Published online Jun 26, 2020. doi: 10.12998/wjcc.v8.i12.2494
Peer-review started: February 5, 2020
First decision: April 21, 2020
Revised: May 8, 2020
Accepted: May 23, 2020
Article in press: May 23, 2020
Published online: June 26, 2020
Processing time: 139 Days and 16.1 Hours
Management of non-neurogenic, non-obstructive dysuria represents one of the most challenging dilemmas in urological practice. The main clinical symptom is the increase in residual urine. Voiding dysfunction is the main cause of dysuria or urinary retention, mainly due to the decrease in bladder contraction (the decrease in contraction amplitude or duration) or the increase in outflow tract resistance. Sacral neuromodulation (SNM) has been used for > 10 years to treat many kinds of lower urinary tract dysfunction. It has become increasingly popular in China in recent years. Consequently, studies focusing on non-neurogenic, non-obstructive dysuria patients treated by SNM are highly desirable.
To assess the outcome of two-stage SNM in non-neurogenic, non-obstructive dysuria.
Clinical data of 54 patients (26 men, 28 women) with non-neurogenic, non-obstructive dysuria treated by SNM from January 2012 to December 2016 in ten medical centers in China were retrospectively analyzed. All patients received two or more conservative treatments. The voiding diary, urgency score, and quality of life score before operation, after implantation of tined lead in stage I (test period), and during short-term follow-up (latest follow-up) after implantation of the implanted pulse generator in stage II were compared to observe symptom improvements.
Among the 54 study patients, eight refused to implant an implanted pulse generator because of the unsatisfactory effect, and 46 chose to embed the implanted pulse generator at the end of stage I. The conversion rate of stage I to stage II was 85.2%. The average follow-up time was 18.6 mo. There were significant differences between baseline (before stage I) and the test period (after stage I) in residual urine, voiding frequency, average voiding amount, maximum voiding amount, nocturia, urgency score, and quality of life score. The residual urine and urgency score between the test period and the latest follow-up time (after stage II) were also significantly different. No significant differences were observed for other parameters. No wound infection, electrode breakage, or other irreversible adverse events occurred.
SNM is effective for patients with non-neurogenic, non-obstructive dysuria showing a poor response to traditional treatment. The duration of continuous stimulation may be positively correlated with the improvement of residual urine.
Core tip: This is the first multicenter retrospective study of sacral neuromodulation (SNM) in China, which can reflect the clinical effects of SNM in the treatment of non-neurogenic, non-obstruction dysuria patients in the past 5 years. Through the study, we can conclude that SNM is effective and safe for patients with non-neurogenic, non-obstructive dysuria showing a poor response to traditional treatment. The duration of continuous stimulation may be positively correlated with the improvement of residual urine.
- Citation: Meng LF, Zhang W, Wang JY, Zhang YG, Zhang P, Liao LM, Lv JW, Ling Q, Wei ZQ, Zhong T, Xu ZH, Wen W, Li JY, Luo DY. Clinical outcomes of sacral neuromodulation in non-neurogenic, non-obstructive dysuria: A 5-year retrospective, multicentre study in China. World J Clin Cases 2020; 8(12): 2494-2501
- URL: https://www.wjgnet.com/2307-8960/full/v8/i12/2494.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i12.2494
Management of non-neurogenic, non-obstructive dysuria represents one of the most challenging dilemmas in urological practice. The main clinical symptom is the increase in residual urine. If patients do not accept the definite diagnosis and effective treatments, they will have a higher risk of urinary retention, upper tract damage, and even renal failure. Voiding dysfunction is the main cause of dysuria or urinary retention, which is mainly due to the decrease in bladder contraction (the decrease in contraction amplitude or duration) or the increase in outflow tract resistance. The decrease in bladder contractile function is usually caused by a temporary or permanent change in the neuromuscular mechanism. The increase in outflow tract resistance is mostly due to obstruction, which can be subdivided into anatomical and functional obstruction.
Sacral neuromodulation (SNM) is a well-recognized therapy for lower urinary tract symptoms. In 1999, SNM was approved by the United States Food and Drug Administration for idiopathic urinary retention[1,2]. It has a two-way regulating effect. It can be used for both detrusor overactivity and underactivity, thus improving the opposing symptoms of voiding dysfunction[3,4]. This provides a new idea in clinical work for treating non-neurogenic, non-obstructive dysuria.
According to our clinical observation, SNM is effective and safe for such patients. Thus, in this retrospective study, its effectiveness and safety are reported. To the best of our knowledge, this is the first multicenter retrospective study of SNM in China.
The clinical data of 54 patients (26 men and 28 women) with non-neurogenic, non-obstructive dysuria were retrospectively analyzed. The average age was 51 years (range, 19-86 years). Urodynamics were performed in all patients; most of them developed dysuria due to sphincter spasm, detrusor underactivity, or detrusor hyperactivity with impaired contractility. All procedures were performed after receiving approval from the ethics department (No. 2019BJYYEC 008 01).
The inclusion criteria were diagnosis of dysuria; history of more than two conservative treatments, but the effect was poor or invalid; treatment by SNM from January 2012 to December 2016; age over 16 years; and being willing to sign an informed consent form. The exclusion criteria were overactive bladder symptoms; pelvic prolapse; bladder outflow obstruction (such as benign prostatic hyperplasia, tumors, and urethral stricture); neurogenic diseases; urinary tract infection; pregnancy; and low compliance bladder (< 10 mL/cm H2O).
The data of voiding diary, urgency score, and quality of life (QoL) score before operation (baseline), after stage I SNM tined lead implantation (test period), and during short-term follow-up after stage II implanted pulse generator implantation (the latest follow-up) for each patient were collected and compared.
In this study, the improvement of clinical symptoms ≥ 50% was defined as effective treatment; the improvement of clinical symptoms < 50% with the presence of positive changes was defined as alleviating treatment; and no improvement or worsening of symptoms was defined as ineffective treatment. The final total improvement rate was defined as the percentage of patients with positive changes in symptoms; thus, the effective and alleviating rates were included in the total improvement rate[5]. All side-effects, complications, and surgical interventions after implantation were recorded.
The main observation index was the improvement rate of different symptoms in patients with non-neurogenic, non-obstructive dysuria (baseline vs test period vs the latest follow-up). Furthermore, the secondary observation indexes were the improvement rate of QoL and the incidence of complications.
Statistical analyses were performed with SPSS, version 22 (SPSS Inc., Chicago, IL, United States). The Kolmogorov-Smirnov test was performed to verify if the data were normally distributed. After confirming that the data followed a normal distribution, the paired-samples t-test was used to compare symptom changes between groups. The data are expressed as the mean ± SD. The non-normally distributed data were compared using the non-parametric two-related sample test. The data are expressed as median (interquartile range). Statistical significance was considered at P < 0.05.
The median treatment time before admission was 4 years. All 54 patients underwent stage-1 SNM for non-neurogenic, non-obstructive dysuria. The average test time was 19.9 d. Of all the patients, 46 (85.2%) subsequently underwent stage II (permanent) InterStim implantation following initial testing. These patients were followed postoperatively to determine efficacy, durability, and device-related complications. At the same time, the improvement rate of symptoms in eight patients without conversion to stage II was analyzed. Additionally, the baseline data, the test period data, and the latest follow-up data of 46 patients, in whom conversion to stage II was observed, were analyzed to observe the improvement of symptoms and scores.
To evaluate the efficacy of the different procedures, both objective criteria (residual urine, average voiding volume, and maximum voiding volume) and subjective criteria (patients’ perception of the clinical improvement, expressed by validated questionnaires or open interview) were considered. Compared to baseline data, the objective and subjective effects of eight patients could be observed intuitively (Table 1). The objective effects were as follows: There was one patient whose residual urine decreased by more than 50%; in all patients, it decreased by an average of 19.6%. There were two patients whose average voiding volume increased by more than 50%, with an average increase of 32.0%. There were four patients with a maximum voiding volume increase of more than 50%, with an average increase of 35.0%.
| Case | Residual urine (mL) | Average voiding volume (mL) | Maximum voiding volume (mL) | QoL score | ||||
| Baseline | Test period | Baseline | Test period | Baseline | Test period | Baseline | Test period | |
| 1 | 250 | 200 | 50 | 70 | 70 | 100 | 5 | 5 |
| 2 | 200 | 100 | 80 | 100 | 100 | 150 | 5 | 4 |
| 3 | 300 | 250 | 60 | 100 | 80 | 150 | 5 | 5 |
| 4 | 50 | 30 | 70 | 100 | 100 | 150 | 6 | 5 |
| 5 | 150 | 130 | 50 | 90 | 80 | 120 | 5 | 5 |
| 6 | 60 | 50 | 150 | 140 | 200 | 200 | 4 | 4 |
| 7 | 250 | 250 | 120 | 130 | 150 | 150 | 4 | 4 |
| 8 | 100 | 100 | 170 | 170 | 200 | 200 | 4 | 4 |
There were significant improvements in the other 46 patients between baseline and the test period in residual urine, voiding frequency, average voiding volume, maximum voiding volume, nocturia, urgency score, and QoL score (Table 2). The residual urine and urgency score between the test period and the latest follow-up time were 15 (0-82.5) mL vs 0 (0-70) mL (P < 0.01) and 2 (0-2) vs 2 (0-2) (P < 0.05), respectively. The other parameters were insignificant (Table 3).
| Baseline | Test period | P value | Z value | |
| Residual urine (mL) | 60 (0-200) | 15 (0-82.5) | < 0.01 | -4.03 |
| Voiding frequency | 13.74 ± 5.85 | 9.32 ± 2.73 | < 0.01 | |
| Average voiding volume (mL) | 114.84 ± 53.23 | 184.35 ± 55.31 | < 0.01 | |
| Maximum voiding volume (mL) | 160 (120-200) | 260 (200-300) | < 0.01 | -5.1 |
| Nocturia | 3 (2-5) | 2 (1-3) | < 0.01 | -4.67 |
| Urgency score | 3 (0-4) | 2 (0-2) | < 0.01 | -4.2 |
| QoL score | 5 (3.25-5) | 3.5 (3-4) | < 0.05 | -1.98 |
| Test period | The latest follow-up time | P value | Z value | |
| Residual urine (mL) | 15 (0-82.5) | 0 (0-70) | < 0.01 | -3.28 |
| Voiding frequency | 10 (7-10) | 8.5 (6.75-10) | > 0.05 | -1.68 |
| Average voiding volume (mL) | 190 (150-210) | 200 (155-240) | > 0.05 | -0.98 |
| Maximum voiding volume (mL) | 260 (200-300) | 255 (220-300) | > 0.05 | -0.72 |
| Nocturia | 2 (1-3) | 2 (1-2.63) | > 0.05 | -0.03 |
| Urgency score | 2 (0-2) | 2 (0-2) | < 0.05 | -2.23 |
| QoL score | 3.5 (3-4) | 4 (2-5) | > 0.05 | -1.08 |
Meanwhile, comparing the latest follow-up data with baseline data, the residual urine of patients was reduced by an average of 46.3%, the average voiding volume increased by an average of 87.2%, and the maximum voiding volume increased by an average of 60.5%. The patient’s main symptoms were effectively improved, which was also a key factor for such patients to be willing to accept the permanent implantation. There was no other significant difference between the test period and latest follow-up. Overall, the symptoms improved steadily.
The average follow-up time was 18.63 ± 13.92 mo (range, 3-63 mo). Of the 46 patients who underwent stage-2 SNM, 40 had sustained remission after surgery. Beyond that, this group had six cases of adverse events, including four cases of fluctuating efficacy, one case of electrode displacement, and one case of pain at the implant site. Among the four patients with fluctuating efficacy, two patients achieved satisfactory results after adjusting the parameters repeatedly. In one case, the SNM device shut down by itself in March 2017, and the other patient was explanted in January 2017. No discomfort was reported at the latest follow-up. The incidence of adverse events was 13.04% (6/46). There were no serious adverse effects or permanent injury associated with the implantable components in the 46 patients.
Fifty-four patients were included in this study, and the conversion rate was 85.2%. At the last follow-up, 57.1% of patients had a residual urine volume reduction of more than 50%. At the same time, combined with the improvement of other symptoms, it was seen that the curative effect was stable and showed a continuous improvement trend. Thus, it can be inferred that SNM has a satisfactory clinical effect in patients with non-neurogenic, non-obstructive dysuria in China. SNM was formally introduced in China in 2012. An increasing number of medical centers in China have applied SNM to treat patients with non-neurogenic, non-obstructive dysuria according to the Chinese expert consensus[6]. SNM significantly reduces residual urine, reduces the possibility of upper urinary tract damage, and achieves good clinical efficacy. The mechanism by which neuromodulation restores bladder function in patients with idiopathic urinary retention is not clearly understood. Idiopathic urinary retention was thought to be psychogenic, but several findings, such as hyperactivity of the pelvic floor and lack of pelvic floor control, in many patients with urinary retention have suggested an organic origin[7]. Some studies also noted that most patients with retention lack pelvic floor control, suggesting that SNM may function by directing the patient to re-localize the pelvic floor and regain the ability to relax it and initiate micturition[8-11]. When abdominal pressure suddenly increases, the defensive reflex regulated by the spinal cord will constrict the urethral sphincter to prevent the occurrence of urinary incontinence. Excessive defensive reflex will undoubtedly increase the incidence of dysuria and urinary retention. However, Dasgupta and Fowler[12] have suggested that this mechanism may be incorrect. They studied 30 women with retention before and after SNM with sphincter electromyography, and found that the electromyographical abnormality present before implantation was unchanged following implantation. A slight increase in detrusor contractility was noted, and they postulated that this increase in contractility was sufficient to overcome the increased sphincter activity and result in voiding.
By analyzing the baseline data and the test period data of eight patients, it can be concluded that the main clinical symptoms, especially residual urine volume, clearly improved. However, the patient’s QoL score still dominates the choice of treatment options. The QoL can be regarded as the subjective effect on patients, and its improvement was not obvious. It can be inferred that although the main clinical symptoms improved, the clinical results expected by the patients were not achieved, and some studies suggest that the effect depends largely on patients’ expectations and treatment compliance[1]. Moreover, economic cost may also be an important factor affecting patients’ treatment choices. This suggests that we need to further increase doctor-patient communication and help patients set reasonable expectations before operation. Furthermore, the retention time of temporary electrodes in patients may be positively correlated with satisfactory clinical results and good prognosis[13]. Similarly, stage-1 SNM may be left in situ for up to 4 wk to ensure the maximum chance of restoring normal bladder function in this complex group of patients. They theorized that patients with non-obstructive chronic urinary retention whose bladder sensation had not occurred for several months or years need a longer period to allow bladder sensation to return to normal and to have a recommencement of efficient voiding[14]. The mean test time was 19.9 d, and the conversion rate was 85.2%. For Asians with relatively small body size, whether prolonging the test time can improve the patient’s symptoms and further improve the conversion rate needs further exploration.
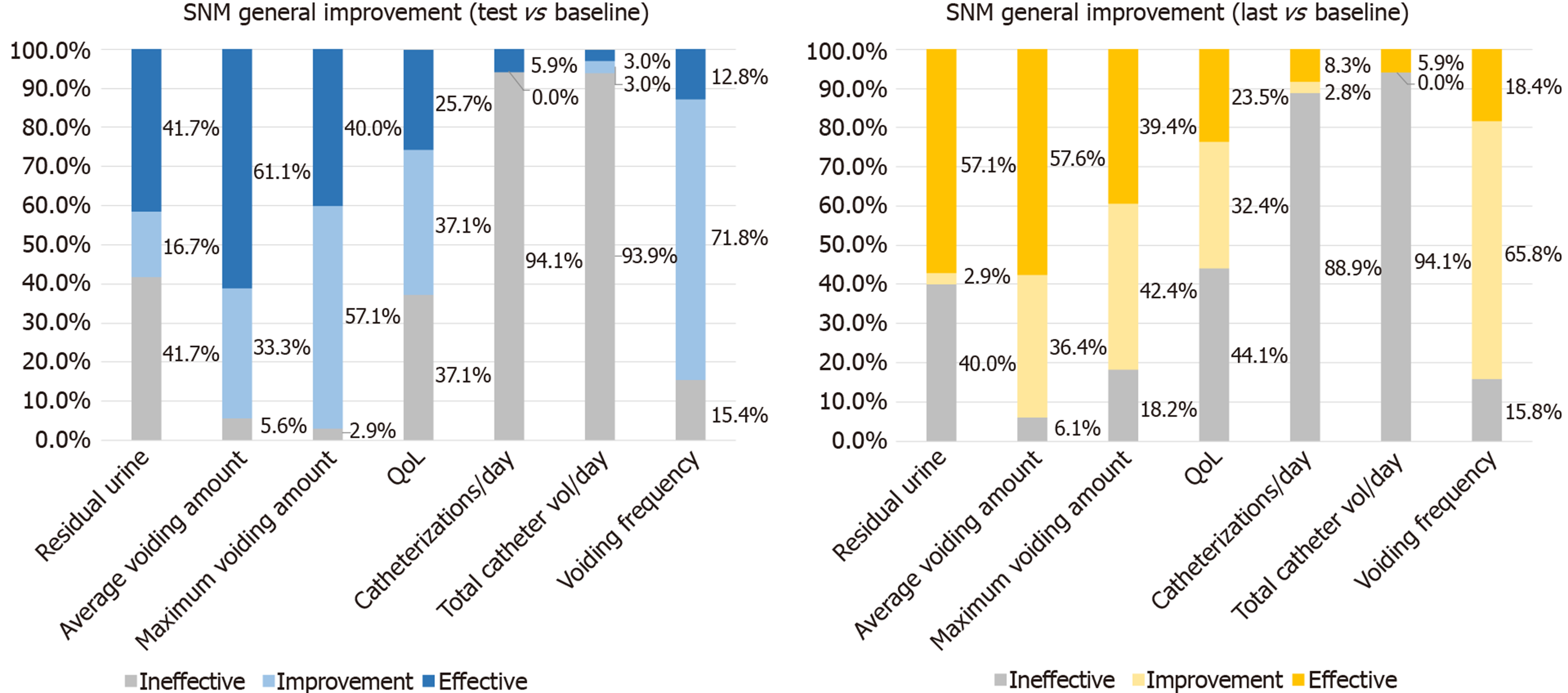
Comparing the latest follow-up data with the baseline data, we can perform therapeutic evaluation for the overall population. Symptoms with a total improvement rate of more than 50% include the total improvement rate of residual urine, average voiding volume, maximum voiding volume, QoL, and voiding frequency at the final follow-up at 60.0%, 93.9%, 82.8%, 55.9%, and 84.2%, respectively. However, the improvement rate of self-catheterization and catheterization volume was less than 15%, suggesting that for patients with severe symptoms or dysuria in the decompensated period, the clinical efficacy of SNM implantation needs to be further verified (Figure 1).
Meanwhile, comparing the parameters of the test period with that of the latest follow-up, the urgency score and residual urine volume were further improved. This improvement may be related to the reduction of residual urine volume and the recovery of bladder function. The proportion of people with an effectiveness rate of ≥ 50% increased from 41.7% to 57.1%, suggesting that the improvement of residual urine was directly proportional to the duration of continuous stimulation. There were no significant differences between the test period and the latest follow-up, suggesting stable improvement of the overall effect, which is consistent with previous reports[15].
The factors influencing the success of treatment for patients with non-neurogenic, non-obstructive dysuria are still unclear. Some studies have noted that the presence of Flower’s syndrome is a positive predictive factor for SNM in women[16]. Patients with non-obstructive urinary retention who were able to void at least 50 cc were more likely to benefit from SNM than those who were unable to void or voided less than 50 cc[13]. This partly explains the high conversion rate of our study. In our study, the average voiding volume of the 46 patients before operation was 114.84 ± 53.23 mL, and the conversion rate was 85.2%, which was higher than the 71% success rate reported previously[2]. These results suggest that a preoperative average voiding volume greater than 50 cc may be a good predictor of the success of SNM treatment.
The cost of SNM therapy is relatively high, and it has not been included in China's medical insurance catalog. Moreover, domestic patients have little knowledge of SNM, limiting the application of SNM therapy in China. The patients in our study had long medical histories, and they had received various treatment methods before SNM implantation. Concerning the current level of medical development, the choice of SNM therapy may be the ultimate choice for such patients. Therefore, further strengthening patient education and developing new devices will benefit more patients with lower urinary tract dysfunction.
This is the first multicenter retrospective study of SNM in China that reflects the clinical effects of SNM in treating patients with non-neurogenic, non-obstruction dysuria at present. The main limitations of our study were its retrospective nature and small number of patients. Potential bias and reporting errors are the main risks of any retrospective study. Another limitation of our study was that subjective data in the form of voiding diaries were recorded sequentially during follow-up at the clinic. These may affect the accuracy of research data and the scientific validity of research conclusions. We look forward to a future prospective study on SNM for lower urinary tract dysfunction to draw a conclusion with a higher level of evidence and further guide clinical diagnosis and treatment.
In conclusion, SNM provides a sustained, prolonged benefit in patients with non-neurogenic, non-obstruction dysuria who are resistant to medical and conservative therapy. Although much has been learned about SNM, there is still a need for ongoing research to know the exact mechanisms of action and proper patient selection and to reduce adverse events and surgical revision rates to save costs. Furthermore, the duration of test stimulation may be positively correlated with the reduction in residual urine.
Dysuria without urinary retention or obvious inducement is a clinically difficult problem to diagnose and treat. In the past, patients suffering from non-neurogenic, non-obstructive dysuria could only rely on intermittent catheterization, transurethral indwelling catheters, or suprapubic cystostomy to drain urine. These treatment methods significantly affected the patients’ quality of life. In 1999, sacral neuromodulation (SNM) was approved by the United States Food and Drug Administration for idiopathic urinary retention. China officially introduced SNM therapy into clinical practice in 2012, providing a new treatment method for non-neurogenic, non-obstructive dysuria.
The traditional treatment methods for non-neurogenic, non-obstructive dysuria are often ineffective, and the best treatment method is still controversial. As its third-line treatment, SNM has been widely used to treat dysuria in China in recent years. This study is the first retrospective, multicenter study of this treatment method in China, reflecting the clinical effect of SNM in the treatment of patients with non-neurogenic, non-obstructive dysuria.
The main purpose of this study was to summarize the experience of SNM in the treatment of non-neurogenic, non-obstructive dysuria.
The clinical data of 54 patients with non-neurogenic, non-obstructive dysuria treated with SNM in ten Chinese medical centers from January 2012 to December 2016 were collected retrospectively, and the paired-samples t-test and two-related sample test were used to compare the differences before and after treatment.
Eight patients refused to implant an implanted pulse generator because of the unsatisfactory effect. The conversion rate was 85.2% (46/54). There were significant differences between baseline and the test period in urgency score, quality of life score, and voiding diary. Only the urgency score and residual urine showed significant differences between the latest follow-up time and the test period.
SNM is a safe and effective, minimally invasive treatment for non-neurogenic, non-obstructive dysuria. The duration of continuous stimulation may be positively correlated with the improvement of residual urine.
Our study reflects the present clinical effects of SNM in the treatment of non-neurogenic, non-obstructive dysuria patients in China. However, this study is limited by its retrospective study design. Therefore, we look forward to a future prospective study on the use of SNM for lower urinary tract dysfunction to draw a conclusion with a higher level of evidence and further guide clinical diagnosis and treatment.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Garg R, Markic D S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Gormley EA, Lightner DJ, Faraday M, Vasavada SP; American Urological Association; Society of Urodynamics, Female Pelvic Medicine. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol. 2015;193:1572-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 400] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 2. | van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, Lycklama á Nijholt AA, Siegel S, Jonas U, Fowler CJ, Fall M, Gajewski JB, Hassouna MM, Cappellano F, Elhilali MM, Milam DF, Das AK, Dijkema HE, van den Hombergh U. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol. 2007;178:2029-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 354] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 3. | Dasgupta R, Wiseman OJ, Kitchen N, Fowler CJ. Long-term results of sacral neuromodulation for women with urinary retention. BJU Int. 2004;94:335-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Kavia RB, Datta SN, Dasgupta R, Elneil S, Fowler CJ. Urinary retention in women: its causes and management. BJU Int. 2006;97:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Zhang P, Wang JY, Zhang Y, Liao L, Lv JW, Ling Q, Wei ZQ, Zhong T, Xu ZH, Wen W, Li JY, Luo DY. Results of Sacral Neuromodulation Therapy for Urinary Voiding Dysfunction: Five-Year Experience of a Retrospective, Multicenter Study in China. Neuromodulation. 2019;22:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Chen GQ, Di W, Ding Y, Fan GS, Gu XY, Hao M, He J, Hu LN, Hua KQ, Huang W, Jin L. Chinese expert consensus on clinical application of sacral neuromodulation. Zhonghua Miniao Waike Zazhi. 2014;35:1-5. [DOI] [Full Text] |
| 7. | Fowler CJ, Christmas TJ, Chapple CR, Parkhouse HF, Kirby RS, Jacobs HS. Abnormal electromyographic activity of the urethral sphincter, voiding dysfunction, and polycystic ovaries: a new syndrome? BMJ. 1988;297:1436-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 157] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Shaker HS, Hassouna M. Sacral root neuromodulation in idiopathic nonobstructive chronic urinary retention. J Urol. 1998;159:1476-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 107] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Leng WW, Chancellor MB. How sacral nerve stimulation neuromodulation works. Urol Clin North Am. 2005;32:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Jonas U, Fowler CJ, Chancellor MB, Elhilali MM, Fall M, Gajewski JB, Grünewald V, Hassouna MM, Hombergh U, Janknegt R, van Kerrebroeck PE, Lylcklama a Nijeholt AA, Siegel SW, Schmidt RA. Efficacy of sacral nerve stimulation for urinary retention: results 18 months after implantation. J Urol. 2001;165:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 235] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Elkelini MS, Abuzgaya A, Hassouna MM. Mechanisms of action of sacral neuromodulation. Int Urogynecol J. 2010;21 Suppl 2:S439-S446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | DasGupta R, Fowler CJ. Urodynamic study of women in urinary retention treated with sacral neuromodulation. J Urol. 2004;171:1161-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Goh M, Diokno AC. Sacral neuromodulation for nonobstructive urinary retention--is success predictable? J Urol. 2007;178:197-199; discussion 199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Elneil S, Abtahi B, Helal M, Digesu A, Gonzales G. Optimizing the duration of assessment of stage-1 sacral neuromodulation in nonobstructive chronic urinary retention. Neuromodulation. 2014;17:66-70; discussion 70-1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Meng LF, Zhang W, Zhang YG, Wang JY, Liao LM, Chen GQ, Ling Q, Zhang P, Wei ZQ, Chen Q. [Sacral neuromodulation preliminary outcomes in male patients with idiopathic dysuria]. Zhonghua Yi Xue Za Zhi. 2019;99:2675-2680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | De Ridder D, Ost D, Bruyninckx F. The presence of Fowler's syndrome predicts successful long-term outcome of sacral nerve stimulation in women with urinary retention. Eur Urol. 2007;51:229-233; discussion 233-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |