Published online Jun 26, 2020. doi: 10.12998/wjcc.v8.i12.2603
Peer-review started: March 8, 2020
First decision: April 22, 2020
Revised: May 18, 2020
Accepted: May 26, 2020
Article in press: May 26, 2020
Published online: June 26, 2020
Processing time: 107 Days and 23.6 Hours
Anti-N-methyl-D-aspartate-receptor (NMDAR) encephalitis is a common type of autoimmune encephalitis characterized by complex clinical signs and variable imaging manifestations. The pathogenesis of the disease is unclear. Syphilis is an infectious disease caused by Treponema pallidum that can invade the nervous and immune systems and cause systemic symptoms. There are few reports of anti-NMDAR encephalitis with syphilis, and the association between them is unknown; both diseases are related to immune system damage. We report a case of anti-NMDAR encephalitis with syphilis.
A 32-year-old man was admitted to our hospital with complaints of cognitive decline, diplopia, and walking instability during the previous 6 mo. He developed dysarthria, difficulty swallowing, and involuntary shaking of his head, neck, and limbs during the month prior to presentation. Cranial magnetic resonance imaging showed symmetrical abnormal signals in the pons, midbrain, and bilateral basal ganglia, and inflammatory demyelination was considered. The diagnosis of syphilis was confirmed based on the syphilis diagnosis test and the syphilis rapid test. He was given anti-syphilis treatment, but the above symptoms gradually worsened. Anti-NMDAR antibody was positive in cerebrospinal fluid but was negative in serum. Due to the cerebrospinal fluid findings, anti-NMDAR encephalitis was a consideration. According to the patient’s weight, he was treated with intravenous methylprednisolone 1 g QD for 5 d, with the dose gradually decreased for 6 mo, and immunoglobulin 25 g QD for 5 d; his symptoms improved after treatment.
This case shows that anti-NMDAR encephalitis may be combined with syphilis, which should be recognized to avoid misdiagnosis and treatment delay.
Core tip: Anti-N-methyl-D-aspartate-receptor (NMDAR) encephalitis is a common type of autoimmune encephalitis characterized by complex clinical signs and various imaging manifestations. We present a rare case of anti-NMDAR encephalitis combined with syphilis, developing in a previously healthy immunocompetent male patient. Anti-NMDAR was detected in the cerebrospinal fluid. Our patient’s symptoms improved after methylprednisolone and immunoglobulin treatment. This case emphasizes that anti-NMDAR encephalitis can present in combination with syphilis. The unique imaging manifestations of anti-NMDAR encephalitis and the clinical manifestations caused by the involvement of the pons, midbrain, and basal ganglia should be recognized.
- Citation: Li XY, Shi ZH, Guan YL, Ji Y. Anti-N-methyl-D-aspartate-receptor antibody encephalitis combined with syphilis: A case report. World J Clin Cases 2020; 8(12): 2603-2609
- URL: https://www.wjgnet.com/2307-8960/full/v8/i12/2603.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i12.2603
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis may be associated with antibodies against neuronal synaptic proteins. Since the discovery of anti-NMDAR encephalitis in 2007, it has been a popular area of research in the field of neurology. Anti-NMDAR encephalitis is primarily diagnosed in children and young adults, with or without an associated tumor; it responds to treatment but can relapse[1]. Malignant tumors associated with anti-NMDAR encephalitis predominantly present between the ages of 12 and 45 years; most cases are ovarian teratomas (94%), followed by extraovarian teratomas (2%) and other tumors (4%)[2]. The presence of a tumor (usually an ovarian teratoma)[3] depends on age, gender, and ethnicity and is more common in women over 18 years of age[1]. Patients with anti-NMDAR encephalitis can present with a variety of clinical symptoms, such as abnormal (psychiatric) behavior or cognitive dysfunction, speech dysfunction, seizures, movement disorder, dyskinesias, decreased level of consciousness, autonomic dysfunction, or central hypoventilation, among others[4]. The most common dyskinesias are orofacial dyskinesias, dance prosthetic deformities, and dystonia[1].
The patient was a 32-year-old man who was admitted to our hospital with complaints of cognitive function decrease, diplopia, and unsteady gait for more than 6 mo.
More than 6 mo prior to admission, he developed diplopia and his right eye showed outward inclination after long-term emotional stress and fatigue. Because the lower limbs were difficult to control when walking, he reported an unstable gait. He also experienced memory loss and personality changes, and his family felt that his personality became naive.
He had a remote history of blood transfusion during leg surgery. He denied a history of infection, diarrhea, fever, or other previous medical history.
The patient was a married 32-year-old man with a height of 180 cm and weight of 62.5 kg. He had no history of drug use, drinking, or bad sexual life, but had a history of smoking. His parents are both in good health.
He lost 15 kg in 1 mo. On assessment, his vital signs and eye movement were normal. Clinical neurological examination revealed slow response, right eye abduction, diplopia, personality change, attention and short-term memory impairments, euphoric mood, static and postural tremor of the head and limbs, positive left Babinski sign, and limb ataxia, without any other pathological signs. Our primary clinical consideration was neurosyphilis, followed by intracranial infection.
There were no significant abnormalities on routine blood tests, including biochemistry, coagulation, high-sensitivity C-reactive protein, and pelvic and liver tumor markers. The syphilis rapid plasma responsive ring card test was positive (1:2). Electroencephalogram, brain electrical activity mapping, and electromyography were normal. Oncologic tests, including chest computed tomography, CA199, and carcinoembryonic antigen, were all negative. The patient’s score on the Montreal Cognitive Assessment examination was 24/30. His Mini-Mental State Examination score was 28/30.
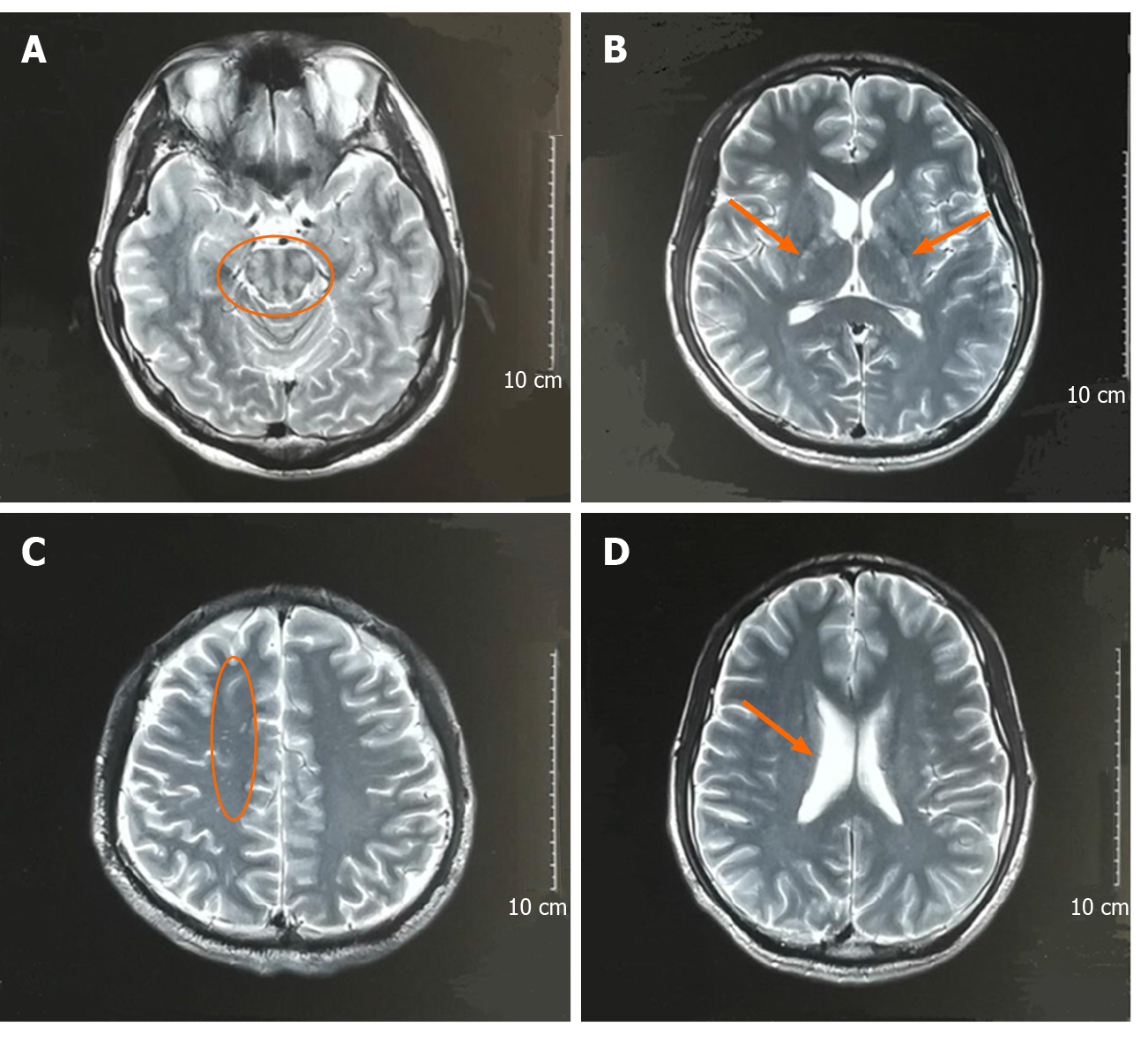
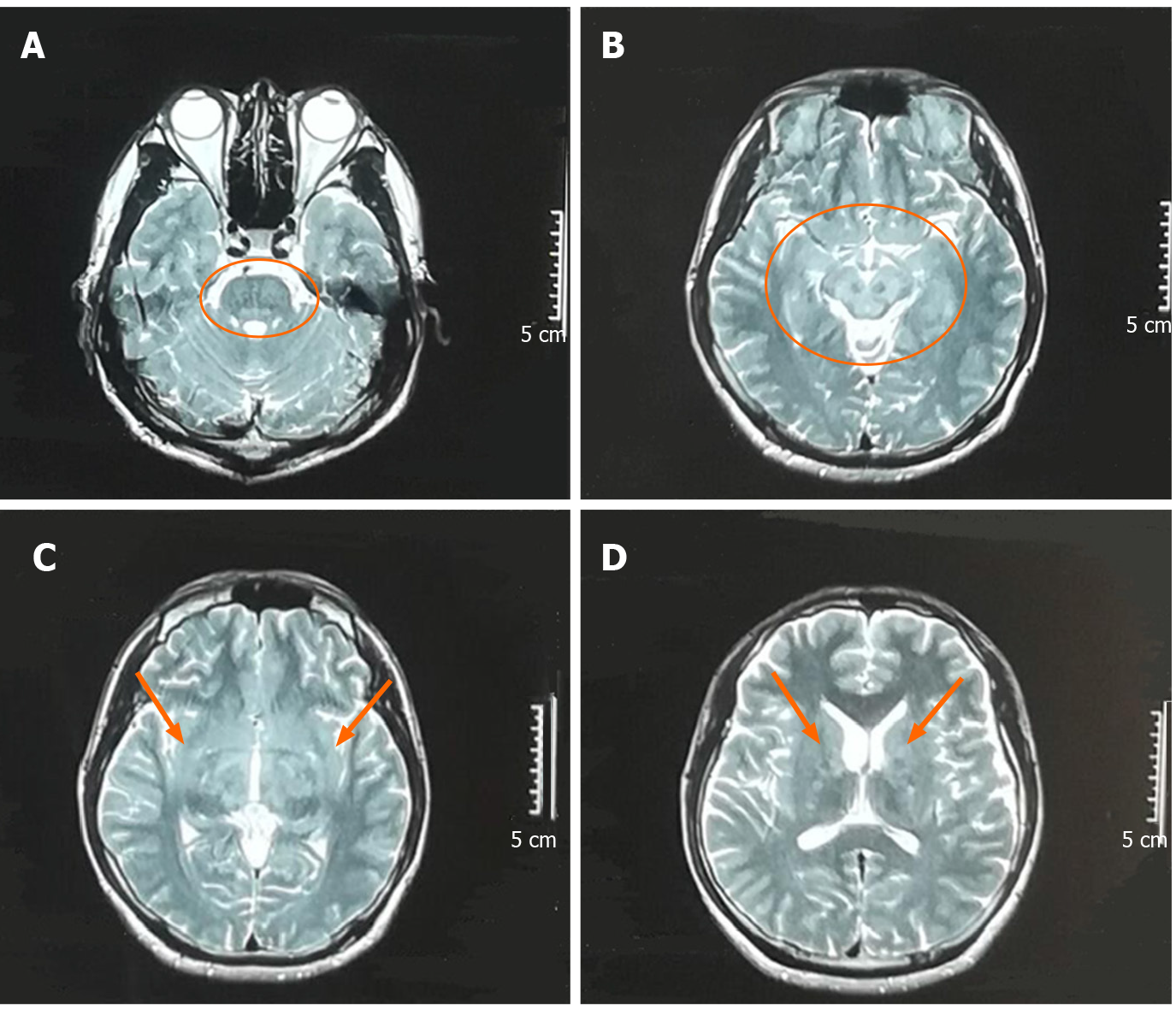
Brain magnetic resonance imaging (MRI) scans 1 mo before admission showed T2 hyperintensity of the brain stem, bilateral internal capsules, and the right frontal lobe; the right ventricle was enlarged (Figure 1). Repeat brain MRI scans on admission showed abnormal symmetrical signals in the pons, mesencephalon, bilateral medial temporal lobe, and bilateral basal ganglia (Figure 2).
Lumbar puncture showed normal cerebrospinal fluid (CSF) with routine testing, including biochemistry and cytology. The immunoglobulin G (IgG) index was 2.95 mg/dL. Because neurosyphilis was considered, ceftriaxone 1.0 g im QD was given for 15 d, but the patient's condition was not improved. Repeat lumbar puncture showed normal CSF (glucose, 2.71 mmol/L; protein, 0.2 g/L; cytology, 3 /mm3). CSF bacterial culture was negative. Central nervous system demyelinating disease serology tests, including anti-aquaporin 4 antibodies, NMO-IgG, anti-MOG-IgG, and anti-MBP-IgG, were negative. His CSF IgG test was positive and oligoclonal bands were present, suggesting intrathecal antibody production. Serum IgG test was negative. Anti-NMDAR CSF test was positive, but serum testing was negative. Other autoimmune encephalitis antibodies were negative in both CSF and serum. Repeat syphilis confirmatory and rapid tests were positive. Repeat lumbar puncture after 1 mo of treatment with immunoglobulin and methylprednisolone therapy showed CSF that was almost acellular (6/mm3) with normal protein (0.43 g/L) and glucose (2.82 mmol/L). CSF bacterial culture was normal. CSF IgG index was elevated (50.6 mg/L). Paraneoplastic tests including Hu, Yo, Ri, CV2, Ma2, Amphiphysin, ANNA-3, Tr, PCA-2, and GAD were negative, except for anti-NMDAR antibody positivity (+1:1) in CSF and negativity in serum. Repeat serum syphilis rapid plasma responsive ring card test was positive and treponemal antibody was elevated (33.4).
Based on the detection of anti-NMDAR antibodies in the CSF, we finally diagnosed the patient with anti-NMDAR encephalitis.
Based on the detection of NMDAR antibody in CSF, combined with the clinical and imaging features of the patient, we considered that the patient may have anti-NMDAR encephalitis. According to the patient’s weight, we treated him with intravenous methylprednisolone 1 g QD and immunoglobulin 25 g QD for 5 d, and his symptoms improved. Five days after immunoglobulin and methylprednisolone therapy, his unsteady gait and cognitive impairment were greatly improved. Diplopia completely resolved, and head and neck tremors improved. After discharge from the hospital, he continued treatment with prednisone 60 mg once per day.
After discharge, the patient continued to take prednisone orally. Prednisone was gradually reduced and his condition remained stable without further aggravation. On follow-up assessment 6 mo later, his gait instability had improved significantly; diplopia was still present, and tremor was alleviated.
The diagnostic criteria[4] for and treatment[5] of anti-NMDAR have been reported[6]. We report a case of anti-NMDAR encephalitis combined with syphilis, which has rarely been reported. Anti-NMDAR encephalitis is autoimmune encephalitis mediated by the NMDA receptor[7]. NMDAR is an important excitatory amino acid in the central nervous system, which regulates neuronal survival and participates in synaptic signaling and plasticity formation. Excessive activation of the NMDAR can lead to excitotoxicity, which may be the underlying pathogenesis of epilepsy, dementia, and stroke[8]; conversely, schizophrenia-like symptoms may occur[9]. At present, the etiology of anti-NMDAR encephalitis is unclear, but it has been reported that IgG antibody can be found in patients with herpes simplex encephalitis[10]. Autoimmune encephalitis should be investigated in cases of atypical herpes simplex encephalitis or other types of viral encephalitis[10].
There are many clinical features of anti-NMDAR encephalitis, but each patient has different symptoms. Our patient’s findings were primarily characterized by cognitive impairment, unsteady gait, diplopia, and tremors. It has been reported that in the course of the disease, adults show more frequent memory impairment and inadequate ventilation than children, and less focal defects (paraplegia and ataxia) and speech or motor disorders[11]. Some patients show symptoms of microcephaly ataxia[12]. In a single-center, longitudinal study in China, the most common clinical manifestations of anti-NMDAR encephalitis were psychosis (82.7%) and epilepsy (80.9%)[13]. Anti-NMDAR encephalitis can show early symptoms of psychosis[14] and be easily misdiagnosed as mental illness[15]. Our patient was initially diagnosed with depression, but there was no improvement in his condition. Therefore, when we evaluate patients with mental disorders, we should recognize the possibility of underlying diagnoses producing psychiatric symptoms. Anti-NMDAR encephalitis showing psychiatric symptoms has been specifically reported[16]. In China, the patients often suffer from mental illness and epilepsy, but the proportion of potential tumors is very low[13]. Our patient showed no abnormality in the whole tumor and chest and abdomen computed tomography, but the patient was requested to undergo additional examination for potential tumors in the future. Tumors are recognized to be associated with anti-NMDAR encephalitis[14,17,18].
Although he was diagnosed with syphilis, the symptoms did not improve after treatment with anti-syphilis medication, indicating that these lesions may not be directly related to syphilis. Despite this, we suspected that this autoimmune encephalitis may be due to irreversible damage to the nervous immune system caused by syphilis; thus it is likely that syphilis and autoimmune encephalitis coexist. Recently, cases of anti-NMDAR encephalitis and neurosyphilis have been published. It is reported that anti-NMDAR encephalitis may be related to neurosyphilis[19]. Although cases of anti-NMDAR encephalitis coexistent with syphilis are rare, we cannot ignore the potential link.
Head MRI in patients with anti-NMDAR encephalitis is not necessarily abnormal, and its imaging findings are not specific. Anti-NMDAR encephalitis is related to extensive superficial white matter damage in patients with incomplete recovery[20]. Data from the largest cohort of patients with anti-NMDAR encephalitis included extensive multimodal MRI findings, which suggest that hippocampal structural damage and related memory loss are important long-term sequelae of encephalitis[21]. Our patients’ brain MRI showed abnormal symmetrical signals in the pons, mesencephalon, bilateral medial temporal lobe, and bilateral basal ganglia. Recently, it has been reported that the head MRI in a patient with anti-NMDAR encephalitis showed extensive lesions in the frontal lobe, temporal lobe, and basal ganglia, with mild mass effect[22].
At present, there are not many methods to treat anti-NMDAR encephalitis, and the curative effect of available treatments is uncertain. Some literature outlined the best treatment for anti-NMDAR encephalitis, including the combination of tumor resection, immunotherapy, intensive care, and rehabilitation including physical therapy[23]. It has been reported that alemtuzumab and methotrexate are used to maintain immunosuppression across the blood-brain barrier, according to the pathogenesis[24,25] of anti-NMDAR, and these treatments have achieved considerable positive effect[26]. After immunoglobulin and hormone therapy, the symptoms of our patient were temporarily relieved during hospitalization, and at the 6-mo follow-up, his walking instability had improved significantly and his tremor was alleviated; his diplopia continued. It has been reported that the disease is characterized by recurrence and incurability[27,28]. We asked the patient to come to the hospital for reexamination every 3-6 mo. Data in the literature showed that immunoglobulin and hormone therapy are effective in the treatment of anti-NMDAR encephaliti[19,29]. It has been reported that the dysfunction of postsynaptic glutamate transmission at the synapse leads to increased release of γ-aminobutyric acid and decreased secretion of glutamate in anti-NMDAR encephalitis, so glutamate therapy can be used in anti-NMDAR encephalitis[30]. In the future, we can try the glutamate therapy in more patients with anti-NMDAR encephalitis to observe the clinical effect.
At present, there are few reported cases of anti-NMDAR encephalitis coexistent with syphilis, and the correlation between the two diseases is unclear. When we understand the characteristics of anti-NMDAR encephalitis combined with syphilis, we can recognize, diagnose, and treat these patients earlier. Additionally, the unique imaging manifestations of anti-NMDAR encephalitis and the clinical manifestations caused by injury of certain parts of the brain, such as the pons, midbrain, and basal ganglia, cannot be ignored.
We are very grateful to the patient and his family.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lin E S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1879] [Cited by in RCA: 1647] [Article Influence: 117.6] [Reference Citation Analysis (0)] |
| 2. | Dutra LA, Abrantes F, Toso FF, Pedroso JL, Barsottini OGP, Hoftberger R. Autoimmune encephalitis: a review of diagnosis and treatment. Arq Neuropsiquiatr. 2018;76:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Yaguchi H, Tsuji T, Yabe I, Hirayama E, Nomura T, Ohashi I, Mito Y, Tanaka K, Tajima Y. Incidence of anti-NMDAR encephalitis in patients undergoing resection of ovarian teratoma in a single institution. J Neurol Sci. 2020;409:116608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, Cortese I, Dale RC, Gelfand JM, Geschwind M, Glaser CA, Honnorat J, Höftberger R, Iizuka T, Irani SR, Lancaster E, Leypoldt F, Prüss H, Rae-Grant A, Reindl M, Rosenfeld MR, Rostásy K, Saiz A, Venkatesan A, Vincent A, Wandinger KP, Waters P, Dalmau J. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2328] [Cited by in RCA: 2656] [Article Influence: 295.1] [Reference Citation Analysis (0)] |
| 5. | Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter MA, Rosenfeld MR, Lynch DR. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2002] [Cited by in RCA: 1720] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 6. | Huang Q, Xie Y, Hu Z, Tang X. Anti-N-methyl-D-aspartate receptor encephalitis: A review of pathogenic mechanisms, treatment, prognosis. Brain Res. 2020;1727:146549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 7. | Alexopoulos H, Dalakas MC. The immunobiology of autoimmune encephalitides. J Autoimmun. 2019;104:102339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 259] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 9. | Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 639] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 10. | Desena A, Graves D, Warnack W, Greenberg BM. Herpes simplex encephalitis as a potential cause of anti-N-methyl-D-aspartate receptor antibody encephalitis: report of 2 cases. JAMA Neurol. 2014;71:344-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Dalmau J, Armangué T, Planagumà J, Radosevic M, Mannara F, Leypoldt F, Geis C, Lancaster E, Titulaer MJ, Rosenfeld MR, Graus F. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18:1045-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 516] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 12. | Poorthuis MHF, van Rooij JLM, Koch AH, Verdonkschot AEM, Leembruggen MM, Titulaer MJ. Cerebellar ataxia as a presenting symptom in a patient with anti-NMDA receptor encephalitis. Neurol Neuroimmunol Neuroinflamm. 2019;6:e579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Xu X, Lu Q, Huang Y, Fan S, Zhou L, Yuan J, Yang X, Ren H, Sun D, Dai Y, Zhu H, Jiang Y, Zhu Y, Peng B, Cui L, Guan H. Anti-NMDAR encephalitis: A single-center, longitudinal study in China. Neurol Neuroimmunol Neuroinflamm. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 14. | Warren N, O'Gorman C, McKeon G, Swayne A, Blum S, Siskind D. Psychiatric management of anti-NMDAR encephalitis: a cohort analysis. Psychol Med. 2019;1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Gastaldi M, Mariotto S, Giannoccaro MP, Iorio R, Zoccarato M, Nosadini M, Benedetti L, Casagrande S, Di Filippo M, Valeriani M, Ricci S, Bova S, Arbasino C, Mauri M, Versino M, Vigevano F, Papetti L, Romoli M, Lapucci C, Massa F, Sartori S, Zuliani L, Barilaro A, De Gaspari P, Spagni G, Evoli A, Liguori R, Ferrari S, Marchioni E, Giometto B, Massacesi L, Franciotta D. Subgroup comparison according to clinical phenotype and serostatus in autoimmune encephalitis: a multicenter retrospective study. Eur J Neurol. 2020;27:633-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Barry H, Hardiman O, Healy DG, Keogan M, Moroney J, Molnar PP, Cotter DR, Murphy KC. Anti-NMDA receptor encephalitis: an important differential diagnosis in psychosis. Br J Psychiatry. 2011;199:508-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Buechner S, Florio I, Sixt GJ, Teatini F. A critical reflection on our first patient presenting with Anti-Nmethyl- D-aspartate receptor encephalitis. Neurol Int. 2019;11:8253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Titulaer MJ, McCracken L, Gabilondo I, Iizuka T, Kawachi I, Bataller L, Torrents A, Rosenfeld MR, Balice-Gordon R, Graus F, Dalmau J. Late-onset anti-NMDA receptor encephalitis. Neurology. 2013;81:1058-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 19. | Qin K, Wu W, Huang Y, Xu D, Zhang L, Zheng B, Jiang M, Kou C, Gao J, Li W, Zhang J, Wang S, Luan Y, Yan C, Xu D, Zheng X. Anti-N-methyl-D-aspartate receptor(NMDAR) antibody encephalitis presents in atypical types and coexists with neuromyelitis optica spectrum disorder or neurosyphilis. BMC Neurol. 2017;17:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Phillips OR, Joshi SH, Narr KL, Shattuck DW, Singh M, Di Paola M, Ploner CJ, Prüss H, Paul F, Finke C. Superficial white matter damage in anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry. 2018;89:518-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Finke C, Kopp UA, Pajkert A, Behrens JR, Leypoldt F, Wuerfel JT, Ploner CJ, Prüss H, Paul F. Structural Hippocampal Damage Following Anti-N-Methyl-D-Aspartate Receptor Encephalitis. Biol Psychiatry. 2016;79:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 22. | Jiang Y, Ma J, Gong T, Hao H, Chen H. The diffuse involvement of anti-N-methyl-D-aspartate receptor encephalitis in brain: a case report. BMC Neurol. 2019;19:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Sansing LH, Tüzün E, Ko MW, Baccon J, Lynch DR, Dalmau J. A patient with encephalitis associated with NMDA receptor antibodies. Nat Clin Pract Neurol. 2007;3:291-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 24. | Tüzün E, Zhou L, Baehring JM, Bannykh S, Rosenfeld MR, Dalmau J. Evidence for antibody-mediated pathogenesis in anti-NMDAR encephalitis associated with ovarian teratoma. Acta Neuropathol. 2009;118:737-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 254] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 25. | Peery HE, Day GS, Dunn S, Fritzler MJ, Prüss H, De Souza C, Doja A, Mossman K, Resch L, Xia C, Sakic B, Belbeck L, Foster WG. Anti-NMDA receptor encephalitis. The disorder, the diagnosis and the immunobiology. Autoimmun Rev. 2012;11:863-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 26. | Liba Z, Sebronova V, Komarek V, Sediva A, Sedlacek P. Prevalence and treatment of anti-NMDA receptor encephalitis. Lancet Neurol. 2013;12:424-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, Honig LS, Benseler SM, Kawachi I, Martinez-Hernandez E, Aguilar E, Gresa-Arribas N, Ryan-Florance N, Torrents A, Saiz A, Rosenfeld MR, Balice-Gordon R, Graus F, Dalmau J. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2139] [Cited by in RCA: 2129] [Article Influence: 177.4] [Reference Citation Analysis (0)] |
| 28. | Liu CY, Zhu J, Zheng XY, Ma C, Wang X. Anti-N-Methyl-D-aspartate Receptor Encephalitis: A Severe, Potentially Reversible Autoimmune Encephalitis. Mediators Inflamm. 2017;2017:6361479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Kong SS, Chen YJ, Su IC, Lin JJ, Chou IJ, Chou ML, Hung PC, Hsieh MY, Wang YS, Chou CC, Wang HS, Lin KL; CHEESE Study Group. Immunotherapy for anti-NMDA receptor encephalitis: Experience from a single center in Taiwan. Pediatr Neonatol. 2019;60:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Tzang RF, Chang CH, Chang YC, Lane HY. Autism Associated With Anti-NMDAR Encephalitis: Glutamate-Related Therapy. Front Psychiatry. 2019;10:440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |