Published online Jun 6, 2020. doi: 10.12998/wjcc.v8.i11.2399
Peer-review started: February 19, 2020
First decision: April 29, 2020
Revised: April 29, 2020
Accepted: May 14, 2020
Article in press: May 14, 2020
Published online: June 6, 2020
Processing time: 110 Days and 0 Hours
Increasing attention has been paid to acute myocardial infarction (AMI) in young female patients for whom secondary factors should be considered during the diagnostic process. Anti-phospholipid syndrome (APS), a rare autoimmune disease that is most common in young female patients, is reportedly related to AMI. To date, coronary interventions, particularly stenting, remains controversial in this special clinical scenario.
A 26-year-old female patient was admitted to hospital for acute chest pain, palpitations, and dyspnea. She had a past medical history of APS and pulmonary embolism. Coronary angiography showed acute occlusion of the proximal left anterior descending artery. After repeated thrombus aspirations, residual thrombus and mild stenosis were found in the proximal left anterior descending artery. Optical coherence tomography (OCT) was done, which confirmed the non-atherosclerosis coronary thrombosis and an intact intima in this patient. Deferring or avoiding stenting based on follow-up intracoronary findings with intensified antithrombotic treatment was chosen. One week later, coronary angiography and OCT confirmed an intact intima with no injury and no residual thrombus. The 3-mo telephone follow-up reported a good prognosis.
APS can cause acute non-atherosclerosis coronary thrombosis which presents as an AMI in young female patients. Intracoronary OCT findings can guide interventional strategies in this special clinical scenario.
Core tip: Acute myocardial infarction secondary to antiphospholipid syndrome in young female patients has been reported but not well studied. Coronary intervention, particularly stenting, is still in controversy in this special clinical scenario. Up to now, no specific management, especially intracoronary findings regarding this disease, has been reported. Here we present the direct evidence of acute coronary thrombosis with no atheroma in a young female acute myocardial infarction patient with antiphospholipid syndrome. Also, this case showed good in-hospital recovery with no stenting and intensified antithrombotic treatment using serial optical coherence tomography examinations.
- Citation: Du BB, Wang XT, Tong YL, Liu K, Li PP, Li XD, Yang P, Wang Y. Optical coherence tomography guided treatment avoids stenting in an antiphospholipid syndrome patient: A case report. World J Clin Cases 2020; 8(11): 2399-2405
- URL: https://www.wjgnet.com/2307-8960/full/v8/i11/2399.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i11.2399
The etiology of acute myocardial infarction (AMI) is primarily local thrombosis caused by atherosclerotic plaque rupture, and there is a trend toward a younger onset. However, for young patients, especially females, the diagnosis should be made cautiously and secondary factors should be considered[1]. For patients with AMI who have a primary cause, coronary stenting must be performed prudently and the primary disease treated[2,3]. Anti-phospholipid syndrome (APS) is a non-infectious autoimmune disease that is related to arterial or venous thrombosis. APS is more common in young female patients. For patients with this special type of AMI, different coronary angiography (CAG) findings were reported, leading to different intervention strategies[4,5]. Although different CAG-based strategies were made, interventional successes and failures have both been reported. Thus, in addition to conventional treatment, intracoronary imaging should be employed to provide more supportive information to guide further intervention strategy. Here we present the optical coherence tomography (OCT)-guided treatment of a young female patient with AMI and APS.
A 26-year-old female patient was admitted to the hospital with chest pain, palpitations, and dyspnea.
The patient’s chest pain started 2 h prior when she was working for which a colleague called an ambulance. The pain was not alleviated after she arrived at the hospital.
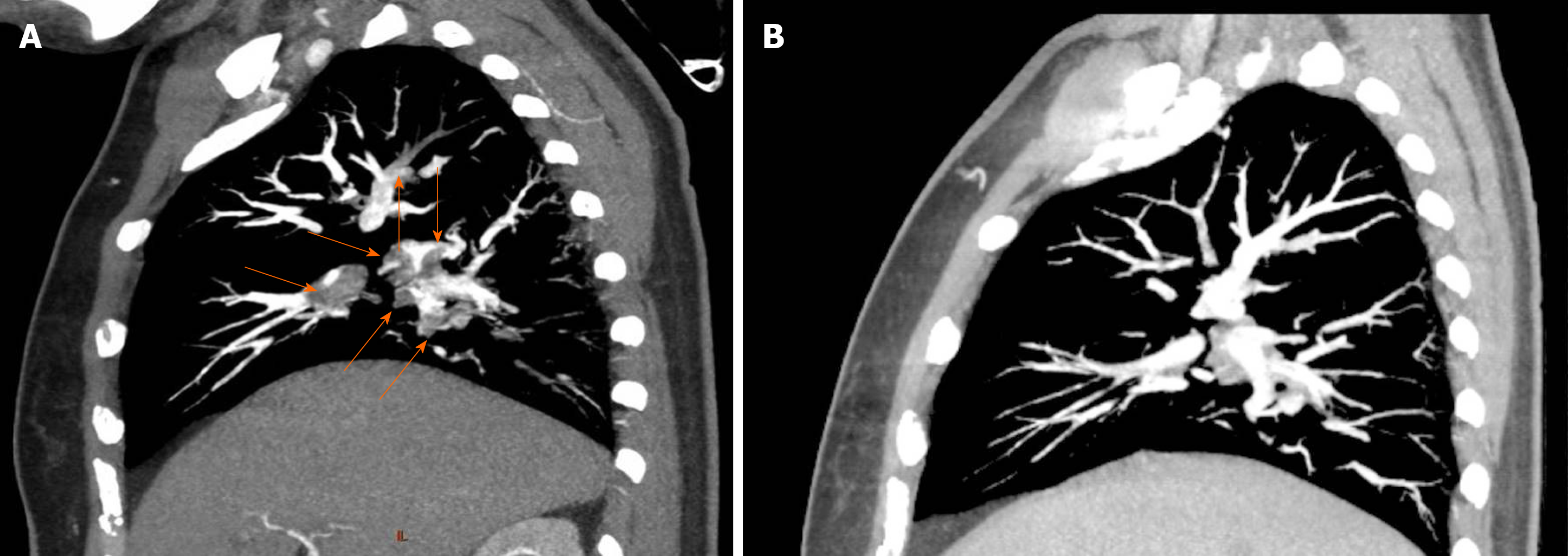
Four years before this admission, the patient was admitted to the hospital with difficult breathing and diagnosed with APS, pulmonary embolism (Figure 1A), and deep vein thrombosis (DVT) in the left lower limb. An inferior vena cava filter was implanted and warfarin 2.5 mg/d and hydroxychloroquine (HCQ) 10 mg/d were prescribed for anticoagulation and anti-inflammatory treatments, respectively. The patient recovered after 6 mo. Follow-up pulmonary computed tomography angiography showed an obvious regression of thrombus (Figure 1B), and lower extremity vascular ultrasound showed that DVT had no progression. However, the patient stopped the warfarin and HCQ 6 mo before this admission. She also had a 2.5-pack-year smoking history and had stopped smoking for 4 years. The patient had no history of hypertension or type 2 diabetes.
The patient married at a young age and had no children. Her family had no early onset history of cardiovascular diseases.
Physical examinations showed no abnormalities, and her blood oxygen saturation was 95%.
A blood gas test showed a slightly decreased partial pressure of oxygen of 78 mmHg (normal range, 83-108 mmHg). D-dimer was negative. Myocardial biomarker testing showed elevated troponin (4.0 ng/mL), myoglobin (430 mg/L), and creatine kinase-MB (46.1 U/L).
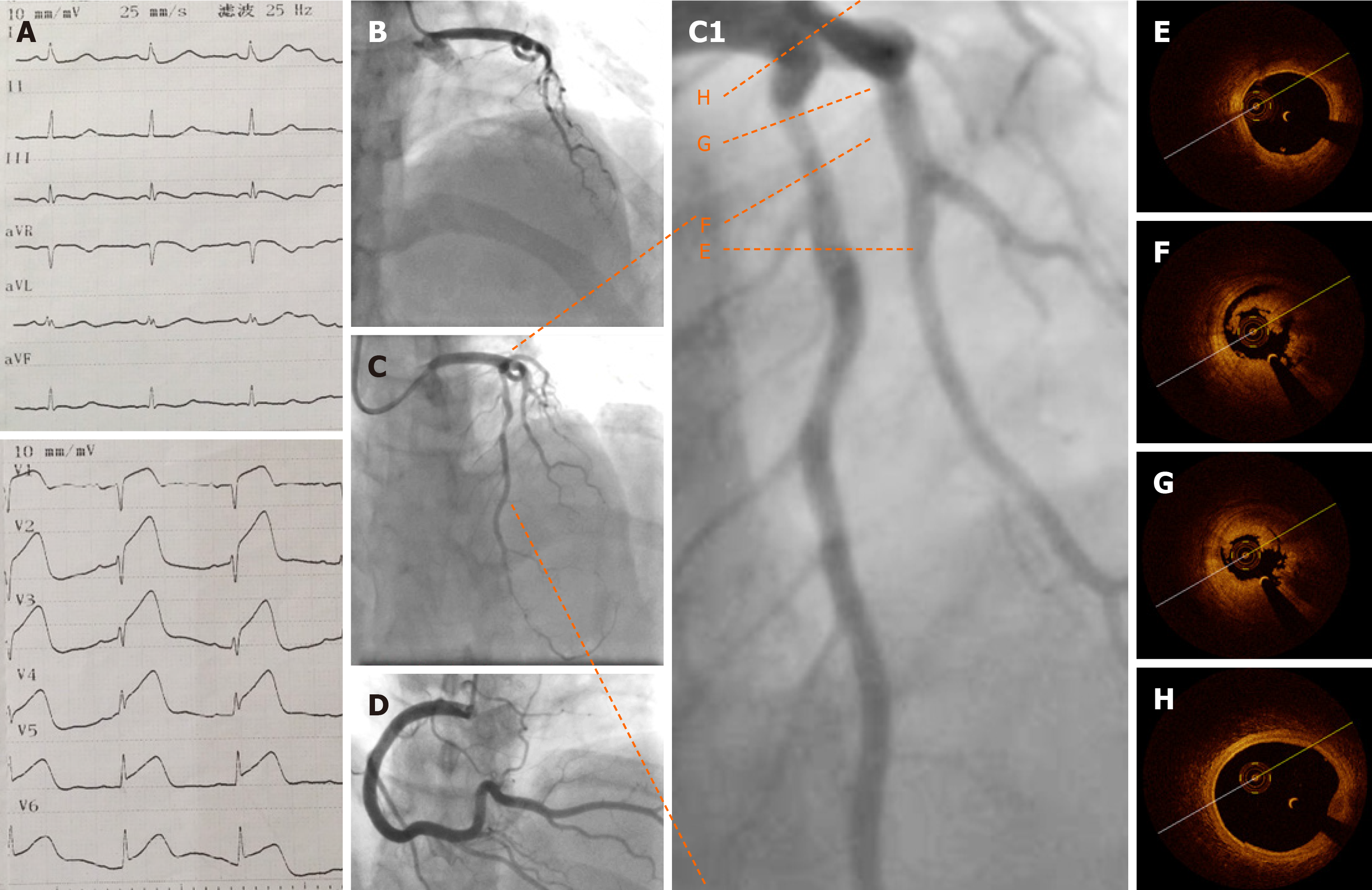
Electrocardiography (ECG) showed an ST segment elevation in the precordial leads (Figure 2A). Emergency CAG showed acute occlusion of the proximal left anterior descending artery (pLAD) and TIMI thrombus grade 5 but a normal circumflex artery and right coronary artery (Figures 2B and D). A 6-F EBU 3.5 guide catheter was engaged and the pLAD was successfully recanalized with a Runthrough guidewire (Terumo, Tokyo, Japan). There was residual thrombus left in the pLAD. Repeated thrombus aspirations were performed and nitroglycerin 200 µg was administered to irradiate the spasm. Subsequent CAG showed mild to moderate stenosis left (Figures 2C and C1). Considering the patient’s age, sex, and the past history of thrombosis, an intracoronary assessment was performed to collect more evidence to support further interventions. OCT was performed and showed residual red and white thrombi and no atheroma in the pLAD, no culprit lesion, no plaque rupture, and no plaque erosion (Figures 2E-H). The minimal lumen area of the pLAD was 2.19 mm2 and the area stenosis was 67.3%; the lumen area was reduced by the residual thrombus around the OCT catheter (Figure 2G).
According to the symptoms, the past medical history, and the laboratory and imaging findings, the patient was diagnosed with acute anterior wall ST elevation myocardial infarction (acute coronary thrombosis), Killip class IV; APS; pulmonary embolism (old); DVT (old); and post-inferior vena cava filter implantation.
Considering the past medical history and OCT evidence for this young female patient, deferring or avoiding stenting based on further CAG and intracoronary imaging assessment findings was chosen. Also, coronary thrombosis was believed to be highly likely related to APS. Intensified antithrombotic treatments with dual antiplatelets (aspirin 100 mg/d + clopidogrel 75 mg/d), anticoagulant (low molecular weight heparin 4200 IU/d), and a glycoprotein IIb/IIIa inhibitor (tirofiban 100 mg until 24 h post-procedure) were performed.
After the procedure, the patient’s chest pain and other symptoms were relieved, and the post-procedure ECG findings returned to normal. Echocardiography showed left ventricular anterior wall hypokinesis and no right-to-left shunt. Lower-extremity venous ultrasonography showed right femoral vein and bilateral iliac vein thrombosis. Coagulation test findings were normal. Both total anticardiolipin antibody (aCL) and immunoglobulin G of aCL levels were elevated (55.6 and 32.5 RU/mL, respectively). Other antinuclear antibody tests were negative.
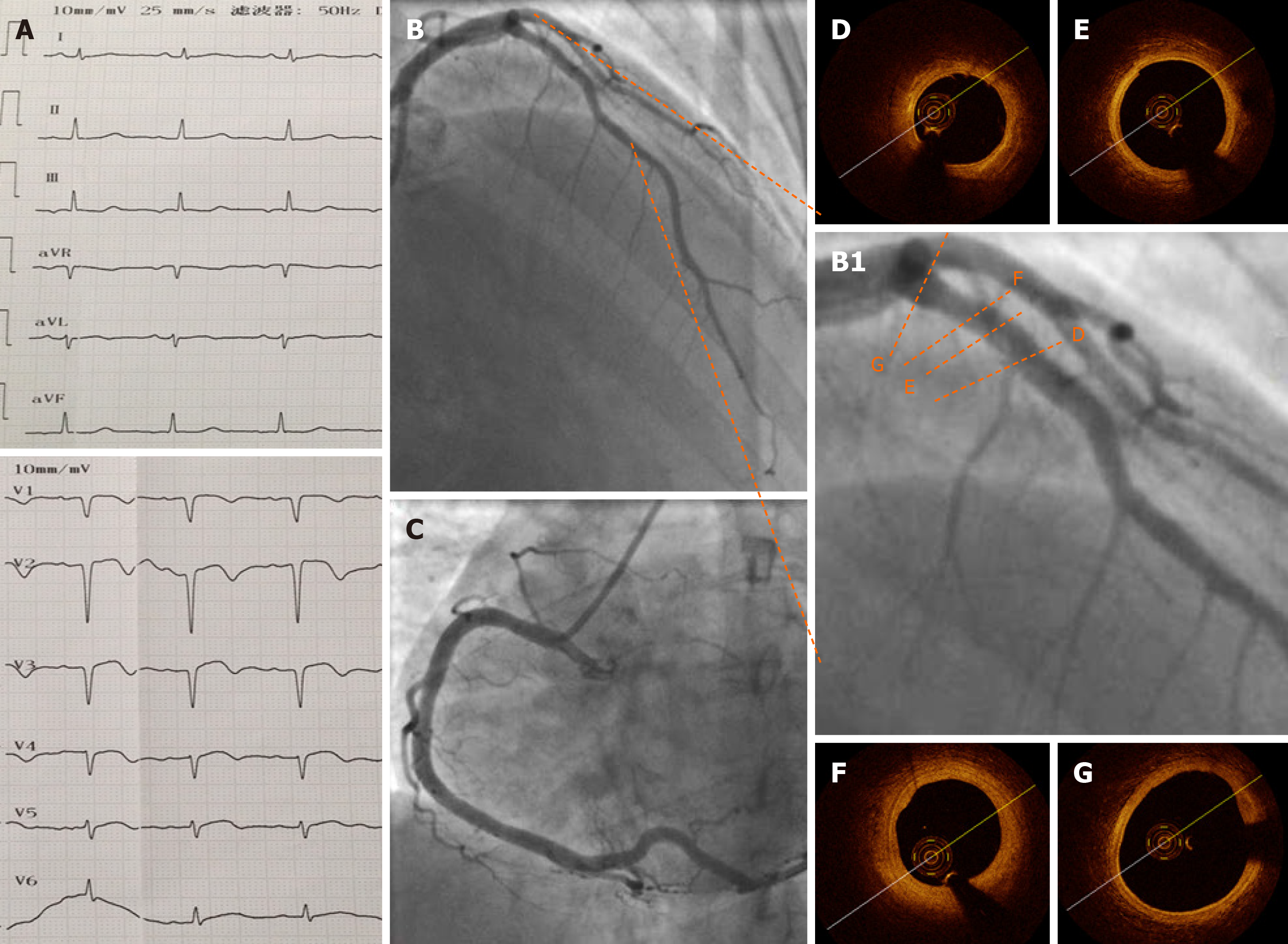
ECG, CAG, and OCT repeated after 1 wk of treatment (Figure 3) showed that no residual thrombus was left in the pLAD and that the intima was intact. No fibrous plaque, lipid plaque, or plaque rupture was detected (Figure 3A-G); the circumflex and right coronary arteries were checked and no obvious atherosclerosis was visible. Bubble study was done and ruled out patent foramen ovale.
For antithrombosis, heparin was replaced with warfarin (2.5 mg/d) 3 d after the procedure combined with dual antiplatelet treatment for 3 mo, followed by a later warfarin-clopidogrel combination until 1 year. After 1 year, lifelong warfarin was recommended with international normalized ratio monitoring and control at 2.0-3.0. Despite other anti-ischemia therapies, HCQ 10 mg/d was re-prescribed to reduce antiphospholipid antibody (aPL) titer. The patient was discharged 1 wk later and recovered well. A good result at the 3-mo follow-up was confirmed by phone.
Here we for the first time present direct evidence of non-atherosclerosis coronary thrombosis and no atheroma in a young female AMI patient with a previous history of APS. Serial OCT examinations demonstrated a good in-hospital clinical outcome after intense antithrombosis treatment without stenting.
APS is reportedly related to various cardiac diseases, such as heart valve disease, coronary artery disease (including AMI), intracardiac thrombosis, and pulmonary hypertension[6].
Although APS is believed to be related to accelerated atherosclerosis due to immunopathological and inflammatory status[6], it is generally accepted that APS-related AMI is caused by in situ coronary thrombosis as a result of a high coagulation condition[7,8]. An increased serum aPL level (including lupus anticoagulant, aCL, and anti-β2-glycoprotein-1 antibody) is considered the main factor leading to thrombosis, but its specific mechanism is unclear[9]. Here we demonstrated direct evidence of non-atherosclerosis coronary thrombosis using serial OCT examinations in a patient with a high aPL level. In fact, based on other traditional risk factors that a patient might have before AMI, there have been reports of severe coronary stenosis and concomitant coronary thrombosis in CAG findings[5,7].
There are limited cases of failed or successful intervention for patients with APS and AMI, which in turn leads to different clinical results and prognosis[10,11]. One major concern related to stenting in these patients is the high risk of stent thrombosis, especially for patients with aPL positivity or poor anticoagulation compliance[7]. To reduce the possibility of thrombosis and recurrent AMI, initial attempt with bare metal stent implantation was made. In one study of 19 APS patients with stent (12 bare metal stents and 7 drug eluting stents) implantation, with up to 3 years of follow-up, higher targeted vessel revascularization (42.1% vs 7.8%) and major adverse cardiac event rate (52.6% vs 18.1%) were found compared to patients without APS[8]. Still there have been only limited data up to now reporting drug eluting stent implantation and the prognosis in APS and AMI patients. OCT evidence for this patient proved the high risk of thrombosis but no signs of intima injury; accordingly, avoiding stenting and an intensive antithrombosis strategy were chosen. One-week follow-up CAG and OCT had proven recovery and safety. However, no standard interventional treatment is available for patients with APS combined with myocardial infarction; thus, further research is needed.
For antithrombotic treatment, no consensus has been achieved in this special clinical setting. We adopted the triple antithrombotic strategy to maximally reduce the risk of thrombosis for this patient with thrombophilia[12]. The use of direct oral anticoagulants has not shown efficacy in the management of this kind of case.
This case demonstrates that, during the diagnosis and treatment of young female patients with AMI, clinicians should be vigilant about the possibility of APS-caused non-atherosclerosis coronary thrombosis. For this special young patient cohort, intracoronary imaging can provide more information and may avoid stenting, which promises a better clinical prognosis.
In young female patients, APS can cause acute non-atherosclerosis coronary thrombosis which presents as an AMI. Intracoronary OCT findings can guide interventional strategies and guarantee a better prognosis in this special clinical scenario.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: The corresponding author is the member of European Society of Cardiology, Acute Cardiovascular Care Association, No. 087109300.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barik R S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Qi LL
| 1. | Dreyer RP, Sciria C, Spatz ES, Safdar B, D'Onofrio G, Krumholz HM. Young Women With Acute Myocardial Infarction: Current Perspectives. Circ Cardiovasc Qual Outcomes. 2017;10:e003480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Kurmann RD, Mankad R. Atherosclerotic Heart Disease in Women With Autoimmune Rheumatologic Inflammatory Conditions. Can J Cardiol. 2018;34:381-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde-Price C, D'Onofrio G, Lichtman JH, Krumholz HM. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 2014;64:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 370] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 4. | de Vries PA, van der Sluis A, van der Horst JC, van Veldhuisen DJ. Myocardial ischaemia with a normal coronary angiogram due to the primary antiphospholipid syndrome. Int J Cardiol. 2002;82:183-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Shan Y, Wang P, Liu J. Antiphospholipid syndrome combined with acute coronary syndrome: Case report. Medicine (Baltimore). 2018;97:e13613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Soltész P, Szekanecz Z, Kiss E, Shoenfeld Y. Cardiac manifestations in antiphospholipid syndrome. Autoimmun Rev. 2007;6:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Correia AF, Oliveira DC, Sanctos M. Coronary Artery Thromboses, Stent Thrombosis and Antiphospholipid Antibody Syndrome: Case Report. Cardiol Res. 2018;9:129-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Perl L, Netzer A, Rechavia E, Bental T, Assali A, Codner P, Mager A, Battler A, Kornowski R, Lev EI. Long-term outcome of patients with antiphospholipid syndrome who undergo percutaneous coronary intervention. Cardiology. 2012;122:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Geller BJ, Mega JL, Morrow DA, Guo J, Hoffman EB, Gibson CM, Ruff CT. Autoantibodies to phosphorylcholine and cardiovascular outcomes in patients with acute coronary syndromes in the ATLAS ACS-TIMI 46 trial. J Thromb Thrombolysis. 2014;37:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Weissman A, Coplan NL. Antiphospholipid antibody syndrome and acute stent thrombosis. Rev Cardiovasc Med. 2006;7:244-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Biceroglu S, Ildizli Demirbas M, Karaca M, Yalcin M, Yilmaz H. Acute thrombotic occlusion of right coronary and left circumflex coronary arteries in a patient with antiphospholipid syndrome: successful stent implantation. Case Rep Med. 2010;2010:198594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Lóczi L, Kappelmayer J, Tarr T, Bagoly Z. Antiphospholipid syndrome and the risk of myocardial infarction: current evidence and uncertainties. Kardiol Pol. 2020;78:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |