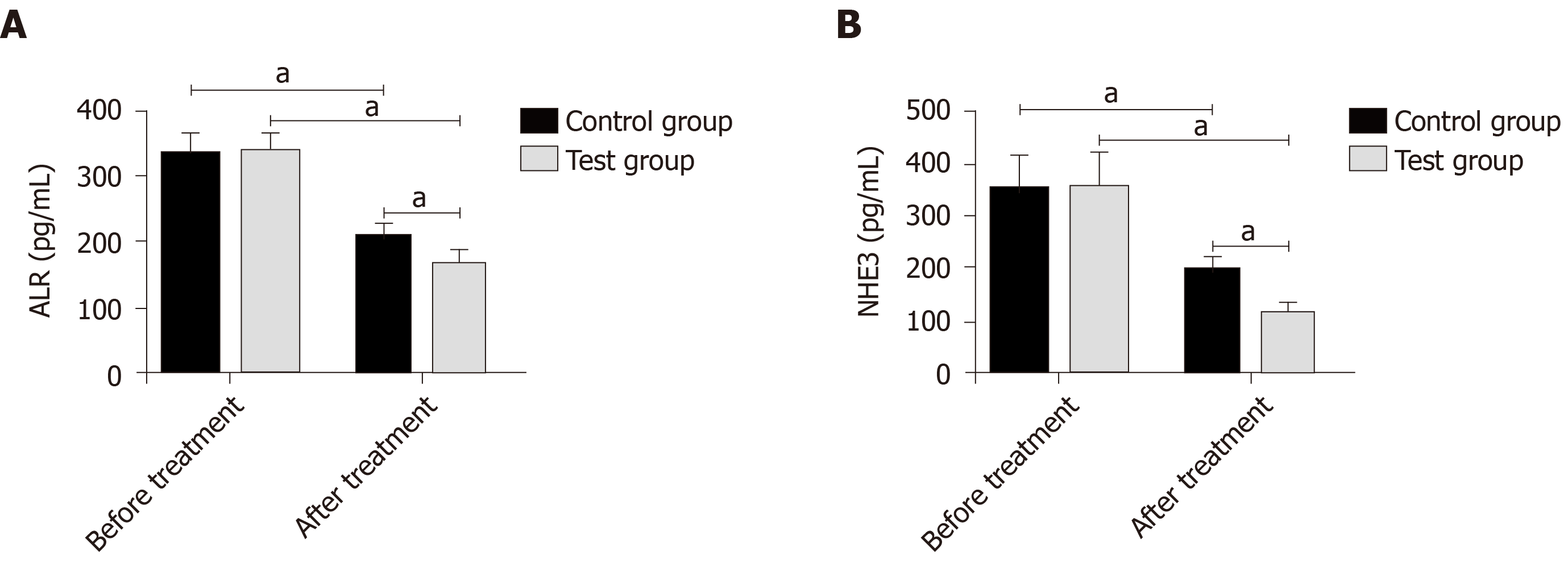
Before treatment, the levels of urinary ALR and NHE3 in the control and experimental groups were approximately the same. After treatment, the levels in all subjects decreased, and the decrease in the experimental group was more significant than that in the control group (P < 0.05) (Figure 1).
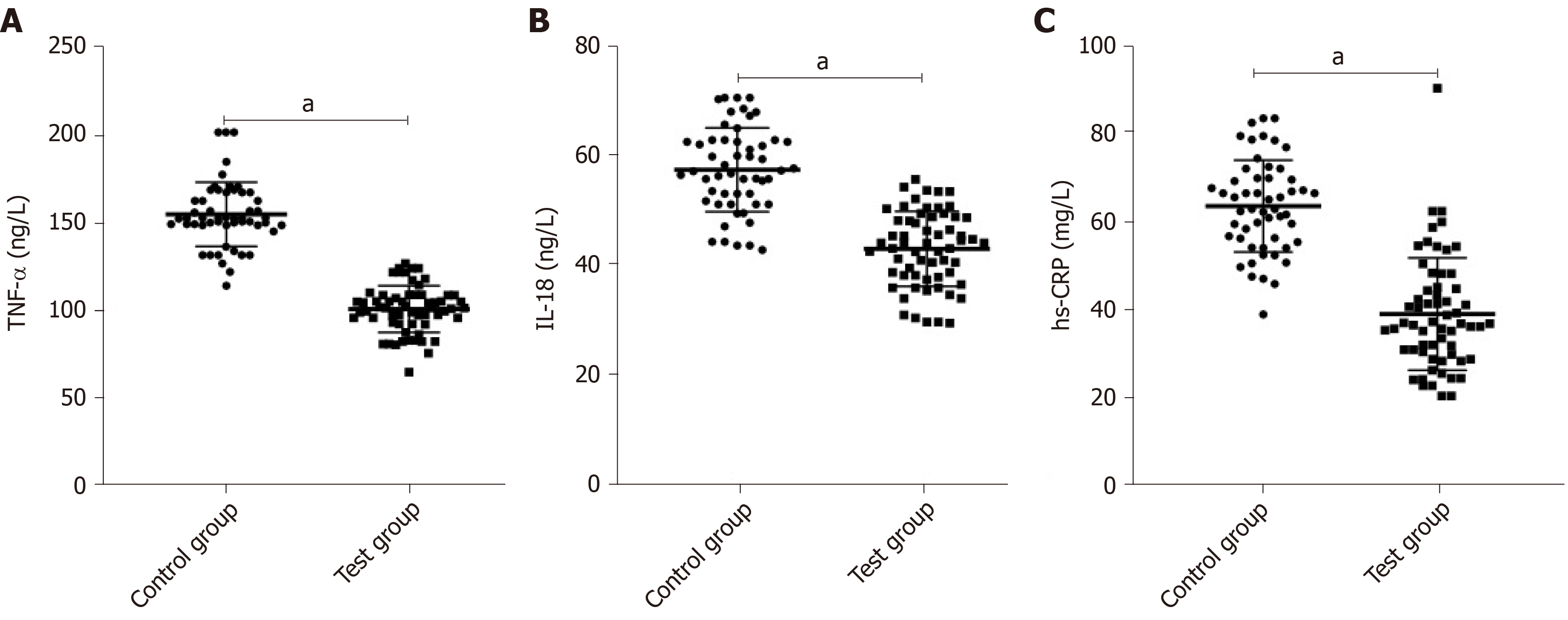
After treatment, the levels of TNF-α, IL-18, and hs-CRP in the experimental group were significantly lower than those in the control group (P < 0.05) (Figure 2).
Before treatment, the levels of BUN and SCr in the control group were about the same as those in the experimental group. After treatment, the levels in all subjects increased, but the increase in the experimental group was smaller than that in the control group (P < 0.05) (Figure 3).
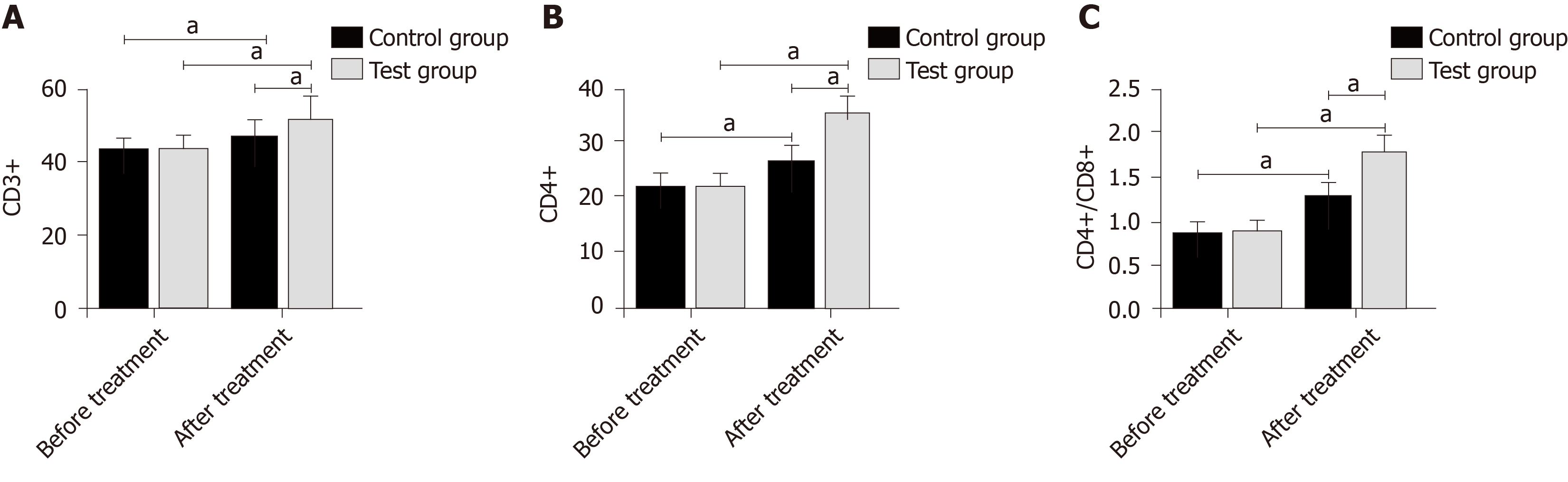
Before treatment, CD3+ T lymphocytes, CD4+ T lymphocytes, and CD4+/CD8+ T lymphocyte ratio in the control and experimental groups were approximately the same. After treatment, the indices in all subjects increased, and the increase in the experimental group was more significant than that in the control group (P < 0.05) (Figure 4).
The time for urine volume recovery and ICU treatment in the experimental group was significantly shorter than that in the control group (P < 0.05), although there was no statistically significant difference in hospital stays between the two groups (Table 2).
| Control group (n = 53) | Experimental group (n = 61) | t | P value | |
| Time for urine volume recovery (d) | 14.37 ± 3.84 | 9.73 ± 2.91 | 7.324 | < 0.001 |
| Time for intensive care unit treatment (d) | 18.83 ± 7.57 | 13.24 ± 5.26 | 4.625 | < 0.001 |
| Hospital stays (d) | 34.53 ± 15.24 | 30.13 ± 14.13 | 1.599 | 0.113 |
The total incidence of adverse reactions in the control and experimental groups was approximately the same (Table 3).
| Control group (n = 53) | Experimental group (n = 61) | χ2 | P vulue | |
| Hypotension | 2 (3.77) | 2 (3.28) | - | - |
| Arrhythmia | 2 (3.77) | 1 (1.64) | - | - |
| Hypertension | 3 (5.66) | 3 (4.92) | - | - |
| Vomiting | 2 (3.77) | 3 (4.92) | - | - |
| Headache | 2 (3.77) | 1 (1.64) | - | - |
| Total incidence of adverse reactions | 11 (20.75) | 10 (16.39) | 0.829 | 0.363 |
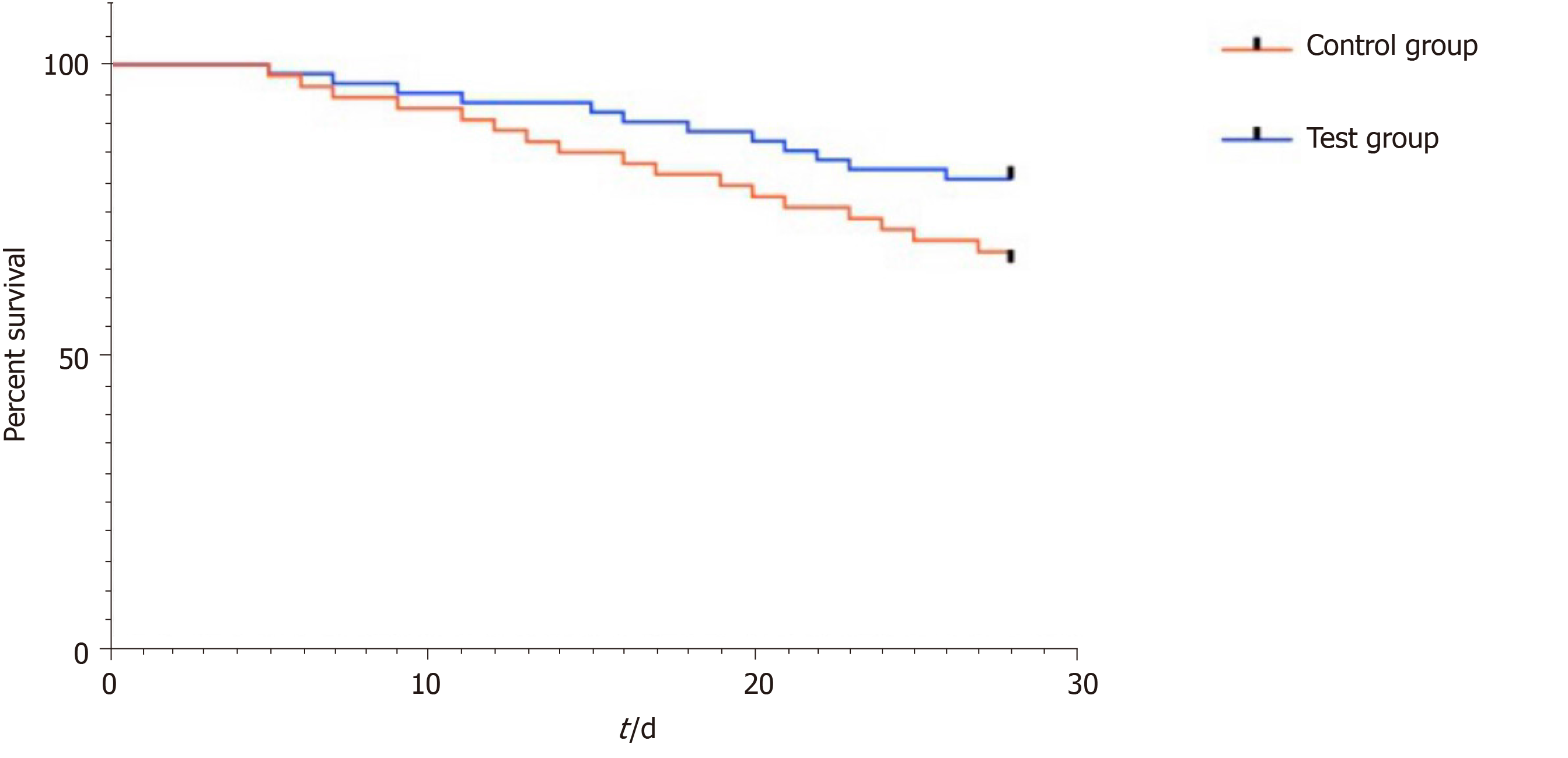
The 28-d survival rate in the experimental group (80.33%) was significantly higher than that in the control group (66.04%) (Figure 5).
Early sepsis stimulates neutrophils to release lysosomal enzymes, a variety of interleukins, and other inflammatory mediators. The massive release and secretion of the mediators through cascade reaction increases body susceptibility and then leads to massive apoptosis of lymphocytes and immune dysfunction in patients, finally resulting in multi-organ dysfunction. The main affected organ is the kidney, whose pathological changes are mainly renal hemodynamic abnormalities, renal ischemia and hypoperfusion, and renal parenchymal damage. Under the strong stimulation of interfering microorganisms, damaged tissues reactivate inflammatory cells such as endothelial cells, monocytes, and macrophages, thereby promoting the release of inflammatory cytokines and causing malignant circulation[15,16]. Taking blocking inflammatory cascade reaction as the final means, CRRT solves the problems of high catabolism, high lactic acids, hypoxemia, and hemodynamic instability in patients with kidney injury[17], but hypotension and renal ischemia still exist during this therapy. In order to provide a more effective therapeutic scheme for patients with SAKI in detail, we formulated a scheme of PGE combined with CRRT for 7-d treatment, and subsequently conducted indicator detection of renal function and inflammatory responses.
The degeneration and necrosis of RTECs may cause renal dysfunction, and the detection of markers for renal tubular injury can directly reflect changes in normal renal function. In this study, the levels of urinary ALR and NHE3 in the control and experimental groups were approximately the same before treatment, while after treatment, the levels in all subjects decreased, and the decrease in the experimental group was more significant. ALR with the function of renal protection mainly promotes liver cell proliferation and protects the liver of patients with hepatic failure. Its expression in injured and regenerated RTECs increases with the aggravation of injury[18,19]. A decrease in NHE3 expression inhibits NaCl transmembrane transport, reduces H+ secretion, and decreases HCO3- reabsorption, which is involved in the processes of urine concentration and urinary sodium excretion[20,21]. The levels of BUN and SCr increased at the stage of disease development but decreased at the stage of disease control. Similarly, the improvement of the levels in the experimental group was better than that in the control group. This suggests that the combined therapy is more effective in the renal function recovery of patients with SAKI. Changes in inflammatory cytokines (TNF-α, IL-18, and hs-CRP) that are the crucial factors leading to SAKI were observed, and we found that the improvement of inflammatory responses in the experimental group was greater than that in the control group after treatment. According to related reports, PGE can regulate endothelial cells and macrophages to produce anti-inflammatory and cell protection functions[22,23], which is consistent with our experimental results. The infection of pathogenic bacteria and the therapeutic effect of drugs are closely related to septic patients’ immune function. The patients’ regulatory T cells were monitored in this study. The results showed that CD3+ T lymphocytes, CD4+ T lymphocytes, and CD4+/CD8+ T lymphocyte ratio in the control and experimental groups were approximately the same before treatment, while after treatment, the indices in all subjects increased, and the increase in the experimental group was more significant than that in the control group. Previous studies have found that CRRT of different doses improves the levels of immunoglobulin ahd the percentages of CD3+ T lymphocytes and CD4+ T lymphocytes in septic patients by clearing inflammatory cytokines, thus being involved in the regulation of immune function[24,25]. The mechanism of PGE on immune function in patients with SAKI has not been determined, but this study showed that the combined therapy can enhance the immune function. The improvement of symptoms, the incidence of adverse reactions, and the prognosis of patients were observed, and the results showed that the combined therapy had better effects on these indices than simple CRRT. This revealed that the combined therapy has higher safety and better long-term efficacy, which may be related to the reduction of inflammatory responses and the improvement of immune function.
In summary, PGE combined with CRRT is clinically effective for treating SAKI, and the combination therapy can significantly improve renal function and reduce inflammatory responses. However, there are still unresolved problems in this study. For instance, a 28-d survival observation was conducted, and the causes of death in patients with SAKI were diverse, but the changes in the patients’ survival rate were not anlyzed in detail to improve the accuracy of selecting therapeutic schemes. Additionally, more reference directions for adverse reactions can be provided so that the safety of the drug can be improved. These are our future research directions, in order to provide effective treatment measures for the early treatment of patients with SAKI.
As a common disease in the intensive care unit (ICU), sepsis refers to a condition that the immune function is affected by inflammatory cells and then involves related multiple organ dysfunction. The disease is uncontrollable and persistent, and there are more new cases and deaths worldwide every year, which have been increasing. Sepsis is usually caused by severe trauma, suppuration, and other infectious conditions. Its severe conditions cause toxins and pathogenic bacteria to rapidly spread from the infected site to blood circulation, finally resulting in systemic infection.
Continuous renal replacement therapy (CRRT) is a continuous and slow blood purification treatment that removes retained water and solute in an adsorptive, convective, and dispersive blood purification mode with extracorporeal circulation. This therapy removes serum pro- and anti-inflammatory mediators and helps the recovery of body immune function in patients. Prostaglandin E (PGE) pharmacologically affects vasodilation and platelet aggregation, so as to protect vascular endothelium, improve microcirculation, and greatly restore the physiological functions of damaged kidneys.
This study aimed to investigate the effects of PGE combined with CRRT on urinary augmenter of liver regeneration (ALR), urinary Na+/H+ exchanger 3 (NHE3), and serum inflammatory cytokines in patients with septic acute kidney injury (SAKI).
Enrolled patients were treated with comprehensive medical treatment such as fluid resuscitation, anti-infective shock, and nutritional support. A bedside hemofiltration apparatus was used for CRRT, adjusted to the continuous veno-venous hemofiltration mode. The flow range was between 20-50 mL/(kg·h) for treatment over 12-24 h each time, and the blood flow was controlled to 200-220 mL/min. The patients were anticoagulated with low molecular heparin, and treated for 5 consecutive days. Patients in the experimental group were additionally treated with PGE, and the mixed solution (20 μg of PGE + 100 mL of normal saline) of standard dose was slowly and intravenously dripped twice per day. They were treated for 7 consecutive days.
Before treatment, urinary ALR, urinary NHE3, blood urea nitrogen (BUN), serum creatinine (SCr), CD3+ T lymphocytes, CD4+ T lymphocytes, and CD4+/CD8+ T lymphocyte ratio in the control and experimental groups were approximately the same (P > 0.05). After treatment, urinary ALR and NHE3 decreased, while BUN, SCr, CD3+ T lymphocytes, CD4+ T lymphocytes, and CD4+/CD8+ T lymphocyte ratio increased in all subjects. Urinary ALR, urinary NHE3, BUN, and SCr in the experimental group were lower than those in the control group, while CD3+ T lymphocytes, CD4+ T lymphocytes, and CD4+/CD8+ T lymphocyte ratio were higher than those in the control group (P < 0.05). After treatment, the levels of tumor necrosis factor-α, interleukin-18, and hs-CRP in the experimental group were lower than those in the control group (P < 0.05). The time for urine volume recovery and ICU treatment in the experimental group was significantly shorter than that in the control group (P < 0.05), although there was no statistically significant difference in hospital stays between the two groups (P > 0.05). The total incidence of adverse reactions did not differ statistically between the two groups (P > 0.05). The 28-d survival rate in the experimental group (80.33%) was significantly higher than that in the control group (66.04%).
PGE combined with CRRT is clinically effective for treating SAKI, and the combination therapy can significantly improve renal function and reduce inflammatory responses.
Since inflammatory responses are a critical factor during sepsis progression, the clearance of inflammatory mediators in vivo is an important direction for the prevention and treatment of SAKI.
STROBE Statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tambuwala M S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Fleischmann C, Thomas-Rueddel DO, Hartmann M, Hartog CS, Welte T, Heublein S, Dennler U, Reinhart K. Hospital Incidence and Mortality Rates of Sepsis. Dtsch Arztebl Int. 2016;113:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 2. | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3146] [Cited by in RCA: 3171] [Article Influence: 264.3] [Reference Citation Analysis (0)] |
| 3. | Mehta RL, Bouchard J, Soroko SB, Ikizler TA, Paganini EP, Chertow GM, Himmelfarb J; Program to Improve Care in Acute Renal Disease (PICARD) Study Group. Sepsis as a cause and consequence of acute kidney injury: Program to Improve Care in Acute Renal Disease. Intensive Care Med. 2011;37:241-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 229] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 4. | Hollinger A, Wittebole X, François B, Pickkers P, Antonelli M, Gayat E, Chousterman BG, Lascarrou JB, Dugernier T, Di Somma S, Struck J, Bergmann A, Beishuizen A, Constantin JM, Damoisel C, Deye N, Gaudry S, Huberlant V, Marx G, Mercier E, Oueslati H, Hartmann O, Sonneville R, Laterre PF, Mebazaa A, Legrand M. Proenkephalin A 119-159 (Penkid) Is an Early Biomarker of Septic Acute Kidney Injury: The Kidney in Sepsis and Septic Shock (Kid-SSS) Study. Kidney Int Rep. 2018;3:1424-1433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Gulla KM, Sachdev A, Gupta D, Gupta N, Anand K, Pruthi PK. Continuous renal replacement therapy in children with severe sepsis and multiorgan dysfunction - A pilot study on timing of initiation. Indian J Crit Care Med. 2015;19:613-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Hanafusa N. Application of Continuous Renal Replacement Therapy: What Should We Consider Based on Existing Evidence? Blood Purif. 2015;40:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Leger PL, Pansiot J, Besson V, Palmier B, Renolleau S, Baud O, Cauli B, Charriaut-Marlangue C. Cyclooxygenase-2-Derived Prostaglandins Mediate Cerebral Microcirculation in a Juvenile Ischemic Rat Model. Stroke. 2016;47:3048-3052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210-4221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1196] [Cited by in RCA: 1473] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 9. | Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J Am Soc Nephrol. 2015;26:1765-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 555] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 10. | Liao XH, Zhang L, Tang XP, Liu Q, Sun H. Expression of augmenter of liver regeneration in rats with gentamicin-induced acute renal failure and its protective effect on kidney. Ren Fail. 2009;31:946-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Liao XH, Chen GT, Li Y, Zhang L, Liu Q, Sun H, Guo H. Augmenter of liver regeneration attenuates tubular cell apoptosis in acute kidney injury in rats: the possible mechanisms. Ren Fail. 2012;34:590-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | McKee JA, Kumar S, Ecelbarger CA, Fernández-Llama P, Terris J, Knepper MA. Detection of Na(+) transporter proteins in urine. J Am Soc Nephrol. 2000;11:2128-2132. [PubMed] |
| 13. | Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3891] [Cited by in RCA: 4132] [Article Influence: 187.8] [Reference Citation Analysis (0)] |
| 14. | Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-c184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 3345] [Article Influence: 257.3] [Reference Citation Analysis (0)] |
| 15. | Gómez H, Kellum JA, Ronco C. Metabolic reprogramming and tolerance during sepsis-induced AKI. Nat Rev Nephrol. 2017;13:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 16. | Vossen MG, Knafl D, Haidinger M, Lemmerer R, Unger M, Pferschy S, Lamm W, Maier-Salamon A, Jäger W, Thalhammer F. Micafungin Plasma Levels Are Not Affected by Continuous Renal Replacement Therapy: Experience in Critically Ill Patients. Antimicrob Agents Chemother. 2017;61:e02425-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Liu S, Cheng QL, Zhang XY, Ma Q, Liu YL, Pan R, Cai XY. Application of continuous renal replacement therapy for acute kidney injury in elderly patients. Int J Clin Exp Med. 2015;8:9973-9978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Nalesnik MA, Gandhi CR, Starzl TE. Augmenter of liver regeneration: A fundamental life protein. Hepatology. 2017;66:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Liao XH, Zhang L, Liu Q, Sun H, Peng CM, Guo H. Augmenter of liver regeneration protects kidneys from ischaemia/reperfusion injury in rats. Nephrol Dial Transplant. 2010;25:2921-2929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Fernández-Llama P, Andrews P, Ecelbarger CA, Nielsen S, Knepper M. Concentrating defect in experimental nephrotic syndrone: altered expression of aquaporins and thick ascending limb Na+ transporters. Kidney Int. 1998;54:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Wang Z, Rabb H, Craig T, Burnham C, Shull GE, Soleimani M. Ischemic-reperfusion injury in the kidney: overexpression of colonic H+-K+-ATPase and suppression of NHE-3. Kidney Int. 1997;51:1106-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Laavola M, Leppänen T, Eräsalo H, Hämäläinen M, Nieminen R, Moilanen E. Anti-inflammatory Effects of Nortrachelogenin in Murine J774 Macrophages and in Carrageenan-Induced Paw Edema Model in the Mouse. Planta Med. 2017;83:519-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Revathikumar P, Estelius J, Karmakar U, Le Maître E, Korotkova M, Jakobsson PJ, Lampa J. Correction: Microsomal prostaglandin E synthase-1 gene deletion impairs neuro-immune circuitry of the cholinergic anti-inflammatory pathway in endotoxaemic mouse spleen. PLoS One. 2018;13:e0196806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Choi SJ, Ha EJ, Jhang WK, Park SJ. Factors Associated With Mortality in Continuous Renal Replacement Therapy for Pediatric Patients With Acute Kidney Injury. Pediatr Crit Care Med. 2017;18:e56-e61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Morimatsu H, Uchino S, Bellomo R, Ronco C. Continuous renal replacement therapy: does technique influence azotemic control? Ren Fail. 2002;24:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |