Published online Jun 26, 2020. doi: 10.12998/wjcc.v8.i12.2502
Peer-review started: February 24, 2020
First decision: March 27, 2020
Revised: April 10, 2020
Accepted: May 19, 2020
Article in press: May 19, 2020
Published online: June 26, 2020
Processing time: 120 Days and 21.5 Hours
Minimal-fat angiomyolipoma (mf-AML) is often misdiagnosed as renal cell carcinoma before surgery.
To analyze the magnetic resonance imaging (MRI) features of mf-AML and the causes of misdiagnosis by MRI before operation.
A retrospective analysis was performed on ten patients with mf-AML confirmed by surgical pathology, all of whom underwent preoperative MRI examination to analyze the morphological characteristics and MRI signals of the tumor.
MRI revealed a circular-like mass in 4/10 (40%) patients, an oval mass in 6/10 patients (60%), a mass with a capsule in 9/10 patients (90%), and a mass with a lipid component in 7/10 patients (70%). The diameter of the masses in all ten patients was from 11 to 47 mm; the diameter was between 11 mm and 40 mm in 8/10 (80%) patients and between 40 mm and 47 mm in 2/10 (20%) patients.
An oval morphological characteristic is strong evidence for the diagnosis of mf-AML, while a capsule and lipids are atypical manifestations of mf-AML.
Core tip: In magnetic resonance imaging, the oval or round-like morphological features, particularly oval or ellipse features, are of great significance for the diagnosis of minimal-fat angiomyolipoma. Capsules, lipid composition, and washout are atypical manifestations of minimal-fat angiomyolipoma.
- Citation: Li XL, Shi LX, Du QC, Wang W, Shao LW, Wang YW. Magnetic resonance imaging features of minimal-fat angiomyolipoma and causes of preoperative misdiagnosis. World J Clin Cases 2020; 8(12): 2502-2509
- URL: https://www.wjgnet.com/2307-8960/full/v8/i12/2502.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i12.2502
Angiomyolipoma (AML) is the most common benign tumor of the kidney and is composed of mature adipose tissue, smooth muscle, and varying amounts of vessels. The typical imaging manifestations of AML make it easy to confirm by magnetic resonance imaging (MRI)[1-5]. However, there are certain AMLs that lack a sufficient amount of fat (a fat content less than 25%) or do not contain adipose tissue, which cannot be distinguished by the naked eye on MRI. These AMLs are called minimal-fat angiomyolipoma (mf-AML). Because mf-AMLs are lack of typical AML imaging characteristic, they are often misdiagnosed as renal cell carcinoma before surgery, thus causing worthless surgical removal of the carcinoma[6,7] and bringing unnecessary burden to the patients. Therefore, this study retrospectively analyzed ten cases of mf-AML confirmed by surgical pathology in combination with the features of MRI manifestations to improve the accuracy of preoperative imaging diagnosis and pathological diagnosis.
The study selected ten patients [five males and five females, with an average age of 50 years (28-65)] with mf-AML confirmed by surgical pathology in the First Medical Center of the People’s Liberation Army General Hospital between January 2018 and December 2018. Among the ten patients, eight were found to have space-occupying lesions on physical examination, and two had urinary retention or lower abdominal pain as the chief complaint. One patient had left renal clear cell carcinoma and right renal angiomyolipoma, one had hyperplasia of the left adrenal gland, and one had left multiple renal angiomyolipoma (Table 1). Clinical data did not indicate tuberous sclerosis or Von Hippel-Lindau syndrome. Among the ten cases, eight were diagnosed with renal cell carcinoma by preoperative MRI, while the diagnosis could not be confirmed in the other two (renal cell carcinoma was likely to be diagnosed by MRI, but AML could not be completely excluded).
| Number | Age | Sex | Maximum diameter | Preoperative diagnosis | Shape | Manifestations |
| 1 | 65 | Male | 31 | The nodule in the right kidney was rich in blood supply with lipids and capsules, indicating the possibility of a malignant tumor, with renal carcinoma (clear cell carcinoma) being most likely | Circular-like | None |
| 2 | 64 | Male | 15 | The nodule in the right kidney was rich in blood supply with lipids, which indicated the possibility of renal cancer, not excluding angiomyolipoma | Oval | None |
| 3 | 63 | Female | 16 | The nodules in posterior portions of the upper pole of the right kidney and the upper pole of the left kidney were rich in blood supply, indicating the possibility of malignant tumors, with renal carcinoma (clear cell carcinoma) being most likely | Oval | None |
| 4 | 28 | Male | 47 | A cystic, solid, space occupying mass was observed in the left kidney, indicating the possibility of a malignant tumor, with renal cancer being most likely. Surgical consultation was recommended | Circular-like | None |
| 5 | 52 | Female | 11 | The nodule in the left kidney was rich in blood supply, indicating the possibility of clear cell carcinoma, not excluding angiomyolipoma | Oval | None |
| 6 | 54 | Male | 17 | The nodule in the right kidney contained lipids and blood supply, indicating the possibility of a malignant tumor, with renal cancer being most likely | Oval | None |
| 7 | 33 | Male | 46 | The mass in the left kidney was rich in blood supply and contained lipids, indicating the possibility of a malignant tumor, with renal carcinoma being most likely | Circular-like | None |
| 8 | 61 | Female | 21 | The nodule in the left kidney was rich in blood supply, indicating the possibility of a malignant tumor, with renal cancer being most likely; a fat embolism had formed in the intrahepatic segment of the inferior vena cava and right renal vein | Circular-like | Superior vena cava and right renal vein fat embolus |
| 9 | 48 | Female | 13 | The nodule in the right kidney was rich in blood supply and contained lipids, indicating the possibility of a malignant tumor, with renal cancer (clear cell carcinoma) being most likely | Oval | None |
| 10 | 34 | Female | 36 | The mass in the left kidney was rich in blood supply, indicating the possibility of a malignant tumor, with renal cancer (clear cell carcinoma) being most likely | Oval | None |
All patients were examined with a GE 3.0 T scanner. The basic scanning methods involved fast spin-echo (FSE,) T2-weighted imaging (T2WI) and T2WI without fat suppression, including transverse and coronal positions, and T1WI in-phase/opposed-phase examination. The parameters were: Time of repeatation: 2000-6000 ms; time of echo: 80-104 ms; matrix: 320 × 224; layer thickness: 5-6 mm; spacing: 1 mm; and field of view (FOV): 36 cm × 36 cm-40 cm × 40 cm. The b value of the diffusion weighted imaging (DWI) sequence was set at 0 and 800-1000 s/mm2. A 3D liver acquisition with volume acceleration (LAVA) sequence was used in the multitemporal dynamic enhancement scan to perform a prescan of fat suppression on T1WI. The contrast agent Gd-diethylenetriamine pentaacetic acid was used in the enhanced scan at a dose of 0.1 mmol/kg (body weight), and the injection was performed with a high-pressure syringe at a rate of 1.5 mL/s. Cortico-medullary scanning was performed 18-21 s after contrast agent injection, while nephrographic phase and excretory phase scanning was performed at 45-60 s and 5-6 min after contrast agent injection, respectively.
The images were analyzed to observe the lesion morphology (circular-like/oval), size, signal characteristics (T2WI/T1WI/DWI/lipid/hemorrhage/cystic changes), surrounding capsule, and tumor enhancement characteristics on MRI images. Among them, tumor enhancement was confirmed based on the renal cortex signals as the reference standard: Nephrographic phase lesions whose signal intensity was hypointense to renal cortex were defined as mild or insignificant enhancements, while lesions whose signal intensity was isointense to renal cortex were defined as moderate enhancements, and lesions whose signal intensity was hyperintense to renal cortex were defined as significant enhancements[8-10].
In this study, nine lesions showed different degrees of exophytic growth. Four cases presented as round-like masses and six as oval masses. Lipid signals were observed in seven cases with different degrees of opposed-phase signal decrease. Lipid composition was not observed in three cases. An identifiable capsule was observed in nine cases but not in one case. The diameter of the tumors ranged from 11 to 47 mm, with a diameter of less than 20 mm in five tumors and less than 40 mm in eight tumors. There were eight cases of solid masses, including one case of a cystic solid mass and one case of a cystic lesion due to massive hemorrhage.
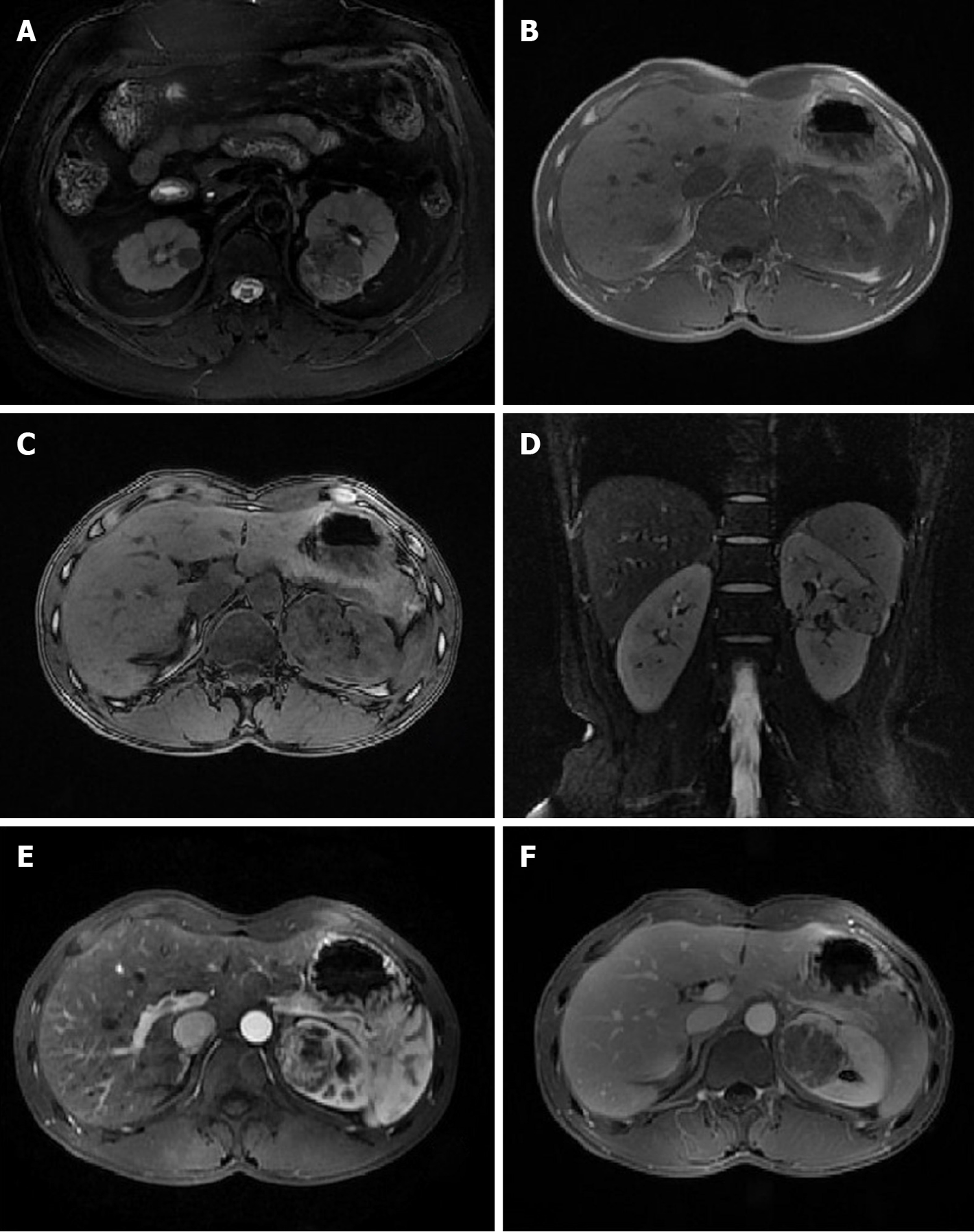
Eight cases of tumors had a homogeneous and slightly low T1W1 signal, and one had a homogeneous isointense T1 signal. One case had a heterogeneous, short T1 signal due to mass bleeding, while the other nine had no bleeding. Eight cases had a homogeneous, low T2 signal or slightly low T2 signal, one had a heterogeneous, low signal due to small-scale cystic changes, and one had a heterogeneous, short T2 signal due to mass bleeding. Six cases had DWI isointense or slightly high signals, and four had a high signal. Seven cases of tumors showed significant heterogeneous enhancement, and three showed moderate enhancement. In the excretory phase, eight cases had tumor washout, and two showed continuous enhancement. Intratumoral vasculature was not observed in eight cases but was visible in the other two (Figure 1).
AML is a benign soft tissue tumor that is susceptible to the influence of adjacent normal kidney tissues. When adjacent normal renal parenchyma or renal mass is squeezed by the membrane, AML will present with typical morphological features, such as angular interface sign, mushrooming, and bubble-over sign[11-15]. However, nine cases in this study, instead of presenting with a typical morphology such as angular interface sign, presented with round-like or oval shapes, indicating the possibility of mf-AML in a solid texture due to the lack of fat and leading to round-like, oval, or ellipse morphological features. In particular, among the six cases of oval or ellipse lesions, the long diameter of the lesions was parallel to the renal medulla, suggesting that the tumors had different degrees of compression. Therefore, this study suggested that round-like or oval lesions could be regarded as the morphological characteristics of AML, which are more common in mf-AML. An oval or ellipse lesion shape has great significance for the diagnosis of mf-AML. A circular-like lesion shape can be considered an atypical morphological feature of mf-AML.
In this study, eight cases of tumors were less than 40 mm in diameter, and the masses were primarily characterized by uniform T1WI and T2WI signals on MRI, a feature of solid masses. Meanwhile, hemorrhage cystic changes were seldom found, indicating little secondary progression of mf-AML. However, a larger sample size is needed to confirm these observations. Regarding enhancement characteristics, in this study there were seven cases of tumors with significant and heterogeneous enhancement, and eight patients had tumor washout in the excretory phase, indicating that mf-AML is rich in blood supply, thus allowing for the enhancement seen on MRI.
The formation of capsule results from ischemia and necrosis caused by the growing tumor pressing on the surrounding normal renal parenchyma, which eventually leads to the formation of fibrous tissue[16-18]. An identifiable capsule is a typical MRI sign of renal cell carcinoma. In this study, nine cases of tumors had capsules, which explained the high rate of preoperative misdiagnosis. This is probably because mf-AML has a lower level of fat, leading to a slightly harder tumor texture and causing compression on the surrounding normal renal parenchyma to form a capsule. Therefore, the identifiable capsule can also be considered an atypical MRI manifestation of mf-AML. However, a larger sample size is needed to confirm whether the capsule thickness, capsule integrity, and enhancement of the mf-AML pseudo capsule are provided with diagnostic significance.
Lipid change, also known as fatty change or steatosis, is defined pathologically as excessive triglyceride accumulation in the cytoplasm of nonfat cells such as liver cells, cardiomyocytes, and renal tubular epithelium and skeletal muscle cells[19-21]. The presence of a small amount of lipid components can be identified on MRI when the opposed-phase signal is reduced. In this study, seven cases were found with small lipid signals in the mass, indicating that lipids could be present in mf-AML. The presence of in-phase and opposed-phase lipid signals is usually a clear and direct sign for the diagnosis of renal cell carcinoma, especially clear cell carcinoma[22-24]. Therefore, the presence of lipid components detected by in-phase and opposed-phase in renal tumors could indicate the possibility of mf-AML based on morphological characteristics.
Based on the above analysis, mf-AML can present with oval or round-like morphological features, particularly oval or ellipse features, which are of great significance for diagnosis. Capsules, lipid composition, and washout are atypical manifestations of mf-AML. The presence of these atypical MRI manifestations should be combined with the morphological characteristics for the preoperative diagnosis of mf-AML.
Minimal-fat angiomyolipoma (mf-AML) is often misdiagnosed as renal cell carcinoma before operation, which leads to unnecessary operation. Improving the rate of preoperative diagnosis is helpful to reduce unnecessary surgical treatment.
The magnetic resonance imaging (MRI) features of mf-AML are different from those of typical AML. Summarizing and analyzing the imaging features of mf-AML are helpful to improve the understanding of the disease and avoid unnecessary surgery.
To summarize the MRI features of mf-AML in order to improve the rate of preoperative diagnosis of mf-AML.
The MRI features of mf-AML confirmed by operation and pathology were retrospectively analyzed, including morphological features, lipids, capsule, washout and so on. These lesions were diagnosed as renal cell carcinoma before operation or could not be diagnosed clearly.
A retrospective analysis of the results of ten cases of AML revealed a circular-like mass in 4/10 (40%) patients, an oval mass in 6/10 (60%), a mass with a capsule in 9/10 (90%), and a mass with a lipid component in 7/10 (70%). But it still needs studies with a larger sample size to prove it.
An oval morphological characteristic is strong evidence for the diagnosis of mf-AML, while a capsule and lipids are atypical manifestations of mf-AML.
The imaging features of mf-AML are not typical, morphological features are very important for the diagnosis of renal tumors, and lipids and capsules can also be MRI findings of mf-AML. Some imaging features of mf-AML overlap with renal cell carcinoma, so it is necessary to comprehensively analyze its imaging features to improve the rate of preoperative diagnosis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Coughlin S, Kmietowicz Z, Kramer JR S-Editor: Wang J L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Sung CK, Kim SH, Woo S, Moon MH, Kim SY, Kim SH, Cho JY. Angiomyolipoma with minimal fat: differentiation of morphological and enhancement features from renal cell carcinoma at CT imaging. Acta Radiol. 2016;57:1114-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Park JJ, Kim CK. Small (< 4 cm) Renal Tumors With Predominantly Low Signal Intensity on T2-Weighted Images: Differentiation of Minimal-Fat Angiomyolipoma From Renal Cell Carcinoma. AJR Am J Roentgenol. 2017;208:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Yan L, Liu Z, Wang G, Huang Y, Liu Y, Yu Y, Liang C. Angiomyolipoma with minimal fat: differentiation from clear cell renal cell carcinoma and papillary renal cell carcinoma by texture analysis on CT images. Acad Radiol. 2015;22:1115-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Park KJ, Kim MH, Kim JK, Cho KS. Sonographic Features of Small (< 4 cm) Renal Tumors With Low Signal Intensity on T2-Weighted MR Images: Differentiating Minimal-Fat Angiomyolipoma From Renal Cell Carcinoma. AJR Am J Roentgenol. 2018;211:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Zhang YY, Luo S, Liu Y, Xu RT. Angiomyolipoma with minimal fat: differentiation from papillary renal cell carcinoma by helical CT. Clin Radiol. 2013;68:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Schieda N, Hodgdon T, El-Khodary M, Flood TA, McInnes MD. Unenhanced CT for the diagnosis of minimal-fat renal angiomyolipoma. AJR Am J Roentgenol. 2014;203:1236-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Liu G, Yao D, Zhang S, Zhao X, Liu T, Li X, Guo H. Minimal fat renal angiomyolipoma with lymph node involvement: A case report and literature review. Can Urol Assoc J. 2015;9:E568-E571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Egbert ND, Caoili EM, Cohan RH, Davenport MS, Francis IR, Kunju LP, Ellis JH. Differentiation of papillary renal cell carcinoma subtypes on CT and MRI. AJR Am J Roentgenol. 2013;201:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Zhao XJ, Pu JX, Ping JG, Zang J, Lu Y, Xi QL, Hou WJ. Angiomyolipoma with minimal fat: differentiation from renal cell carcinoma at helical CT. Chin Med J (Engl). 2013;126:991-992. [PubMed] |
| 10. | Hindman N, Ngo L, Genega EM, Melamed J, Wei J, Braza JM, Rofsky NM, Pedrosa I. Angiomyolipoma with minimal fat: can it be differentiated from clear cell renal cell carcinoma by using standard MR techniques? Radiology. 2012;265:468-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 11. | Jhaveri KS, Elmi A, Hosseini-Nik H, Hedgire S, Evans A, Jewett M, Harisinghani M. Predictive Value of Chemical-Shift MRI in Distinguishing Clear Cell Renal Cell Carcinoma From Non-Clear Cell Renal Cell Carcinoma and Minimal-Fat Angiomyolipoma. AJR Am J Roentgenol. 2015;205:W79-W86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Lu Q, Wang W, Huang B, Li C, Li C. Minimal fat renal angiomyolipoma: the initial study with contrast-enhanced ultrasonography. Ultrasound Med Biol. 2012;38:1896-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Siegel C. Re: Small (< 4 cm) Renal Tumors with Predominantly Low Signal Intensity on T2-Weighted Images: Differentiation of Minimal-Fat Angiomyolipoma from Renal Cell Carcinoma. J Urol. 2017;198:231-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Buj Pradilla MJ, Martí Ballesté T, Torra R, Villacampa Aubá F. Recommendations for imaging-based diagnosis and management of renal angiomyolipoma associated with tuberous sclerosis complex. Clin Kidney J. 2017;10:728-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Li H, Li A, Zhu H, Hu Y, Li J, Xia L, Hu D, Kamel IR, Li Z. Whole-Tumor Quantitative Apparent Diffusion Coefficient Histogram and Texture Analysis to Differentiation of Minimal Fat Angiomyolipoma from Clear Cell Renal Cell Carcinoma. Acad Radiol. 2019;26:632-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Wan Y, Guo H, Ji L, Li Z, Gao J. Gemstone spectral imaging dual-energy computed tomography for differentiation of renal cell carcinoma and minimal-fat renal angiomyolipoma. J Cancer Res Ther. 2018;14:S394-S399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Li A, Xing W, Li H, Hu Y, Hu D, Li Z, Kamel IR. Subtype Differentiation of Small (≤ 4 cm) Solid Renal Mass Using Volumetric Histogram Analysis of DWI at 3-T MRI. AJR Am J Roentgenol. 2018;211:614-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Deniffel D, Boutelier T, Labani A, Ohana M, Pfeiffer D, Roy C. Computed Tomography Perfusion Measurements in Renal Lesions Obtained by Bayesian Estimation, Advanced Singular-Value Decomposition Deconvolution, Maximum Slope, and Patlak Models: Intermodel Agreement and Diagnostic Accuracy of Tumor Classification. Invest Radiol. 2018;53:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Razik A, Das CJ, Sharma S. Angiomyolipoma of the Kidneys: Current Perspectives and Challenges in Diagnostic Imaging and Image-Guided Therapy. Curr Probl Diagn Radiol. 2019;48:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Cong X, Zhang J, Xu X, Zhang M, Chen Y. Renal epithelioid angiomyolipoma: magnetic resonance imaging characteristics. Abdom Radiol (NY). 2018;43:2756-2763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Lim RS, McInnes MDF, Siddaiah M, Flood TA, Lavallee LT, Schieda N. Are growth patterns on MRI in small (< 4 cm) solid renal masses useful for predicting benign histology? Eur Radiol. 2018;28:3115-3124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Chen LS, Zhu ZQ, Wang ZT, Li J, Liang LF, Jin JY, Wang ZQ. Chemical shift magnetic resonance imaging for distinguishing minimal-fat renal angiomyolipoma from renal cell carcinoma: a meta-analysis. Eur Radiol. 2018;28:1854-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Li H, Liang L, Li A, Hu Y, Hu D, Li Z, Kamel IR. Monoexponential, biexponential, and stretched exponential diffusion-weighted imaging models: Quantitative biomarkers for differentiating renal clear cell carcinoma and minimal fat angiomyolipoma. J Magn Reson Imaging. 2017;46:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Schieda N, Dilauro M, Moosavi B, Hodgdon T, Cron GO, McInnes MD, Flood TA. MRI evaluation of small (< 4 cm) solid renal masses: multivariate modeling improves diagnostic accuracy for angiomyolipoma without visible fat compared to univariate analysis. Eur Radiol. 2016;26:2242-2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |