Published online Jun 6, 2020. doi: 10.12998/wjcc.v8.i11.2081
Peer-review started: January 2, 2020
First decision: February 19, 2020
Revised: March 27, 2020
Accepted: April 24, 2020
Article in press: April 24, 2020
Published online: June 6, 2020
Processing time: 157 Days and 13.8 Hours
Isoflavones constitute a class of plant hormones including genistein, daidzein, glycitein, formononetin, biochanin A, and irilone, and the major source of human intake is soybeans. Inflammatory bowel disease (IBD) is a chronic recurrent inflammatory disease including ulcerative colitis, Crohn’s disease, and indeterminate colitis, which seriously affects the quality of life of patients and has become a global health problem. Although the pathogenesis of IBD is not very clear, many factors are thought to be related to the occurrence and development of IBD such as genes, immunity, and intestinal flora. How to control IBD effectively for a long time is still a problem for gastroenterologists. Diet has an important effect on IBD. Patients with IBD should pay more attention to diet. To date, many studies have reported that isoflavones have both good and bad effects on IBD. Isoflavones have many activities such as regulating the inflammatory signal pathways and affecting intestinal barrier functions and gut flora. They can also act through estrogen receptors, as they have a similar structure to estrogen. Isoflavones are easy to get from diet for human. Whether they are valuable to be applied to the treatment of IBD is worth studying. This review summarizes the relationship between isoflavones and IBD.
Core tip: Isoflavones constitute a class of plant hormones, and the major source of human intake is soybeans. Inflammatory bowel disease (IBD) is a chronic recurrent inflammatory disease, which seriously affects the quality of life of patients. To date, many studies have reported that isoflavones have effects on IBD. Isoflavones have many activities, such as regulating the inflammatory signal pathways, intestinal barrier function, and gut flora. They can also act through estrogen receptors, because they have a similar structure to estrogen. This review summarizes the relationship between isoflavones and IBD.
- Citation: Wu ZY, Sang LX, Chang B. Isoflavones and inflammatory bowel disease. World J Clin Cases 2020; 8(11): 2081-2091
- URL: https://www.wjgnet.com/2307-8960/full/v8/i11/2081.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i11.2081
Inflammatory bowel disease (IBD) is a chronic recurrent inflammatory disease. There are three forms of IBD: Ulcerative colitis (UC), Crohn’s disease (CD), and indeterminate colitis[1,2]. The etiology is not very clear, and this disease has a close relationship with genes, immunity, intestinal flora, etc[3]. The incidence of IBD is increasing[4,5]. IBD has become a global health problem[6]. Studies found that diet can affect the intestinal flora of the human body and then affect human immunity[7-9]. Therefore, diet plays an important role in the occurrence and development of IBD.
A Westernized diet can promote the occurrence and development of IBD[10]. A large intake of total fats, polyunsaturated fatty acids, meat, and omega-6 fatty acids increased the risk of UC and CD, while a high intake of dietary fiber, fruits, and vegetables can reduce the risk[11]. Some foods, such as omega-3 fatty acid-rich fish, monounsaturated fat, fruits, and vegetables, are recommended as part of the diet of IBD patients[7]. The Asian population has its own dietary structure and soy products are an integral part, thus the intake of isoflavones in Asians is high[12]. Isoflavones are a class of phytoestrogens, the structure of which is similar to that of estrogen[13], and they were reported to have effects on many diseases, including osteoporosis[14], cardiovascular disease[15], breast cancer[16], colorectal cancer[17], and Alzheimer’s disease[18]. In recent years, studies have reported the effect of isoflavones on IBD. In this paper, we review the relationship between isoflavones and IBD.
Isoflavones constitute a class of phytoestrogens, which include genistein, daidzein, glycitein, formononetin, biochanin A, and irilone. The first three are from soybeans, and the last three are from red clover[13,19]. The content of isoflavones is the most abundant in soybeans and their derivatives, which are the major source of isoflavones for humans. In addition, the content of isoflavones in different derivatives is related to the processing methods of soybeans[20,21]. There are two forms of isoflavones: Glucosides and aglycones[13,22], which are absorbed in the human body in both the small and large intestine[23]. Isoflavones in the form of aglycones are absorbed faster than glucosides, which can be hydrolyzed into bioactive aglycones in the proximal intestine for better absorption[24,25]. Isoflavones are mainly in the form of glucuronide in human blood, accounting for 75%; 24% in the form of sulfate; and ≤ 1% in the form of aglycone[26]. Isoflavones are similar to estrogen in structure, thus, isoflavones can bind to estrogen receptors[27].
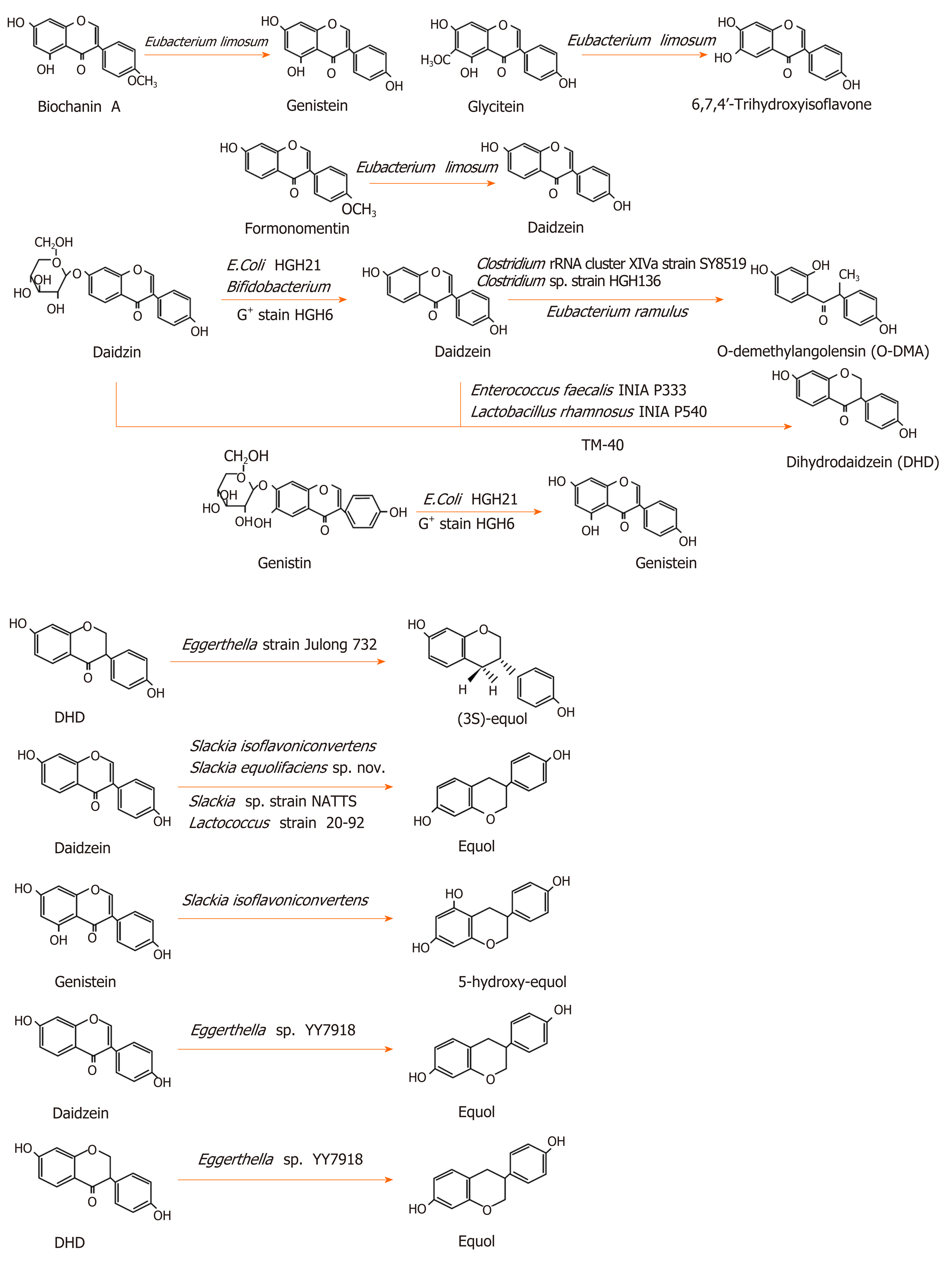
Isoflavones can be metabolized into equol and O-demethylangolensin(O-DMA)[28]. The metabolism for these is different in different individuals. In China, Korea, and Japan, more than 80% of the population can produce equol, while only 25% of the population in North America and Europe can produce it[25]. There are a large number of intestinal floras that play an important role in metabolism. Many intestinal floras can metabolize isoflavones. Some lactic acid bacteria and bifidobacteria with β-glucosidase activity can transform glycoside into aglycone isoflavone[29]. Lactic acid bacteria and bifidobacteria prefer to transform genistin into genistein[30]. However, not all isoflavones can be metabolized by intestinal floras. Irilone, a red clover isoflavone, is difficult for intestinal bacteria to degrade[19].
Eubacterium limosum is an absolute anaerobic bacterium in the human intestinal tract, which was shown to O-demethylate isoflavones and convert biochanin A, formononetin, and glycitein into genistein, daidzein, and 6,7,4’-trihydroxyisoflavone, respectively, but it could not further transform these three products[31]. Bifidobacterium was demonstrated to convert daidzin into daidzein, but not into equol[32]. Escherichia coli HGH21 and a Gram-positive (G+) strain HGH6 transformed daidzin and genistin into daidzein and genistein, respectively[33]. Clostridium sp. strain HGH 136, a G+ anaerobic bacterium in the gut, transformed daidzein into O-DMA by cutting off the C-ring of isoflavones[34]. Eubacterium ramulus also converted daidzein to O-DMA[35]. Strain SY8519, a strain belonging to Clostridium rRNA cluster XIVa in the human intestine, produced O-DMA from daidzein[36].
Some intestinal microfloras were shown to convert daidzein and genistein into dihydrodaidzein and dihydrogenistein, two intermediate metabolites, re-spectively[29,37]. Tamura et al[38] isolated a Clostridium-like bacterial strain, TM-40, from the feces of a 7-year-old boy. It could transform isoflavones both daidzein and daidzin into dihydrodaidzein but did not produce equol. The strain was 93% similar to Coprobacillus catenaformis[38]. Enterococcus faecalis INIA P333 and Lactobacillus rhamnosus INIA P540 converted daidzein into DHD[29]. In an anaerobic environment, the G+ strain HGH6 further converted daidzein and genistein into dihydrodaidzein and dihydrogenistein, respectively, but HGH6 could not further convert dihydrodaidzein and dihydrogenistein[33].
Certain bacteria can convert isoflavones into equol. The rod-shaped Gram-negative bacterium, Eggerthella strain Julong 732, in the human intestine, was demonstrated to transform dihydrodaidzein into S-equol in an anaerobic environment. Full 16S RNA gene sequence analysis indicated that the bacterium was 92.8% similar to Eggerthella hongkongenis HKU10[39,40]. Eggerthella sp. YY7918, a bacterium that is 93.3% similar to Eggerthella hongkongenis HKU10, also converted daidzein and dihydrodaidzein into equol[41]. Slackia isoflavoniconvertens converted daidzein into equol and genistein into 5-hydroxy-equol[42]. Slackia equolifaciens sp. nov., Slackia sp. strain NATTS, and Lactococcus strain 20-92 converted daidzein into equol[43-45]. The metabolic process of isoflavones is shown in Figure 1.
UC and CD are the two main forms of IBD. UC can affect the end of the ileocecum, the whole colon, and rectum, and CD can affect the whole digestive tract, which seriously affects the quality of life for patients. The changes of IBD can occur in the intestinal mucosal barrier, the intestinal flora, and their metabolites, cytokines.
The intestinal barrier of IBD patients is abnormal, and the mucous layer and epithelial cells of the intestinal tract are changed. Mucin 2 and IgG Fc-binding protein, the main components of the mucus layer of intestinal barrier, decrease in the active UC, leading to a thinning of the mucus layer. This change occurs in the early stage of UC, even before the inflammation[46,47]. Intestinal mucosal barrier damage and increased intestinal mucosal permeability are associated with the occurrence of UC and CD[48] and abnormal intestinal tight junctions are one of mechanisms leading to these changes[49].
There are abnormalities in the cytokines of IBD patients. For example, studies found that interleukin (IL)-17A and IL-23 were increased in IBD patients, and they were increased more in UC than in CD[50]. Lena Öhman et al[51] found that the level of serum IL-17A was related to the severity of newly diagnosed, untreated UC. Cytokines, such as the IL-1 family and IL-17, play an important role in the development of IBD[52].
An imbalance of intestinal flora is closely related to the incidence of IBD. The abundance, evenness, and diversity of intestinal flora in UC patients are reduced[53]. Some gut microfloras that are very important for maintaining intestinal homeostasis show dysbiosis, such as the butyrate-producing microflora, human Roseburia hominis, and Faecalibacterium prausnitzii (F. prausnitzii), which are reduced in UC patients[54]. Even in the first-degree relatives of UC patients, F. prausnitzii is decreased[55]. In addition to UC patients, healthy people living with UC patients also have certain intestinal dysbiosis[56]. The dysbiosis in CD is more serious than that in UC[57], mainly causing a reduction of the phylum Firmicutes[58]. The dysbiosis of viruses and fungi also plays a role in the development of IBD[59,60].
An imbalance of metabolites, caused by the imbalance of intestinal flora, also affects the occurrence and development of IBD. Short-chain fatty acids (SCFAs), including acetic acid, propionic acid, and butyric acid, are produced by intestinal microflora and play an important role in the intestinal tract[61]. SCFAs were reported to promote the production of IL-10, an anti-inflammatory cytokine, by Th1 cells and to have anti-inflammatory effects[61,62]. In a colitis mouse model, it was found that butyrate, an SCFA, can maintain the balance of Th17/Treg in the intestinal tract and has an anti-inflammatory effect[63]. A decrease of SCFA can also increase the intestinal permeability[64]. Therefore, the regulation of intestinal microflora and its metabolites is of great significance to the recovery of IBD.
In recent years, studies have reported the effects of isoflavones on IBD. In a mouse model, daidzein and glyceolins (isopentehexene isoflavone, a derivative of daidzein, produced by soybeans under stress), relieved dextran sulfate sodium (DSS)-induced colitis, therefore, they may be used in the treatment of UC[65,66]. A moderate isoflavone intake by UC patients in the remission period was beneficial[67]. Isoflavones demonstrated effects on the symptoms of UC patients in the remission period. Isoflavones intake may contribute to reducing the incidence of abdominal pain[67]. A high intake of daidzein and total isoflavones helped to reduce the mucus in the feces of UC patients; however, a high intake of daidzein alone may increase fecal pus[68]. It was reported that soybean isoflavones may have a preventive effect on CD[69].
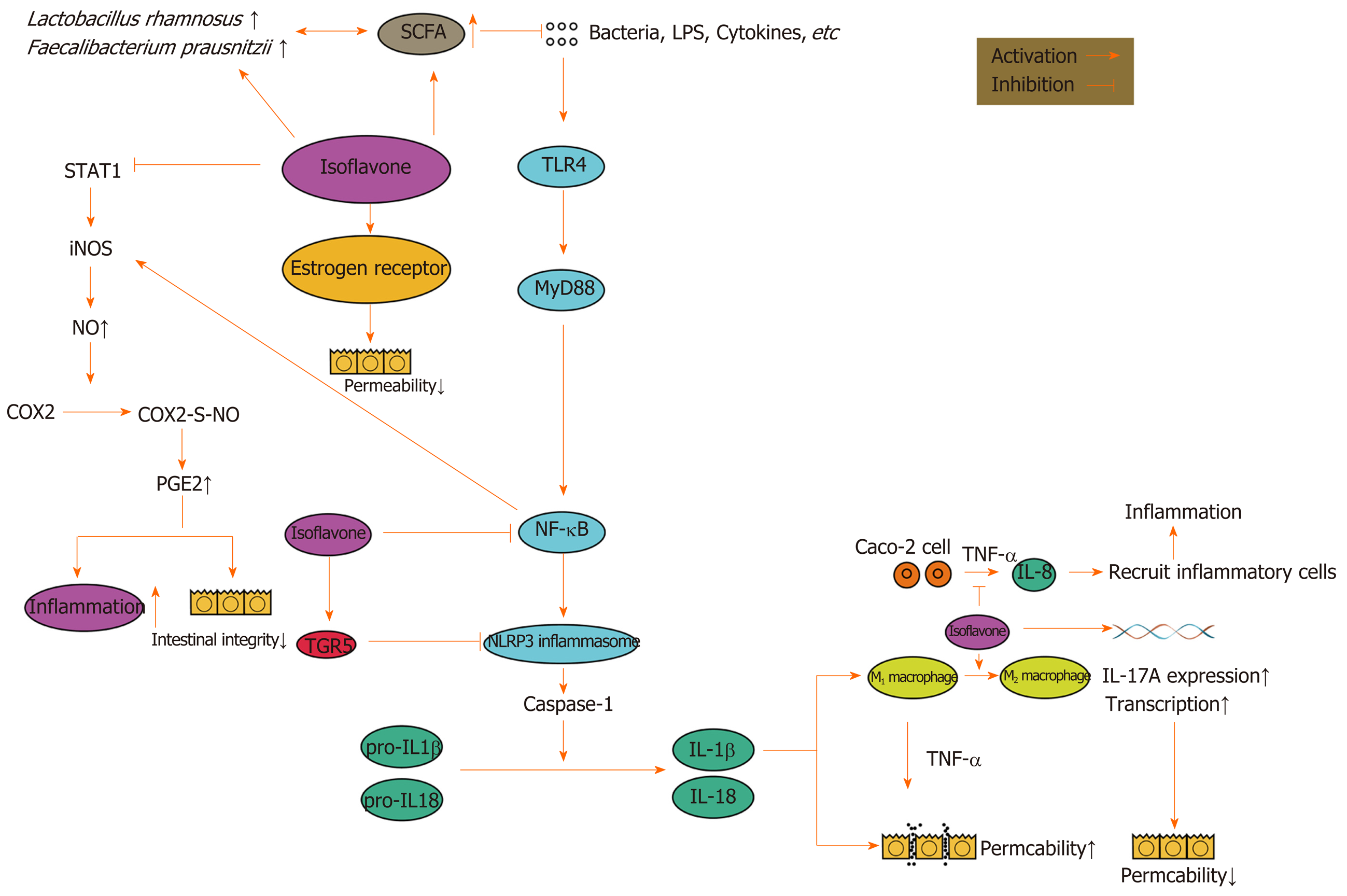
Isoflavones have an anti-inflammatory effect[70]. After treatment with genistein, an isoflavone, inflammation was reduced in a 2,4,6-trinitrobenzenesulfonic acid-induced colitis rat model[71]. Isoflavones play a role in many aspects of inflammation. The expression and activation of the signal translator and activator of transcription 1 increased in the intestines of patients with IBD[72]. Daidzein and genistein were shown to inhibit signal translator and activator of transcription 1 and then to reduce the expression of inducible nitric oxide synthase (iNOS)[73]. The overproduction of NO caused by the induction of iNOS could lead to massive prostaglandin E2 (PGE2) production through cyclooxygenase-2 S-nitrosylation[74]. PGE2 has a proinflammatory effect[75] and massive PGE2 could damage the intestinal integrity[76].
Isoflavones were shown to inhibit the tumor necrosis factor α (TNF-α) induced production of chemokine IL-8 by Caco-2 cells[77], reduce the inflammatory cell infiltration in the intestines, decrease the level of myeloperoxidase, inhibit the nuclear factor-κB (NF-κB) pathway and the expression of inflammatory factors (such as IL-6, IL-1 β, and TNF-α), and reduce the levels of NO and PGE2 in colonic tissues[65].
NF-κB is a family of transcription factors, including RelA (p65), c-Rel, RelB, p105/p50, and p100/p52[78], and almost all cells in the human body can express NF-κB[79]. The activity of NF-κB is controlled by the inhibitory κB protein and the inhibitory κB protein kinase.There are two ways to activate NF-κB. One is the classical way: Bacterial products and proinflammatory factors, such as TNF-α and IL-1, can phosphorylate and degrade IκB α and activate p65/p50 through the IκB kinase (IKK) complex. Another is the nonclassical pathway: The TNF cytokine family, additional to TNF-α, can phosphorylate p100 and activate p52/RelB through the IKK complex[79,80]. The NF-κB signaling pathway plays a dual role in the gut[81]. On the one hand, the activation of NF-κB induces the expression of chemokines, cytokines, and adhesion factors to promote the development of inflammation[78]. On the other hand, NF-κB is important in maintaining the integrity of the intestinal mucosal barrier and intestinal mucosal homeostasis[82].
Daidzein reduced the secretion of pro-inflammatory factors by inhibiting the NF-κB signaling pathway[65]. Kim et al[83] found that soybean isoflavones inhibited the activation of macrophages by inhibiting the phosphorylation of the IκB protein and the degradation of IKK in a DSS-induced colitis mouse model. M1 macrophages damaged the intestinal epithelial cell barrier through TNF-α[84]; however, genistein promoted the transformation of M1 macrophages into M2, reduced cytokines, and reduced T cells in the lamina propria of the colon[85]. It was reported that soybean isoflavones may reduce DSS-induced intestinal inflammation by inhibiting the TLR4/MyD88 signal[86]. Stimuli in the intestinal tract could activate NF-κB through TLR4/MyD88[78].
In addition, isoflavones can inhibit the NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome. Abnormal activation of the NLRP3 inflammasome may be an important mechanism of IBD occurrence and development. The NF-κB pathway could induce the expression of the NLRP3 inflammasome[87]. Bauer et al[88] used DSS to induce colitis in mice and found that the NLRP3 inflammasome played an important role in the development of intestinal inflammation. DSS activated caspase-1 through NLRP3 inflammasome and transformed IL-1β and IL-18 into their active forms. IL-1β and IL-18 are important pro-inflammatory cytokines[89], and IL-1β can increase intestinal tight junction permeability through the classical NF-κB pathway[90,91].
However, the effect of IL-18 on the intestinal mucosal barrier is controversial[92,93]. Both a protective effect and a destructive effect have been reported. Genistein inhibited the NLRP3 inflammasome in macrophages through the G-protein-coupled bile acid receptor 1, also known as TGR5[94]. However, Hirota et al[95] found that, in mice that had lost the NLRP3 inflammasome, experimental colitis was likely to appear. A lack of the NLRP3 inflammasome can damage the intestinal barrier function and cause inflammation, which may be related to promoting epithelial cell apoptosis and reducing epithelial cell proliferation[96].
IL-17 also plays an important role in IBD. IL-17A and IL-17C can regulate occludin and maintain the integrity of the barrier[97,98]. Inhibiting IL-17 weakens the barrier function of intestinal epithelial cells. Therefore, anti-IL-17A treatment will aggravate IBD[99,100]. Isoflavones increased the gene expression of IL-17A in immune cells[101]. Biochanin A activated IL-17 transcription, which is dependent on retinoic acid receptor-related orphan receptor γ[102]. However, IL-17 by itself could gather inflammatory cells and promote the development of inflammation in coordination with other cytokines[103], and IL-17 increased in the serum and intestinal mucosa of IBD patients[104]. As a result, the up-regulation of IL-17 by isoflavones may play a role in promoting the development of IBD.
The estrogen-like effect of isoflavones also has an effect on IBD. Estrogen was found to alleviate endoplasmic reticulum stress, reduce the production of pro-inflammatory factors through epithelial cells, and improve the barrier function of intestinal epithelial cells (by up-regulating the tight junction proteins)[105]. The colon estrogen β receptor signaling pathway up-regulated the expression of occludin and the junctional adhesion molecule A in epithelial cells and reduced the intestinal permeability[106]. Therefore, the estrogen-like effect of soybean isoflavones could improve the gut barrier function[107].
Soybean isoflavones can also regulate the intestinal flora. Genistein reduced the growth rate of Lactococcus lactis subsp. lactis, Slackia equolifaciens, and Bacteroides fragilis, while genistein and equol increased the growth rate of F. prausnitzii and Lactobacillus rhamnosus[108]. Lactobacillus rhamnosus GG had an anti-inflammatory effect both in vivo and in vitro[109], and Lactobacillus rhamnosus CNCM I-3690 had a protective effect on the intestinal barrier[110]. F. prausnitzii is a flora in the human intestinal tract, which was shown to produce butyrate and play an anti-inflammatory role by regulating the balance of Th17/Treg in the intestinal tract. Butyrate is a kind of SCFA, which is very important for intestinal health. Isoflavone could increase butyrate and acetate[63,111]. In addition, isoflavones promoted the production of SCFA in vitro[112]. Therefore, isoflavones can increase intestinal SCFA, and SCFA can further promote the growth of flora that can produce butyrate, have an anti-inflammatory effect, and protect the intestinal barrier function. This may form a virtuous cycle and finally promote the recovery of IBD.
However, different opinions have been expressed in certain studies. An increased intake of isoflavones increased the risk of UC, especially in the female population[113]. Moreover, the early adoption of an isoflavone-rich diet did not avoid the occurrence and development of IBD in a rat model[114]. This may be related to the estrogen effect of isoflavones. The effect of estrogen on IBD has been reported. One study found that postmenopausal women who take estrogen or progesterone supplements had an increased risk of UC[115], and another study also found that patients who take oral contraceptives had an increased risk of IBD[116]. The effects of isoflavones on IBD are shown in Figure 2.
The incidence of IBD is increasing worldwide. The quality of life of IBD patients is seriously impacted and IBD cannot be cured at present. How to control IBD effectively for a long time is, therefore, of particular importance. Isoflavones have certain therapeutic effects on IBD through inhibiting inflammation, as well as regulating intestinal flora and its metabolites. Therefore, isoflavones may have a certain potential in the treatment of IBD. However, the two-way effect of the NLRP3 inflammasome and IL-17 would lead to a dual effect of isoflavones on IBD. The effects of isoflavones may be related to the dosage, frequency of use, intestinal flora, and the type and severity of IBD. Therefore, we suggest that future research should focus on the dosage and frequency of use of isoflavones in relation to their positive effects and compare the disease characteristics and intestinal flora characteristics of people with different effects, so as to determine how to take advantage of the beneficial role of isoflavones for IBD.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chiu KW, Slomiany BL, Tomizawa M S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2019] [Cited by in RCA: 1883] [Article Influence: 134.5] [Reference Citation Analysis (2)] |
| 2. | Guindi M, Riddell RH. Indeterminate colitis. J Clin Pathol. 2004;57:1233-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Loddo I, Romano C. Inflammatory Bowel Disease: Genetics, Epigenetics, and Pathogenesis. Front Immunol. 2015;6:551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 304] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 4. | Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1157] [Cited by in RCA: 1874] [Article Influence: 187.4] [Reference Citation Analysis (1)] |
| 5. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3527] [Article Influence: 271.3] [Reference Citation Analysis (5)] |
| 6. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4109] [Article Influence: 513.6] [Reference Citation Analysis (110)] |
| 7. | Kakodkar S, Mutlu EA. Diet as a Therapeutic Option for Adult Inflammatory Bowel Disease. Gastroenterol Clin North Am. 2017;46:745-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2376] [Cited by in RCA: 2165] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 9. | De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107:14691-14696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3584] [Cited by in RCA: 4034] [Article Influence: 268.9] [Reference Citation Analysis (0)] |
| 10. | Chiba M, Nakane K, Komatsu M. Westernized Diet is the Most Ubiquitous Environmental Factor in Inflammatory Bowel Disease. Perm J. 2019;23:18-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 11. | Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 693] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 12. | Viggiani MT, Polimeno L, Di Leo A, Barone M. Phytoestrogens: Dietary Intake, Bioavailability, and Protective Mechanisms against Colorectal Neoproliferative Lesions. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Křížová L, Dadáková K, Kašparovská J, Kašparovský T. Isoflavones. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 354] [Cited by in RCA: 426] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 14. | Akhlaghi M, Ghasemi Nasab M, Riasatian M, Sadeghi F. Soy isoflavones prevent bone resorption and loss, a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2019;1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Yamagata K. Soy Isoflavones Inhibit Endothelial Cell Dysfunction and Prevent Cardiovascular Disease. J Cardiovasc Pharmacol. 2019;74:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Chen M, Rao Y, Zheng Y, Wei S, Li Y, Guo T, Yin P. Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: a meta-analysis of epidemiological studies. PLoS One. 2014;9:e89288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Yu Y, Jing X, Li H, Zhao X, Wang D. Soy isoflavone consumption and colorectal cancer risk: a systematic review and meta-analysis. Sci Rep. 2016;6:25939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Essawy AE, Abdou HM, Ibrahim HM, Bouthahab NM. Soybean isoflavone ameliorates cognitive impairment, neuroinflammation, and amyloid β accumulation in a rat model of Alzheimer's disease. Environ Sci Pollut Res Int. 2019;26:26060-26070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Braune A, Maul R, Schebb NH, Kulling SE, Blaut M. The red clover isoflavone irilone is largely resistant to degradation by the human gut microbiota. Mol Nutr Food Res. 2010;54:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Sakai T, Kogiso M. Soy isoflavones and immunity. J Med Invest. 2008;55:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Dixon RA, Sumner LW. Legume natural products: understanding and manipulating complex pathways for human and animal health. Plant Physiol. 2003;131:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Cornwell T, Cohick W, Raskin I. Dietary phytoestrogens and health. Phytochemistry. 2004;65:995-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 338] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 23. | Franke AA, Lai JF, Halm BM. Absorption, distribution, metabolism, and excretion of isoflavonoids after soy intake. Arch Biochem Biophys. 2014;559:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000;130:1695-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 642] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 25. | Shor D, Sathyapalan T, Atkin SL, Thatcher NJ. Does equol production determine soy endocrine effects? Eur J Nutr. 2012;51:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Gu L, House SE, Prior RL, Fang N, Ronis MJ, Clarkson TB, Wilson ME, Badger TM. Metabolic phenotype of isoflavones differ among female rats, pigs, monkeys, and women. J Nutr. 2006;136:1215-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Vitale DC, Piazza C, Melilli B, Drago F, Salomone S. Isoflavones: estrogenic activity, biological effect and bioavailability. Eur J Drug Metab Pharmacokinet. 2013;38:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 313] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 28. | Tomás-Barberán FA, Selma MV, Espín JC. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr Opin Clin Nutr Metab Care. 2016;19:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 251] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 29. | Gaya P, Peirotén Á, Medina M, Landete JM. Isoflavone metabolism by a collection of lactic acid bacteria and bifidobacteria with biotechnological interest. Int J Food Sci Nutr. 2016;67:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Gaya P, Medina M, Sánchez-Jiménez A, Landete JM. Phytoestrogen Metabolism by Adult Human Gut Microbiota. Molecules. 2016;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 31. | Hur H, Rafii F. Biotransformation of the isoflavonoids biochanin A, formononetin, and glycitein by Eubacterium limosum. FEMS Microbiol Lett. 2000;192:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Raimondi S, Roncaglia L, De Lucia M, Amaretti A, Leonardi A, Pagnoni UM, Rossi M. Bioconversion of soy isoflavones daidzin and daidzein by Bifidobacterium strains. Appl Microbiol Biotechnol. 2009;81:943-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 33. | Hur HG, Lay JO Jr, Beger RD, Freeman JP, Rafii F. Isolation of human intestinal bacteria metabolizing the natural isoflavone glycosides daidzin and genistin. Arch Microbiol. 2000;174:422-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 190] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Hur HG, Beger RD, Heinze TM, Lay JO Jr, Freeman JP, Dore J, Rafii F. Isolation of an anaerobic intestinal bacterium capable of cleaving the C-ring of the isoflavonoid daidzein. Arch Microbiol. 2002;178:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Schoefer L, Mohan R, Braune A, Birringer M, Blaut M. Anaerobic C-ring cleavage of genistein and daidzein by Eubacterium ramulus. FEMS Microbiol Lett. 2002;208:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Yokoyama S, Niwa T, Osawa T, Suzuki T. Characterization of an O-desmethylangolensin-producing bacterium isolated from human feces. Arch Microbiol. 2010;192:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Rowland I, Faughnan M, Hoey L, Wähälä K, Williamson G, Cassidy A. Bioavailability of phyto-oestrogens. Br J Nutr. 2003;89 Suppl 1:S45-S58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 263] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 38. | Tamura M, Tsushida T, Shinohara K. Isolation of an isoflavone-metabolizing, Clostridium-like bacterium, strain TM-40, from human faeces. Anaerobe. 2007;13:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Wang XL, Hur HG, Lee JH, Kim KT, Kim SI. Enantioselective synthesis of S-equol from dihydrodaidzein by a newly isolated anaerobic human intestinal bacterium. Appl Environ Microbiol. 2005;71:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 40. | Kim M, Kim SI, Han J, Wang XL, Song DG, Kim SU. Stereospecific biotransformation of dihydrodaidzein into (3S)-equol by the human intestinal bacterium Eggerthella strain Julong 732. Appl Environ Microbiol. 2009;75:3062-3068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Yokoyama S, Suzuki T. Isolation and characterization of a novel equol-producing bacterium from human feces. Biosci Biotechnol Biochem. 2008;72:2660-2666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 42. | Matthies A, Blaut M, Braune A. Isolation of a human intestinal bacterium capable of daidzein and genistein conversion. Appl Environ Microbiol. 2009;75:1740-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 43. | Jin JS, Kitahara M, Sakamoto M, Hattori M, Benno Y. Slackia equolifaciens sp. nov., a human intestinal bacterium capable of producing equol. Int J Syst Evol Microbiol. 2010;60:1721-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Tsuji H, Moriyama K, Nomoto K, Miyanaga N, Akaza H. Isolation and characterization of the equol-producing bacterium Slackia sp. strain NATTS. Arch Microbiol. 2010;192:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Shimada Y, Takahashi M, Miyazawa N, Ohtani T, Abiru Y, Uchiyama S, Hishigaki H. Identification of two novel reductases involved in equol biosynthesis in Lactococcus strain 20-92. J Mol Microbiol Biotechnol. 2011;21:160-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | van der Post S, Jabbar KS, Birchenough G, Arike L, Akhtar N, Sjovall H, Johansson MEV, Hansson GC. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut. 2019;68:2142-2151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 327] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 47. | Strugala V, Dettmar PW, Pearson JP. Thickness and continuity of the adherent colonic mucus barrier in active and quiescent ulcerative colitis and Crohn's disease. Int J Clin Pract. 2008;62:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 48. | Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:362-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 450] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 49. | Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 437] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 50. | Olsen T, Rismo R, Cui G, Goll R, Christiansen I, Florholmen J. TH1 and TH17 interactions in untreated inflamed mucosa of inflammatory bowel disease, and their potential to mediate the inflammation. Cytokine. 2011;56:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 51. | Ohman L, Dahlén R, Isaksson S, Sjöling A, Wick MJ, Sjövall H, Van Oudenhove L, Simrén M, Strid H. Serum IL-17A in newly diagnosed treatment-naive patients with ulcerative colitis reflects clinical disease severity and predicts the course of disease. Inflamm Bowel Dis. 2013;19:2433-2439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1545] [Cited by in RCA: 1979] [Article Influence: 179.9] [Reference Citation Analysis (1)] |
| 53. | Michail S, Durbin M, Turner D, Griffiths AM, Mack DR, Hyams J, Leleiko N, Kenche H, Stolfi A, Wine E. Alterations in the gut microbiome of children with severe ulcerative colitis. Inflamm Bowel Dis. 2012;18:1799-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 54. | Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, Ferrante M, Verhaegen J, Rutgeerts P, Vermeire S. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63:1275-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1336] [Article Influence: 121.5] [Reference Citation Analysis (3)] |
| 55. | Varela E, Manichanh C, Gallart M, Torrejón A, Borruel N, Casellas F, Guarner F, Antolin M. Colonisation by Faecalibacterium prausnitzii and maintenance of clinical remission in patients with ulcerative colitis. Aliment Pharmacol Ther. 2013;38:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 187] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 56. | Chen GL, Zhang Y, Wang WY, Ji XL, Meng F, Xu PS, Yang NM, Ye FQ, Bo XC. Partners of patients with ulcerative colitis exhibit a biologically relevant dysbiosis in fecal microbial metacommunities. World J Gastroenterol. 2017;23:4624-4631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Pascal V, Pozuelo M, Borruel N, Casellas F, Campos D, Santiago A, Martinez X, Varela E, Sarrabayrouse G, Machiels K, Vermeire S, Sokol H, Guarner F, Manichanh C. A microbial signature for Crohn's disease. Gut. 2017;66:813-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 454] [Cited by in RCA: 597] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 58. | Rajca S, Grondin V, Louis E, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, Dupas JL, Pillant H, Picon L, Veyrac M, Flamant M, Savoye G, Jian R, Devos M, Paintaud G, Piver E, Allez M, Mary JY, Sokol H, Colombel JF, Seksik P. Alterations in the intestinal microbiome (dysbiosis) as a predictor of relapse after infliximab withdrawal in Crohn's disease. Inflamm Bowel Dis. 2014;20:978-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 59. | Sokol H, Leducq V, Aschard H, Pham HP, Jegou S, Landman C, Cohen D, Liguori G, Bourrier A, Nion-Larmurier I, Cosnes J, Seksik P, Langella P, Skurnik D, Richard ML, Beaugerie L. Fungal microbiota dysbiosis in IBD. Gut. 2017;66:1039-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 658] [Cited by in RCA: 900] [Article Influence: 112.5] [Reference Citation Analysis (0)] |
| 60. | Norman JM, Handley SA, Baldridge MT, Droit L, Liu CY, Keller BC, Kambal A, Monaco CL, Zhao G, Fleshner P, Stappenbeck TS, McGovern DP, Keshavarzian A, Mutlu EA, Sauk J, Gevers D, Xavier RJ, Wang D, Parkes M, Virgin HW. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell. 2015;160:447-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 916] [Article Influence: 91.6] [Reference Citation Analysis (0)] |
| 61. | Sun M, Wu W, Chen L, Yang W, Huang X, Ma C, Chen F, Xiao Y, Zhao Y, Ma C, Yao S, Carpio VH, Dann SM, Zhao Q, Liu Z, Cong Y. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9:3555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 432] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 62. | Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2007] [Cited by in RCA: 2399] [Article Influence: 149.9] [Reference Citation Analysis (0)] |
| 63. | Zhou L, Zhang M, Wang Y, Dorfman RG, Liu H, Yu T, Chen X, Tang D, Xu L, Yin Y, Pan Y, Zhou Q, Zhou Y, Yu C. Faecalibacterium prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflamm Bowel Dis. 2018;24:1926-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 327] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 64. | Laffin M, Fedorak R, Zalasky A, Park H, Gill A, Agrawal A, Keshteli A, Hotte N, Madsen KL. A high-sugar diet rapidly enhances susceptibility to colitis via depletion of luminal short-chain fatty acids in mice. Sci Rep. 2019;9:12294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 65. | Shen J, Li N, Zhang X. Daidzein Ameliorates Dextran Sulfate Sodium-Induced Experimental Colitis in Mice by Regulating NF-κB Signaling. J Environ Pathol Toxicol Oncol. 2019;38:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 66. | Seo H, Oh J, Hahn D, Kwon CS, Lee JS, Kim JS. Protective Effect of Glyceollins in a Mouse Model of Dextran Sulfate Sodium-Induced Colitis. J Med Food. 2017;20:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Głąbska D, Guzek D, Grudzińska D, Lech G. Influence of dietary isoflavone intake on gastrointestinal symptoms in ulcerative colitis individuals in remission. World J Gastroenterol. 2017;23:5356-5363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Skolmowska D, Głąbska D, Guzek D, Lech G. Association between Dietary Isoflavone Intake and Ulcerative Colitis Symptoms in Polish Caucasian Individuals. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 69. | Wiseman A. Crohn's disease leading to bowel cancer may be avoided by consumption of soya isoflavones: adjunct-chemotherapy with oxaliplatin. Med Hypotheses. 2006;66:934-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 70. | Milán-Noris AK, Gutiérrez-Uribe JA, Santacruz A, Serna-Saldívar SO, Martínez-Villaluenga C. Peptides and isoflavones in gastrointestinal digests contribute to the anti-inflammatory potential of cooked or germinated desi and kabuli chickpea (Cicer arietinum L.). Food Chem. 2018;268:66-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 71. | Seibel J, Molzberger AF, Hertrampf T, Laudenbach-Leschowski U, Diel P. Oral treatment with genistein reduces the expression of molecular and biochemical markers of inflammation in a rat model of chronic TNBS-induced colitis. Eur J Nutr. 2009;48:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 72. | Schreiber S, Rosenstiel P, Hampe J, Nikolaus S, Groessner B, Schottelius A, Kühbacher T, Hämling J, Fölsch UR, Seegert D. Activation of signal transducer and activator of transcription (STAT) 1 in human chronic inflammatory bowel disease. Gut. 2002;51:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 172] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 73. | Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007;2007:45673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 614] [Article Influence: 36.1] [Reference Citation Analysis (1)] |
| 74. | Slomiany BL, Slomiany A. Role of LPS-elicited signaling in triggering gastric mucosal inflammatory responses to H. pylori: modulatory effect of ghrelin. Inflammopharmacology. 2017;25:415-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 75. | Sheibanie AF, Yen JH, Khayrullina T, Emig F, Zhang M, Tuma R, Ganea D. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23-->IL-17 axis. J Immunol. 2007;178:8138-8147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 76. | Lejeune M, Leung P, Beck PL, Chadee K. Role of EP4 receptor and prostaglandin transporter in prostaglandin E2-induced alteration in colonic epithelial barrier integrity. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1097-G1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 77. | Satsu H, Hyun JS, Shin HS, Shimizu M. Suppressive effect of an isoflavone fraction on tumor necrosis factor-alpha-induced interleukin-8 production in human intestinal epithelial Caco-2 cells. J Nutr Sci Vitaminol (Tokyo). 2009;55:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Wullaert A. Role of NF-kappaB activation in intestinal immune homeostasis. Int J Med Microbiol. 2010;300:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Mitchell JP, Carmody RJ. NF-κB and the Transcriptional Control of Inflammation. Int Rev Cell Mol Biol. 2018;335:41-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 356] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 80. | Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2497] [Cited by in RCA: 3632] [Article Influence: 227.0] [Reference Citation Analysis (0)] |
| 81. | Spehlmann ME, Eckmann L. Nuclear factor-kappa B in intestinal protection and destruction. Curr Opin Gastroenterol. 2009;25:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 82. | Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, Gumucio D, Neurath MF, Pasparakis M. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 792] [Cited by in RCA: 945] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 83. | Kim SE, Kawaguchi K, Hayashi H, Furusho K, Maruyama M. Remission Effects of Dietary Soybean Isoflavones on DSS-Induced Murine Colitis and an LPS-Activated Macrophage Cell Line. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 84. | Lissner D, Schumann M, Batra A, Kredel LI, Kühl AA, Erben U, May C, Schulzke JD, Siegmund B. Monocyte and M1 Macrophage-induced Barrier Defect Contributes to Chronic Intestinal Inflammation in IBD. Inflamm Bowel Dis. 2015;21:1297-1305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 176] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 85. | Abron JD, Singh NP, Price RL, Nagarkatti M, Nagarkatti PS, Singh UP. Genistein induces macrophage polarization and systemic cytokine to ameliorate experimental colitis. PLoS One. 2018;13:e0199631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 86. | Wang B, Wu C. Dietary soy isoflavones alleviate dextran sulfate sodium-induced inflammation and oxidative stress in mice. Exp Ther Med. 2017;14:276-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 87. | Franchi L, Muñoz-Planillo R, Núñez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 837] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 88. | Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E, Schnurr M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 721] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 89. | Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1162] [Cited by in RCA: 1428] [Article Influence: 95.2] [Reference Citation Analysis (0)] |
| 90. | Al-Sadi R, Ye D, Dokladny K, Ma TY. Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J Immunol. 2008;180:5653-5661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 335] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 91. | Al-Sadi R, Ye D, Said HM, Ma TY. IL-1beta-induced increase in intestinal epithelial tight junction permeability is mediated by MEKK-1 activation of canonical NF-kappaB pathway. Am J Pathol. 2010;177:2310-2322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 92. | Siegmund B. Interleukin-18 in intestinal inflammation: friend and foe? Immunity. 2010;32:300-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 93. | Williams MA, O'Callaghan A, Corr SC. IL-33 and IL-18 in Inflammatory Bowel Disease Etiology and Microbial Interactions. Front Immunol. 2019;10:1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 94. | Chen Y, Le TH, Du Q, Zhao Z, Liu Y, Zou J, Hua W, Liu C, Zhu Y. Genistein protects against DSS-induced colitis by inhibiting NLRP3 inflammasome via TGR5-cAMP signaling. Int Immunopharmacol. 2019;71:144-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 95. | Hirota SA, Ng J, Lueng A, Khajah M, Parhar K, Li Y, Lam V, Potentier MS, Ng K, Bawa M, McCafferty DM, Rioux KP, Ghosh S, Xavier RJ, Colgan SP, Tschopp J, Muruve D, MacDonald JA, Beck PL. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis. 2011;17:1359-1372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 357] [Cited by in RCA: 362] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 96. | Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 820] [Cited by in RCA: 820] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 97. | Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, Blumenschein WM, Judo M, Ayanoglu G, McClanahan TK, Li X, Cua DJ. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity. 2015;43:727-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 593] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 98. | Reynolds JM, Martinez GJ, Nallaparaju KC, Chang SH, Wang YH, Dong C. Cutting edge: regulation of intestinal inflammation and barrier function by IL-17C. J Immunol. 2012;189:4226-4230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 99. | Hohenberger M, Cardwell LA, Oussedik E, Feldman SR. Interleukin-17 inhibition: role in psoriasis and inflammatory bowel disease. J Dermatolog Treat. 2018;29:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 100. | Maxwell JR, Zhang Y, Brown WA, Smith CL, Byrne FR, Fiorino M, Stevens E, Bigler J, Davis JA, Rottman JB, Budelsky AL, Symons A, Towne JE. Differential Roles for Interleukin-23 and Interleukin-17 in Intestinal Immunoregulation. Immunity. 2015;43:739-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 275] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 101. | Kojima H, Takeda Y, Muromoto R, Takahashi M, Hirao T, Takeuchi S, Jetten AM, Matsuda T. Isoflavones enhance interleukin-17 gene expression via retinoic acid receptor-related orphan receptors α and γ. Toxicology. 2015;329:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 102. | Takahashi M, Muromoto R, Kojima H, Takeuchi S, Kitai Y, Kashiwakura JI, Matsuda T. Biochanin A enhances RORγ activity through STAT3-mediated recruitment of NCOA1. Biochem Biophys Res Commun. 2017;489:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 103. | Bunte K, Beikler T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 352] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 104. | Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1234] [Cited by in RCA: 1356] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 105. | van der Giessen J, van der Woude CJ, Peppelenbosch MP, Fuhler GM. A Direct Effect of Sex Hormones on Epithelial Barrier Function in Inflammatory Bowel Disease Models. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 106. | Braniste V, Leveque M, Buisson-Brenac C, Bueno L, Fioramonti J, Houdeau E. Oestradiol decreases colonic permeability through oestrogen receptor beta-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J Physiol. 2009;587:3317-3328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 107. | Moussa L, Bézirard V, Salvador-Cartier C, Bacquié V, Lencina C, Lévêque M, Braniste V, Ménard S, Théodorou V, Houdeau E. A low dose of fermented soy germ alleviates gut barrier injury, hyperalgesia and faecal protease activity in a rat model of inflammatory bowel disease. PLoS One. 2012;7:e49547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 108. | Vázquez L, Flórez AB, Guadamuro L, Mayo B. Effect of Soy Isoflavones on Growth of Representative Bacterial Species from the Human Gut. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 109. | Pagnini C, Corleto VD, Martorelli M, Lanini C, D'Ambra G, Di Giulio E, Delle Fave G. Mucosal adhesion and anti-inflammatory effects of Lactobacillus rhamnosus GG in the human colonic mucosa: A proof-of-concept study. World J Gastroenterol. 2018;24:4652-4662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 110. | Martín R, Chamignon C, Mhedbi-Hajri N, Chain F, Derrien M, Escribano-Vázquez U, Garault P, Cotillard A, Pham HP, Chervaux C, Bermúdez-Humarán LG, Smokvina T, Langella P. The potential probiotic Lactobacillus rhamnosus CNCM I-3690 strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response. Sci Rep. 2019;9:5398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 111. | Luo Q, Cheng D, Huang C, Li Y, Lao C, Xia Y, Liu W, Gong X, Hu D, Li B, He X, Chen Z. Improvement of Colonic Immune Function with Soy Isoflavones in High-Fat Diet-Induced Obese Rats. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 112. | Guadamuro L, Dohrmann AB, Tebbe CC, Mayo B, Delgado S. Bacterial communities and metabolic activity of faecal cultures from equol producer and non-producer menopausal women under treatment with soy isoflavones. BMC Microbiol. 2017;17:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 113. | Ohfuji S, Fukushima W, Watanabe K, Sasaki S, Yamagami H, Nagahori M, Watanabe M, Hirota Y; Japanese Case-Control Study Group for Ulcerative Colitis. Pre-illness isoflavone consumption and disease risk of ulcerative colitis: a multicenter case-control study in Japan. PLoS One. 2014;9:e110270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 114. | Seibel J, Molzberger AF, Hertrampf T, Laudenbach-Leschowski U, Degen GH, Diel P. In utero and postnatal exposure to a phytoestrogen-enriched diet increases parameters of acute inflammation in a rat model of TNBS-induced colitis. Arch Toxicol. 2008;82:941-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 115. | Khalili H, Higuchi LM, Ananthakrishnan AN, Manson JE, Feskanich D, Richter JM, Fuchs CS, Chan AT. Hormone therapy increases risk of ulcerative colitis but not Crohn's disease. Gastroenterology. 2012;143:1199-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 116. | Ortizo R, Lee SY, Nguyen ET, Jamal MM, Bechtold MM, Nguyen DL. Exposure to oral contraceptives increases the risk for development of inflammatory bowel disease: a meta-analysis of case-controlled and cohort studies. Eur J Gastroenterol Hepatol. 2017;29:1064-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 117. | Ko KP. Isoflavones: chemistry, analysis, functions and effects on health and cancer. Asian Pac J Cancer Prev. 2014;15:7001-7010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |