Published online Mar 6, 2019. doi: 10.12998/wjcc.v7.i5.623
Peer-review started: December 20, 2018
First decision: January 19, 2019
Revised: February 3, 2019
Accepted: February 18, 2019
Article in press: February 18, 2019
Published online: March 6, 2019
Processing time: 77 Days and 18.3 Hours
The practice of Indian Ayurvedic medicine is spreading in Western countries and Shilajit is one of the most used drugs, for its antioxidant activities and immunomodulatory effects. Albeit Shilajit has showed a high degree of safety, it can act as cofactor of anaphylaxis, especially in condition at high risk, such as mast cell activation syndrome (MCAS). We reported this case to sensitize practitioners to investigate to the use of complementary and alternative medicine, in case of exercise-induced anaphylaxis (EIAn).
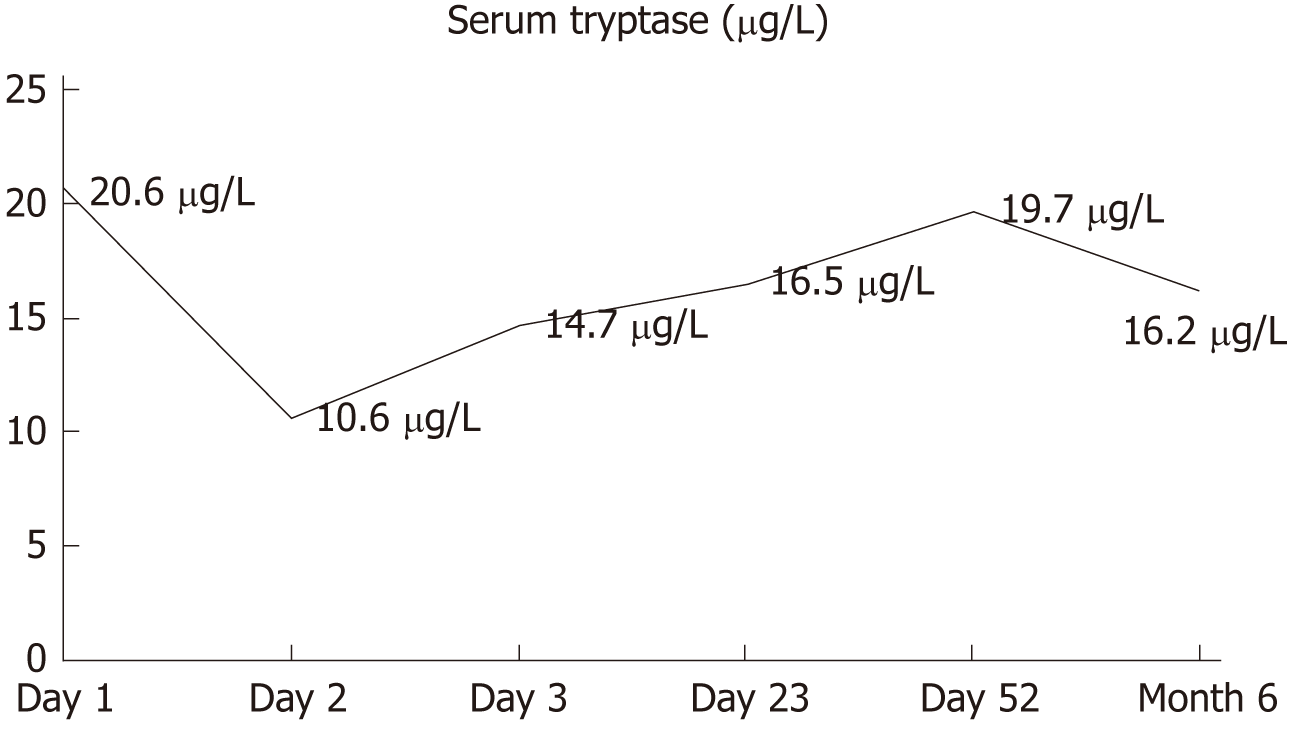
A 43-year-old woman, working as a dance teacher, developed urticaria after ingestion of rice, tuna and Shilajit, which did not respond to intramuscular corticosteroids. Subsequently, she developed dyspnoea and hypotension with loss of consciousness that arose 1 h after sexual activity. The patient did not refer personal history of atopy. Specific IgE for main food allergens resulted negative, with total IgE levels of 14 IU/L. Oral provocation test with Shilajit was not perfomed because the patient refused, but we performed prick-by-prick and patch test that resulted negative. Serum tryptase at the time of anaphylaxis was 20.6 μg/L that fell down to of 10.6 μg/L after therapy, but has remained at the high value after two days and during the follow-up. We performed an analysis of the c-KIT gene in peripheral blood, which was negative. We felt the diagnosis consistent with EIAn in a patient with a possible MCAS.
In Western countries the use of drugs from Ayurvedic medicine is more common than in the past. These substances can be cofactors of anaphylaxis in patients with risk factors.
Core tip: This case report describes, for the first time, the role of drugs belonging to complementary and alternative medicine (CAM) in triggering anaphylaxis. Owing to the increase in their consumption in Western countries, these drugs should be considered as potential cofactor in conditions with a high risk of anaphylaxis, such as exercise-induced anaphylaxis and mast cell activation syndrome. This experience may be useful to give insight to practitioners about CAM and potential adverse drug reactions.
- Citation: Losa F, Deidda M, Firinu D, Martino MLD, Barca MP, Giacco SD. Exercise-induced anaphylaxis with an Ayurvedic drug as cofactor: A case report. World J Clin Cases 2019; 7(5): 623-627
- URL: https://www.wjgnet.com/2307-8960/full/v7/i5/623.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i5.623
Anaphylaxis is defined as a “severe, life-threatening systemic hypersensitivity reaction”[1]. The most common triggers are medications, insect stings, and foods. Exercise-induced anaphylaxis (EIAn) is a rare, unpredictable, and potentially fatal syndrome in which the occurrence of anaphylaxis coincides with exercise or physical activity. EIAn may occur after the ingestion of food, either pre- or post-exercise (Food-Dependent Exercise-Induced Anaphylaxis - FDEIAn), or independently of food ingestion (Non-Food Dependent Exercise-Induced Anaphylaxis[2]. When a Non-Food-Dependent form is identified, patients should be evaluated for mast cell activation syndrome (MCAS), which encompasses a group of diseases characterized by symptoms due to release of mast-cell mediators[3]. Shilajit is a herbo-mineral compound, derived from mountain regions of Asia, and consists of organic substances, divided into variable humic and non-humic proportions. The active components of Shilajit are dibenzo-alpha-pyrones and related metabolites; organic acids, such as fulvic acid; and inorganic constituents. It is one of most commonly used drugs in Indian Ayurvedic medicine, a complementary and alternative medicine (CAM). No data are available regarding anaphylaxis or allergic reactions elicited by Shilajit[4]. Here, we report a case of a woman who developed anaphylaxis after intense physical activity and ingestion of Shilajit.
A 43-year-old woman, a dance teacher, started taking a drug containing Shilajit in April 2017, at a dose of 400 mg/d. In July, she decided to increase the dose to 800 mg/d. The first day of the Shilajit dose increase, taken with a meal of rice and tuna, she developed urticaria, after one hour from the meal, immediately subsequent to intense physical activity. The hives appeared on thorax, neck, and arms and decreased after intramuscular corticosteroids, but did not disappear completely. The patient decided to stop taking Shilajit. Despite the symptoms, the patient also went to the beach that day and sunbathed for hours. The next day, one hour after sexual activity and a meal based again on rice, she developed angioedema, dyspnea, and hypotension with syncope that resolved after the administration of adrenaline, intravenous corticosteroids, and H1 and H2 antihistamines in the Emergency Department. She denied the assumption of alcohol, menses and she did not present sign or symptoms of infection.
The patient’s personal history and laboratory results were not suggestive of allergy, as summarized in Table 1.
| Patient characteristics | Laboratory data |
| Age, yr | 43 |
| BMI, kg/m2 | 23 |
| Family history of atopy | Negative |
| ADR (antibiotics, NSAID, anesthetics, contrast agents) | No |
| Bone mineral density | T score - 0.7 |
| FEV1 | Normal |
| Chest radiograph | Normal |
| Abdomen ultrasonography | Normal |
| Skin Prick Test (foods and aeroallergens) | Negative |
| Blood test results | |
| Total IgE | 14.8 KU/L |
| Specific IgE1 | |
| Wheat | 0.00 KU/L |
| rTri a 19 | 0.00 KU/L |
| rTri a 14 LPT | 0.00 KU/L |
| Alpha-amilase | 0.00 KU/L |
| Rice | 0.00 KU/L |
| Oats | 0.00 KU/L |
| Barley | 0.00 KU/L |
| Tuna | 0.00 KU/L |
| Soy | 0.00 KU/L |
| rPru p 3 | 0.00 KU/L |
| Nut | 0.00 KU/L |
| Walnut | 0.00 KU/L |
| Latex | 0.04 KU/L |
Serum tryptase (ST) level at the time of anaphylaxis was 20.6 μg/L (normal level: 1–15 μg/L), and its progression is shown in Figure 1. Prick-by-prick and patch-testing with Shilajit were negative in the patient and in the controls, which were tested to exclude a false-positive result. Exercise test after a meal without Shilajit was negative, and an oral provocation test was not performed, due to patient refusal. To evaluate the hypothesis of an underlying MCAS, despite being the first anaphylaxis episode, we performed an analysis of the c-KIT gene in peripheral blood, which was negative (allele-specific real time quantitative PCR assay, ABI PRISM 7500 FAST, ThermoScientific, Uppsala, Sweden). We did not perform bone marrow biopsy, due to a low probability of clonal mast-cell syndrome, according to the Spanish Network on Mastocytosis (REMA) protocol[5]. During hospitalization, oral prednisone and antihistamines were administered and rapidly tapered until discontinuation.
Urticarial.
At discharge, we prescribed epinephrine auto-injector and she was advised to avoid potential triggers, such as intense physical activity, for at least four hours after meals.
In the following months, the patient did not experience further episodes of urticaria or other symptoms. She did not change her eating habits, including the intake of rice and tuna, or physical activity. ST remained elevated during the follow-up.
The use of CAM drugs from Asia is rapidly increasing in Western countries. Shilajit is one of the substances most used in Ayurvedic medicine for its therapeutic antioxidant and anti-inflammatory properties, as well as its alleged antioxidant and immunomodulatory effects[4]. There has been only one in vitro study where fulvic acid has shown an inhibitory effect on human leukemia basophil (KU812) cells[6]. The active constituent of Shilajit are dibenzo-α-pyrones and related metabolites and fulvic acid. The pure extract of Shilajit is often made impure by contaminations of mycotoxins, heavy metal ions or reactive free radicals, so it is mandatory purify the substance before the use[7]. No studies on the pharmacokinetics and pharmacodynamics are available and there are no reports of adverse or hypersensitivity reactions to Shilajit, its components, or other drugs belonging to CAM[8].
MCAS is characterized by recurrent symptoms due to the release of mast-cell mediators, which correspond to an increase of ST level. Moreover, a prompt response to anti-mediator therapy and the exclusion of primary and secondary causes of mast cell activation are required for diagnosis[9]. MCAS is one of the differential diagnoses in cases of anaphylaxis. Although hymenoptera stings are the triggers responsible for most of the life-threatening reactions, drugs and foods can also cause anaphylaxis, in association with physical activity or exposure to heat. The case we here report was challenging for the time interval between the ingestion of Shilajit at an increased dose and the physical activity, in conjunction with a negative diagnostic work-up, which made a diagnosis of FEIAn not likely. On the contrary, the time course of the reaction and the presence of several triggers made more likely a diagnosis of EIAn. Moreover, the lack of other episodes of anaphylaxis in the past twelve months and during the follow-up is not consistent with a diagnosis of idiopathic anaphylaxis[10]. The persistent increase of ST level over time raises the suspicion of MCAS.
Both elevated basal ST level (> 20 μg/L) and an increase of 20% plus 2 μg/L over the basal level are suggestive of mast cell activation and satisfy MCAS criteria. We performed a c-KIT mutational analysis by rtPCR using peripheral blood, and the results were negative. The sensitivity of this technique on peripheral blood is quite low. Indeed, some MCAS patients may carry the D816V KIT mutation but there are also cases of mastocytosis that do not[11]. In the presence of a single episode of anaphylaxis and with no c-KIT mutation detectable, we chose not to perform a bone marrow biopsy and to monitor our patient over time according to the REMA indications. The REMA group has proposed a scoring model to predict the presence of clonal mast cells in patients with history of anaphylaxis without skin mastocytosis, before performing a bone marrow study. Our patient had a score of 1, which indicated a low probability of clonal mast-cell activation disorder[5].
Considering the whole clinical picture and the laboratory outcomes, the persistence of elevated ST and the lack of anaphylaxis recurrences after suspending the culprit drug we felt the diagnosis consistent with EIAn in a patient with a possible MCAS.
The main limitations in the diagnostic work-up were the lack of information about the pharmacokinetics of Shilajit and the refuse of the patient to undergo to an oral challenge with the drug. Nevertheless, the occurrence of urticaria immediately after the increase in Shilajit dose and anaphylaxis in association with well-known triggers brought us to consider Shilajit as a key cofactor for anaphylaxis. Data from the literature suggest that augmenting factors take place in more than 30% of occurrence of anaphylaxis, so physicians have to investigate during anamnesis about cofactors[12].
This case is the first example reported of a CAM drug potentially eliciting anaphylaxis. Thus, because the consumption of these drugs is increasing in Western countries, Shilajit might be regarded as a potential cofactor, especially in people with features of mast-cells related disorders.
CAM drugs can potentially elicit anaphylaxis. In case of EIAn it is mandatory to investigate the presence of mast cell disorders. More studies are necessary about pharmacokinetics and pharmacodynamics of CAM drugs
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Exbrayat JM, Moneim AA, Rangel-Corona R S- Editor: Ji FF L- Editor: A E- Editor: Bian YN
| 1. | Muraro A, Roberts G, Worm M, Bilò MB, Brockow K, Fernández Rivas M, Santos AF, Zolkipli ZQ, Bellou A, Beyer K, Bindslev-Jensen C, Cardona V, Clark AT, Demoly P, Dubois AE, DunnGalvin A, Eigenmann P, Halken S, Harada L, Lack G, Jutel M, Niggemann B, Ruëff F, Timmermans F, Vlieg-Boerstra BJ, Werfel T, Dhami S, Panesar S, Akdis CA, Sheikh A; EAACI Food Allergy and Anaphylaxis Guidelines Group. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 637] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 2. | Ansley L, Bonini M, Delgado L, Del Giacco S, Du Toit G, Khaitov M, Kurowski M, Hull JH, Moreira A, Robson-Ansley PJ. Pathophysiological mechanisms of exercise-induced anaphylaxis: an EAACI position statement. Allergy. 2015;70:1212-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Onnes MC, Tanno LK, Elberink JN. Mast Cell Clonal Disorders: Classification, Diagnosis and Management. Curr Treat Options Allergy. 2016;3:453-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Wilson E, Rajamanickam GV, Dubey GP, Klose P, Musial F, Saha FJ, Rampp T, Michalsen A, Dobos GJ. Review on shilajit used in traditional Indian medicine. J Ethnopharmacol. 2011;136:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Alvarez-Twose I, González-de-Olano D, Sánchez-Muñoz L, Matito A, Jara-Acevedo M, Teodosio C, García-Montero A, Morgado JM, Orfao A, Escribano L. Validation of the REMA score for predicting mast cell clonality and systemic mastocytosis in patients with systemic mast cell activation symptoms. Int Arch Allergy Immunol. 2012;157:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Motojima H, O Villareal M, Han J, Isoda H. Microarray analysis of immediate-type allergy in KU812 cells in response to fulvic acid. Cytotechnology. 2011;63:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Agarwal SP, Khanna R, Karmarkar R, Anwer MK, Khar RK. Shilajit: a review. Phytother Res. 2007;21:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Bielory L. Complementary and Alternative Medicine in Allergy-Immunology: More Information is Needed. J Allergy Clin Immunol Pract. 2018;6:99-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Akin C. Mast cell activation syndromes presenting as anaphylaxis. Immunol Allergy Clin North Am. 2015;35:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Nwaru BI, Dhami S, Sheikh A. Idiopathic Anaphylaxis. Curr Treat Options Allergy. 2017;4:312-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Kristensen T, Vestergaard H, Møller MB. Improved detection of the KIT D816V mutation in patients with systemic mastocytosis using a quantitative and highly sensitive real-time qPCR assay. J Mol Diagn. 2011;13:180-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 12. | Muñoz-Cano R, Pascal M, Araujo G, Goikoetxea MJ, Valero AL, Picado C, Bartra J. Mechanisms, Cofactors, and Augmenting Factors Involved in Anaphylaxis. Front Immunol. 2017;8:1193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |