Published online Jun 26, 2020. doi: 10.12998/wjcc.v8.i12.2566
Peer-review started: February 24, 2020
First decision: April 24, 2020
Revised: May 20, 2020
Accepted: May 23, 2020
Article in press: May 23, 2020
Published online: June 26, 2020
Processing time: 120 Days and 7.7 Hours
Lymphoplasmacytic lymphoma is a rare non-Hodgkin’s lymphoma, occurring mostly in the elderly. It develops slowly and leads to malignant proliferation of lymphoid line cells in the bone marrow, lymph nodes and spleen. It may also affect nerve roots and meninges; some patients develop sensorimotor polyneuropathy which may precede general symptoms of lymphoma.
We present a case of a 36-year-old man diagnosed in 2012 with chronic inflammatory demyelinating polyneuropathy (CIDP), then he was hospitalized in 2019 due to progressive symptoms of heart failure and significant weight loss over the previous four months. Based on clinical and laboratory findings a diagnosis of lymphoplasmacytic lymphoma was suspected and confirmed by bone marrow flow cytometry. There was no improvement in the results of laboratory tests and the patient's condition after immediate implementation of chemotherapy. Patient died on the fifth day of treatment.
While CIDP and malignant disease co-occurrence is rare, it should be suspected and investigated in patients with atypical neuropathy symptoms.
Core tip: Lymphoplasmacytic lymphoma is a low-grade B cell type of non-Hodgkin's lymphoma. It occurs mainly in elderly, develops slowly and leads to malignant proliferation of lymphoid line cells in the bone marrow. In some cases, patients develop polyneuropathy, which may precede the general symptoms of lymphoma. We present a case of a young man with chronic inflammatory demyelinating polyneuropathy since 2012. His neurological condition worsened rapidly seven years later, which turned out to be paraneoplastic symptom of aggressive lymphoplasmacytic lymphoma. The lack of typical hematological symptoms resulted in delaying the diagnosis and fatal outcome.
- Citation: Rozłucka L, Semik-Grabarczyk E, Pietrukaniec M, Żak-Gołąb A, Grabarczyk M, Grosicki S, Holecki M. Demyelinating polyneuropathy and lymphoplasmacytic lymphoma coexisting in 36-year-old man: A case report. World J Clin Cases 2020; 8(12): 2566-2573
- URL: https://www.wjgnet.com/2307-8960/full/v8/i12/2566.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i12.2566
Chronic inflammatory demyelinating polyneuropathy (CIDP), the most common chronic autoimmune polyneuropathy, is a rare neuromuscular disorder of spinal nerve roots, major plexuses, and the proximal nerve trunks. Its incidence is estimated to be 1.6/100000 per year with a prevalence of 8.9/100000[1]. CIDP mainly occurs between the 3th and 6th decade, slightly more often in men[2,3]. It may be associated with viral infections (e.g., hepatitis C, human immune deficiency virus), connective tissue disease, hyperthyroidism, diabetes mellitus, but also with hematological diseases, the diagnosis of which may often be preceded by CIDP[4]. The clinical manifestations of CIDP include progressive, symmetric paresis and weakness of the proximal and distal parts of upper and lower limbs. Paresis effects roots, plexuses or nerve trunks, which results in general paresthesia, sensation (touch, pain, temperature) loss and balance problems. Cranial nerves are occasionally affected. The complete clinical symptoms develop gradually within two months[5]. The course of the disease can be: (1) Monophasic; (2) Chronic and progressive; and (3) Variant with periods of remission and relapses. Due to the similar clinical and pathological course, the differential diagnosis of CIDP should include Guillaine-Barré (GB) syndrome[2,6]. Lymphoplasmacytic lymphoma (LPL) is a rare B-cell type of non-Hodgkin’s lymphoma. It originates from small mature B cells, that can differentiate into plasma cells[7,8]. The prevalence is about 2% of blood cancers, and the annual incidence is six per million people. The disease usually affects older adults (the average age at the moment of diagnosis is 63-68), it develops slowly and leads to malignant proliferation of lymphoid line cells in the bone marrow[9,10]. Approximately 30% of patients develop symmetrical sensorimotor polyneuropathy (multifocal or mononeuropathy), which may precede general symptoms of lymphoma[11].
CIDP has been described in association with different malignancies. The most common are lymphomas. Thirteen patients with lymphoma described by Viala et al[12] suffered from CIDP. Seven of them had non-Hodgkin B-cell lymphoma, two non-Hodgkin T-cell lymphoma and remaining four Hodgkin Lymphoma[12]. Another study analyzed fifty-three patients with lymphoma and various paraneoplastic neurologic symptoms. While cerebral degeneration was mainly associated with Hodgkin lymphomas, in patients with non-Hodgkin lymphomas (all B-cell) the most common manifestation of neurologic symptoms was acute or chronic demyelinating polyradiculoneuropathy[13].
A 36-year-old man diagnosed with CIDP and hypothyroidism seven years ago, was admitted in April 2019 to the Department of Internal Medicine, Autoimmune and Metabolic Diseases due to progressive weakness, peripheral edema, weight loss (approximately 10 kg within the previous four months, 31 kg in total since CIDP diagnosis), deterioration of appetite and impairment of the muscular system (the patient moved around the house with the help of others). On admission, the patient complained of "stiffness of the torso", sensory disturbances affecting hands and feet in a glove and stocking distribution.
The patient’s condition deteriorated significantly about five months before admittance, with particular aggravation of symptoms during the last month. In December 2018 patient was hospitalized due to concomitant edema, protein deficiency and heart failure. Despite intensive diuretic treatment, the improvement was temporary. In the meantime, due to increasing abdominal pain, patient underwent gastroscopy and was diagnosed with gastritis. Proton pomp inhibitors treatment resulted in only partial improvement. The patient’s neurological condition continued to deteriorate despite intravenous immunoglobulin and glucocorticosteroid treatment leading to his necessity of hospitalization.
Patient was diagnosed with CIDP and hypothyroidism in 2012. Since then he was treated using INN–human normal immunoglobulin (Kiovig 60 mg), with the last administration one month before current hospitalization and methylprednisolone (in intravenous pulses followed by an oral therapy). Hypothyroidism was treated with Levothyroxine (50 μg/d).
Patients’ grandfather died of cancer of unknown localization and mother was treated in the past due to thyroid cancer.
Physical examination revealed dry, thickened, slightly flaky skin of the whole body and swelling of the lower limbs was also noticeable. Peripheral lymph nodes were not palpable. His body weight was 55 kg, height 178 cm (BMI 17.3 kg/m2). Neurological examination revealed disturbances of deep sensation and vibration sense in the lower limbs, reduction of muscle tone in the limbs except for the hands, symmetrical muscle wasting in the face, upper and lower limbs.
The results of laboratory tests are presented in Table 1. The overall clinical picture, as well as the results of laboratory and imaging studies suggested a secondary nature of polyradiculoneuropathy. Despite the negative opinion of a neurologist, the patient underwent a bone marrow aspiration. Bone marrow cytology showed an increased percentage of plasmocytes, which strongly suggested a hematological background.
| Result | Normal range | |
| Hematology | ||
| White blood cell count | 20.31 × 103/µL | (4.0-10.0) |
| Red blood cell count | 4.93 × 106/µL | (4.2-5.7) |
| Hemoglobin | 12.3 g/dL | (13.5-6.5) |
| Hematocrit | 38.7% | (40-53) |
| MCV | 78.5 fL | (84-98) |
| MCH | 24.9 pg | (27-31) |
| MCHC | 31.8 g/dL | (32-36) |
| Platelets | 704 × 103/µL | (130-400) |
| Lymphocytes | 47.6% | (20-45) |
| Monocytes | 7.1% | (3–9) |
| Neutrophils | 44.2% | (45-70) |
| Eosinophils | 0.20% | (1.00-5.00) |
| Basophils | 0.4% | (0-1) |
| ESR | 9 mm/h | (3-8) |
| Coagulation | ||
| aPTT | 29.0 s | (25.4-36.9) |
| Thrombin time | 16.6 s | (10.3-16.6) |
| Prothrombin time | 11.3 s | (9.4-12.5) |
| INR | 1.00 ng/mL | (0.80-1.20) |
| D-dimer | 1744.0 | (< 500.0) |
| Biochemistry | ||
| C-reactive protein | 7.3 mg/L | (< 5.0) |
| Glucose | 116.00 mg/dL | (74.00-106.00) |
| Creatinine | 1.10 mg/dL | (0.84-1.25) |
| MDRD GFR | > 60 mL/min | (> 60) |
| Uric acid | 6.37 mg/dL | (3.50-7.20) |
| Lactate dehydrogenase | 196 U/I | (< 248) |
| Lactate (Lac) | 2.10 mmol/L | (1.10-2.40) |
| Alkaline phosphatase | 95 U/L | (38-126) |
| Alpha amylase | 75.0 U/L | (< 90.0) |
| Lipase | 44.00 U/I | (21.00-67.00) |
| Serum iron | 26.00 µg/dL | (60.00-180.00) |
| UIBC | 150.00 µg/dL | (155.00-300.00) |
| TIBC | 176.0 µg/dL | (250.0-450.0) |
| Total cholesterol | 133.0 mg/dL | (< 200) |
| Triglycerides | 160.00 mg/dL | (0.00-150.00) |
| Hormones | ||
| Anti-TPO | < 5.00 IU/mL | (< 34.00) |
| TSH | 5.230 µIU/mL | (0.27-4.2) |
| Cortisol | 12.60 µg/dL | (4.82-19.50) |
| Electrolytes | ||
| Sodium | 134.2 mmol/L | (136.0-146.0) |
| Potassium | 4.95 mmol/L | (3.50-5.10) |
| Calcium-total | 7.70 mg/dL | (8.80-10.60) |
| Phosphates inorganic | 3.95 mg/dL | (2.50-4.50) |
| Liver enzymes | ||
| ALT | 17.0 U/L | (< 45.0) |
| AST | 20.0 U/L | (< 35.0) |
| GGT | 15.00 U/L | (< 55.00) |
| Total bilirubin | 0.19 mg/dL | (0.30-1.20) |
| Cardiac biomarkers | ||
| Troponin T high sensitivity | 47.74 ng/L | (0.00-14.00) |
| Creatine Kinase | 41 U/L | (< 170) |
| Creatine Kinase -MB | 18 U/L | (< 25) |
| CKMB | 43.9% | (< 6.0) |
| Pro-BNP | 288.30 pg/mL | (0-125) |
| Serum protein electrophoresis | ||
| Albumin | 49.2% | (53.8-65.2) |
| Alpha 1 | 7.2% | (1.1-3.7) |
| Alpha 2 | 21.1% | (8.5-14.5) |
| Beta | 17.1% | (8.6-14.8) |
| Gamma | 5.4% | (9.2-18.2) |
| A/G ratio | 0.97 | (1.27-1.96) |
| Total protein | 4.30 g/dL | (6.60-8.30) |
| Albumin | 2.30 g/dL | (3.5-5.2) |
| Virology tests | ||
| Anti-HCV | Negative | |
| HbsAg | Negative | |
| Anti-HIV | Negative | |
| Immunoglobulins | ||
| Immunoglobulin A | 25 mg/dL | (70-400) |
| Immunoglobulin G | 193 mg/dL | (700-1600) |
| Immunoglobulin M | 142 mg/dL | (40-230) |
| Rheumatology profile | ||
| ANA profile | Negative | |
| Anti- CCP | 0.47 RU/mL | (< 5.00) |
| Anti- dsDNA | 13.9 IU/mL | (< 100) |
| p-ANCA | Negative | |
| c-ANCA | Negative | |
| Complement C3 | 134 mg/dL | (90-180) |
| Complement C4 | 35 mg/dL | (10-40) |
| Onconeural antibodies | ||
| Chromogranin A | 363.250 µg/L | (< 100) |
| Anti-neuronal autoantibodies | ||
| (Hu. Yo. Ri. CV2. Ma2/Ta. amphiphysin. anti-GAD) | Negative | |
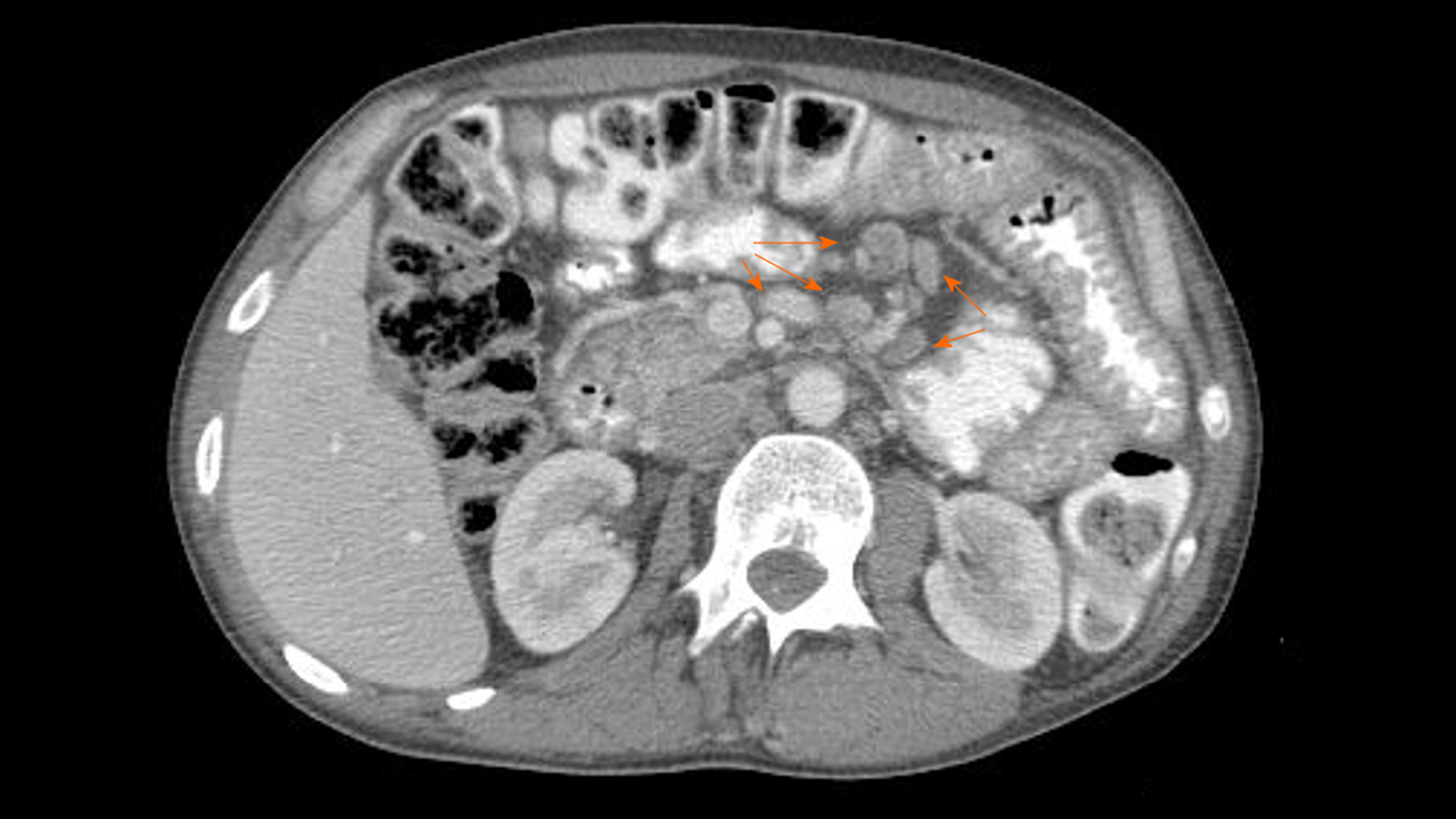
The abdomen ultrasonography revealed abdominal lymphadenopathy and accumulated pericardial and peritoneal fluid. Computed tomography (CT) scan of the chest, abdomen and pelvis revealed streaky fibrous changes at the base of the left lung, and numerous enlarged mesenteric lymph nodes (up to 14 mm × 29 mm, Figure 1).
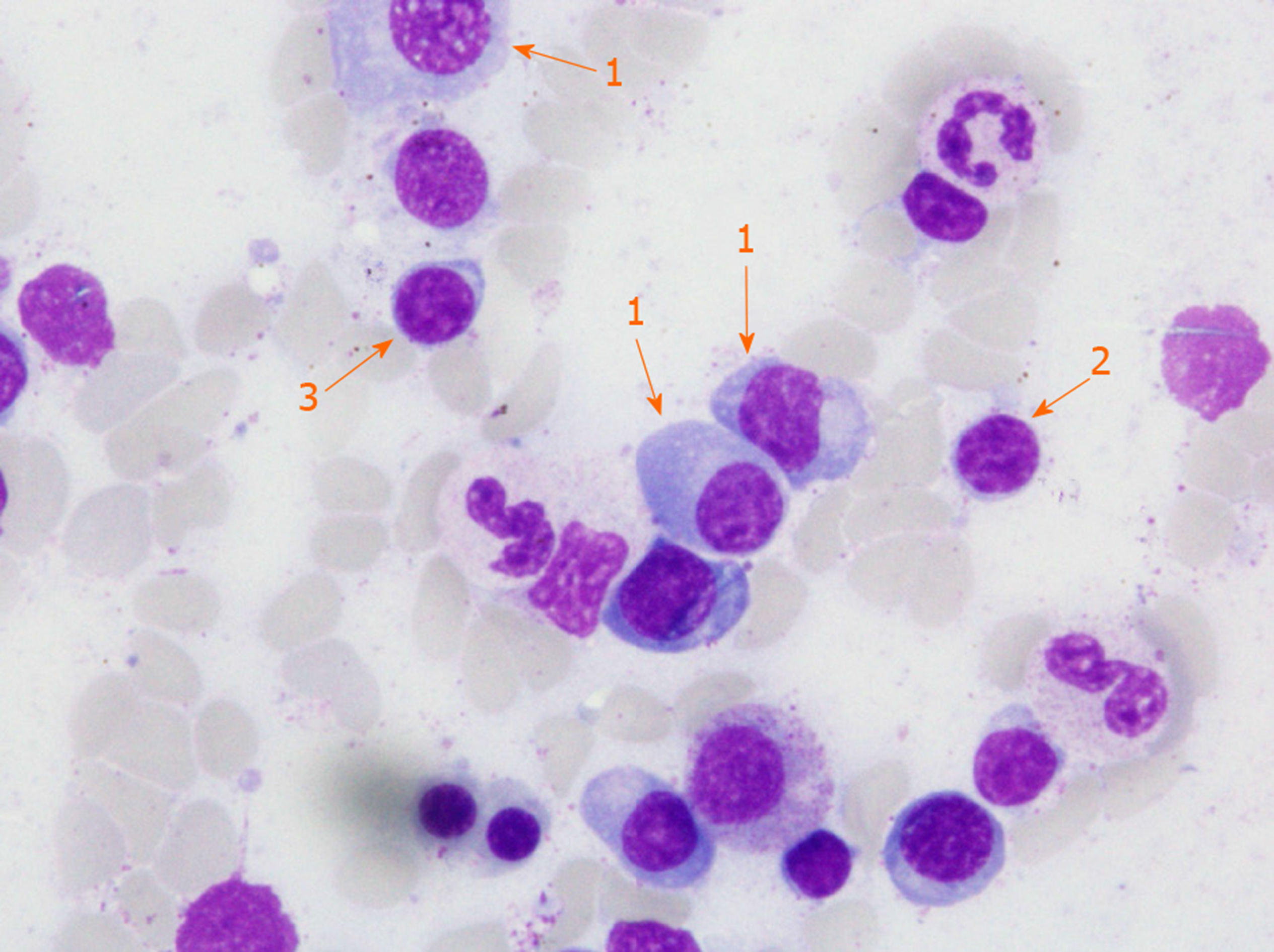
In the course of diagnostics, an increased pool of lymphoid cells in the myelogram was found and the patient was referred for further diagnosis in the Hematology Department. Fifty-five percent infiltration with clonal plasma, lymphoplasmic and B cells was confirmed in immunophenotype and bone marrow morphology (Figure 2). Increased concentration of free kappa light chains in peripheral blood serum and urine, and the presence of M protein in the IgM kappa class in serum was confirmed. In peripheral blood counts, leukocytosis 21.9 × 103/μL (normal range 4.0 × 103/μL-10.0 × 103/μL) with granulocytosis, microcytic, sideropenic anemia and reactive thrombocytosis have been noted.
Taking into account all the symptoms, a diagnosis of LPL was stated.
Given the rapidly worsening clinical condition of the patient, R-COP (rituximab 600 mg iv 1 d, vincristin 2 mg iv 1 d, cyclophosphamide 1200 mg iv 1-5 d, methylprednisolone 125 mg iv 1-5 d,) immunochemotherapy as an emergency treatment was started. Unfortunately, despite intensive supportive treatment the general clinical and neurological condition of the patient deteriorated.
The patient died on the fifth day of treatment in the Hematology Department.
Lymphomas, more commonly B-cell lymphomas including LPL may cause axonal damage or infiltrate peripheral nerves and cause asymmetric mononeuropathy or CIDP[14]. Therefore, there are a number of reports regarding occurrence of CIDP in the course of lymphoma, but only a few describing paraneoplastic CIDP, which precede the lymphoma diagnosis[15-17]. Vial et al[12] presented nine cases in which CIDP symptoms preceded lymphomas, but none of them where LPL. However, they described a case of 39-year-old man with axonal multiple mononeuropathy which occurs six months before LPL diagnosis and 79-year-old woman with radiculopathy, in whom LPL was diagnosed five months after the first symptoms of radiculopathy. In the first case treatment included doxorubicin-based therapy, intrathecal methotrexate/cyclophosphamide and fludarabine. The second patient was treated with doxorubicin, intrathecal methotrexate and prednisone. In both cases hematological remission and neurological improvement were observed[12].
The patient described by us is an example of paraneoplastic polyradiculoneuropathy and lymphoplasmacytic lymphoma. CIDP was initially diagnosed in 2012, since when remissions and relapses were observed alternately. The patient was treated monthly with intravenous immunoglobulins (Kiovig), and with intermittent intravenous pulses of methylprednisolone sodium succinate (Solu-Medrol). This treatment was initially effective – the patient was able to function normally in everyday life (he worked as a mechanic in the workshop, often travelled abroad, actively spending his spare-time). However, in December 2018 his general health condition (especially neurological) deteriorated. The rapid weight loss was attributed to the intensive diuretic treatment due to concomitant edema, which in turn were caused by protein deficiency and heart failure (not typical for such a young man). Due to increasing abdominal pain, patient underwent gastroscopy and was diagnosed with gastritis. Proton pomp inhibitors treatment resulted in only partial improvement. The patient’s neurological condition continued to deteriorate despite intravenous immunoglobulin and glucocorticosteroid treatment leading to his necessity of hospitalization.
The overall clinical symptoms, the results of laboratory tests and CT scan suggested the secondary background of polyradiculoneuropathy. Despite the different opinion of the neurologists, we stayed with our suspicion of a diagnosis of lymphoma, which was finally confirmed. It seems that the lack of typical symptoms, such as peripheral lymphadenopathy, splenomegaly or low-grade fever, resulted in delaying the diagnosis of lymphoma and worsening its’ prognosis. Disturbing symptoms reported by the patient were not considered to have paraneoplastic origin. It seems reasonable, that such asymptomatic patients should have regular basic laboratory tests such as blood count, blood biochemistry and proteinogram, as well as chest X-ray and an abdominal ultrasound. In case of any abnormalities bone marrow biopsy and hematologist consultation should be done. Polyradiculoneuropathy, while mostly primary, may also by secondary to LPL. Such a situation, when symmetrical sensorimotor polyneuropathy (multifocal or mononeuropathy) precedes the diagnosis of lymphoma, is observed even in 30% of patients. This case should be a reminder to consider an oncological diagnosis in patients with CIDP, especially those with an atypical or rapidly progressive course of the disease.
CIDP and malignant diseases co-occurrence is rare, nevertheless patients with atypical symptoms of neuropathy should have extended diagnostics and remain under constant supervision of specialists.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sridharan G S-Editor: Wang J L-Editor: A E-Editor: Liu JH
| 1. | Laughlin RS, Dyck PJ, Melton LJ, Leibson C, Ransom J, Dyck PJ. Incidence and prevalence of CIDP and the association of diabetes mellitus. Neurology. 2009;73:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 2. | Dalakas MC; Medscape. Advances in the diagnosis, pathogenesis and treatment of CIDP. Nat Rev Neurol. 2011;7:507-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 3. | Peltier AC, Donofrio PD. Chronic inflammatory demyelinating polyradiculoneuropathy: from bench to bedside. Semin Neurol. 2012;32:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Vallat JM, Sommer C, Magy L. Chronic inflammatory demyelinating polyradiculoneuropathy: diagnostic and therapeutic challenges for a treatable condition. Lancet Neurol. 2010;9:402-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Dimachkie MM, Barohn RJ. Chronic inflammatory demyelinating polyneuropathy. Curr Treat Options Neurol. 2013;15:350-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Ruts L, Drenthen J, Jacobs BC, van Doorn PA; Dutch GBS Study Group. Distinguishing acute-onset CIDP from fluctuating Guillain-Barre syndrome: a prospective study. Neurology. 2010;74:1680-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117:5019-5032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1446] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 8. | NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Waldenstrom’s Macroglobulinemia/Lymphoplasmacytic Lymphoma. Version 2.2011. National Comprehensive Cancer Network. Available from: http://williams.medicine.wisc.edu/waldenstroms.pdf. |
| 9. | Anagnostopoulos A, Hari PN, Pérez WS, Ballen K, Bashey A, Bredeson CN, Freytes CO, Gale RP, Gertz MA, Gibson J, Goldschmidt H, Lazarus HM, McCarthy PL, Reece DE, Vesole DH, Giralt SA. Autologous or allogeneic stem cell transplantation in patients with Waldenstrom's macroglobulinemia. Biol Blood Marrow Transplant. 2006;12:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Ansell SM, Kyle RA, Reeder CB, Fonseca R, Mikhael JR, Morice WG, Bergsagel PL, Buadi FK, Colgan JP, Dingli D, Dispenzieri A, Greipp PR, Habermann TM, Hayman SR, Inwards DJ, Johnston PB, Kumar SK, Lacy MQ, Lust JA, Markovic SN, Micallef IN, Nowakowski GS, Porrata LF, Roy V, Russell SJ, Short KE, Stewart AK, Thompson CA, Witzig TE, Zeldenrust SR, Dalton RJ, Rajkumar SV, Gertz MA. Diagnosis and management of Waldenström macroglobulinemia: Mayo stratification of macroglobulinemia and risk-adapted therapy (mSMART) guidelines. Mayo Clin Proc. 2010;85:824-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Baldini L, Nobile-Orazio E, Guffanti A, Barbieri S, Carpo M, Cro L, Cesana B, Damilano I, Maiolo AT. Peripheral neuropathy in IgM monoclonal gammopathy and Wäldenstrom's macroglobulinemia: a frequent complication in elderly males with low MAG-reactive serum monoclonal component. Am J Hematol. 1994;45:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Viala K, Béhin A, Maisonobe T, Léger JM, Stojkovic T, Davi F, Leblond V, Bouche P. Neuropathy in lymphoma: a relationship between the pattern of neuropathy, type of lymphoma and prognosis? J Neurol Neurosurg Psychiatry. 2008;79:778-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Briani C, Vitaliani R, Grisold W, Honnorat J, Graus F, Antoine JC, Bertolini G, Giometto B; PNS Euronetwork. Spectrum of paraneoplastic disease associated with lymphoma. Neurology. 2011;76:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Kelly JJ, Karcher DS. Lymphoma and peripheral neuropathy: a clinical review. Muscle Nerve. 2005;31:301-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Viala K, Stojkovic T, Doncker AV, Maisonobe T, Lenglet T, Bruneteau G, Musset L, Neil J, Léger JM, Leblond V. Heterogeneous spectrum of neuropathies in Waldenström's macroglobulinemia: a diagnostic strategy to optimize their management. J Peripher Nerv Syst. 2012;17:90-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Levine T, Pestronk A, Florence J, Al-Lozi MT, Lopate G, Miller T, Ramneantu I, Waheed W, Stambuk M, Stone MJ, Choksi R. Peripheral neuropathies in Waldenström's macroglobulinaemia. J Neurol Neurosurg Psychiatry. 2006;77:224-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Vallat JM, De Mascarel HA, Bordessoule D, Jauberteau MO, Tabaraud F, Gelot A, Vallat AV. Non-Hodgkin malignant lymphomas and peripheral neuropathies--13 cases. Brain. 1995;118:1233-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 3.7] [Reference Citation Analysis (0)] |