Published online Mar 6, 2019. doi: 10.12998/wjcc.v7.i5.562
Peer-review started: December 23, 2018
First decision: December 29, 2018
Revised: January 13, 2019
Accepted: January 29, 2019
Article in press: January 30, 2019
Published online: March 6, 2019
Processing time: 74 Days and 19.6 Hours
The off-label use of various devices has been reported for the transcatheter closure of perimembranous ventricular septal defects (PmVSD) because of serious complications, such as heart block and tricuspid regurgitation (TR), associated with conventional ventricular septal defect devices. However, whether certain defects such as PmVSD with abnormally attached tricuspid are fit for interventional treatment is still disputable.
To explore the feasibility and safety of transcatheter closure of PmVSD with abnormally attached tricuspid chordae tendineae using an improved patent ductus arteriosus (PDA) occluder.
We retrospectively analyzed 20 patients diagnosed with PmVSD with abnormally attached tricuspid chordae tendineae who underwent interventional treatment using an improved PDA occluder at our center from January 2012 to January 2016. Baseline characteristics and procedural and follow-up data were analyzed.
All 20 patients achieved procedure success. No heart block occurred during the operation. One patient had a transient complete right bundle branch block within 48 h post-procedure and reverted to normal rhythm after intravenous injections of dexamethasone for 3 d. For all 20 patients, no residual shunt was observed by transthoracic echocardiography post-procedure. During the average follow-up period of 2.4 years, no severe TR was observed.
Using of the improved PDA occluder for the transcatheter closure of PmVSD with abnormally attached tricuspid chordae tendineae is a safe and promising treatment option. However, long-term follow-up in a large group of patients is still warranted.
Core tip: The aim of this study was to explore the feasibility and safety of transcatheter closure of perimembranous ventricular septal defects (PmVSD) with abnormally attached tricuspid chordae tendineae using an improved PDA occluder. A total of 20 patients diagnosed with PmVSD with abnormally attached tricuspid chordae tendineae who underwent interventional treatment using the improved patent ductus arteriosus (PDA) occluder were observed. All 20 patients achieved procedure success, and no residual shunt or severe tricuspid regurgitation was observed. Our study suggested that using of the improved PDA occluder for the transcatheter closure of PmVSD with abnormally attached tricuspid chordae tendineae is a safe and promising treatment option.
- Citation: He L, Du YJ, Cheng GS, Zhang YS. Safety of an improved patent ductus arteriosus occluder for transcatheter closure of perimembranous ventricular septal defects with abnormally attached tricuspid chordae tendineae. World J Clin Cases 2019; 7(5): 562-571
- URL: https://www.wjgnet.com/2307-8960/full/v7/i5/562.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i5.562
Transcatheter interventional therapy for certain types of perimembranous ventricular septal defects (PmVSD) is a substitute for surgical treatment. However, post-procedure complications of interventional therapy such as heart block[1-4] and tricuspid regurgitation (TR)[5,6] are often reported and appear serious, thus limiting the clinical application of this procedure. Furthermore, PmVSD are not infrequently complicated by the presence of aneurysmal fibrous tissue from the septal leaflet of the tricuspid valve, making the use of the device technically challenging and increasing the potential risk of inducing increased tricuspid insufficiency[7].
In some countries, such as China and India, tens of thousands of patients diagnosed with PmVSD are treated by transcatheter closure each year. However, with the increase in the number of interventional treatments, tricuspid insufficiency has been observed during follow-up. Although epidemiological data are lacking, these problems are not less serious than heart block. Some cases even require surgery to repair the tricuspid valve. Because the bilateral disc of the traditional symmetrical ventricular septal defect occluder is 2 mm longer than the waist, it is generally believed that interventional therapy is feasible when the attachment point of the tricuspid chordae tendineae to the defect on the right ventricular (RV) side is greater than 2 mm. However, during follow-up, after using of the traditional symmetrical occluder, tricuspid chordae tendineae rupture[6,8,9] or tricuspid valve stenosis[10,11] caused by the compression of the RV disc may occur even if the attachment point of the tricuspid chordae tendineae to the defect on the RV side is greater than 2 mm.
Using of the Amplatzer ductus occluder (ADO) in transcatheter closure of PmVSD has also been reported[12-14]. Because the ADO has no RV disc, theoretically, there is no special requirement for the distance between the attachment point of the tricuspid chordae tendineae and the RV side of the defect. Thus, the ADO can maximally avoid abrasion of the tricuspid chordae tendineae by the RV disc. However, the ADO has a longer waist (5-8 mm) than the traditional symmetrical VSD occluder. After occluder implantation, the waist will become longer because of the squeezing effect of the surrounding tissue. This longer waist may compress the tricuspid chordae tendineae, resulting in a moderate to large amount of TR. For patients diagnosed with PmVSD with abnormally attached tricuspid chordae tendineae, the indications for interventional treatment and the most appropriate type of occluder remain controversial. Therefore, in the present study, we used an improved patent ductus arteriosus (PDA) occluder, which has no RV disc and a shorter waist, to explore the feasibility and safety of transcatheter closure of PmVSD with abnormally attached tricuspid chordae tendineae.
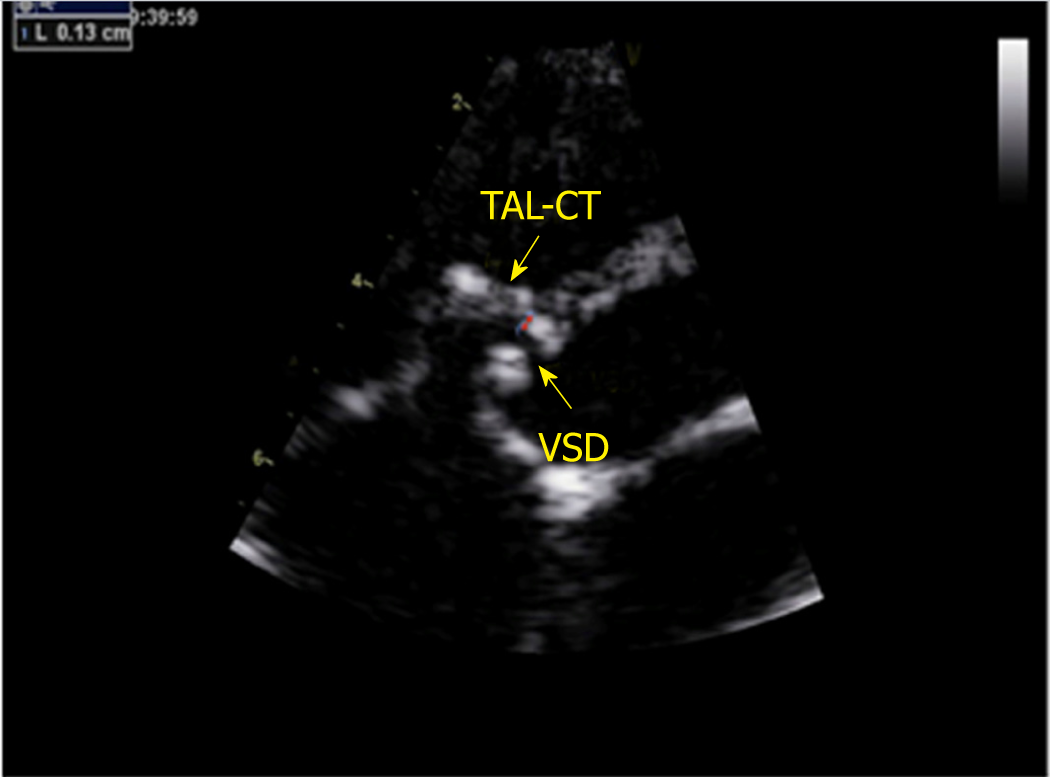
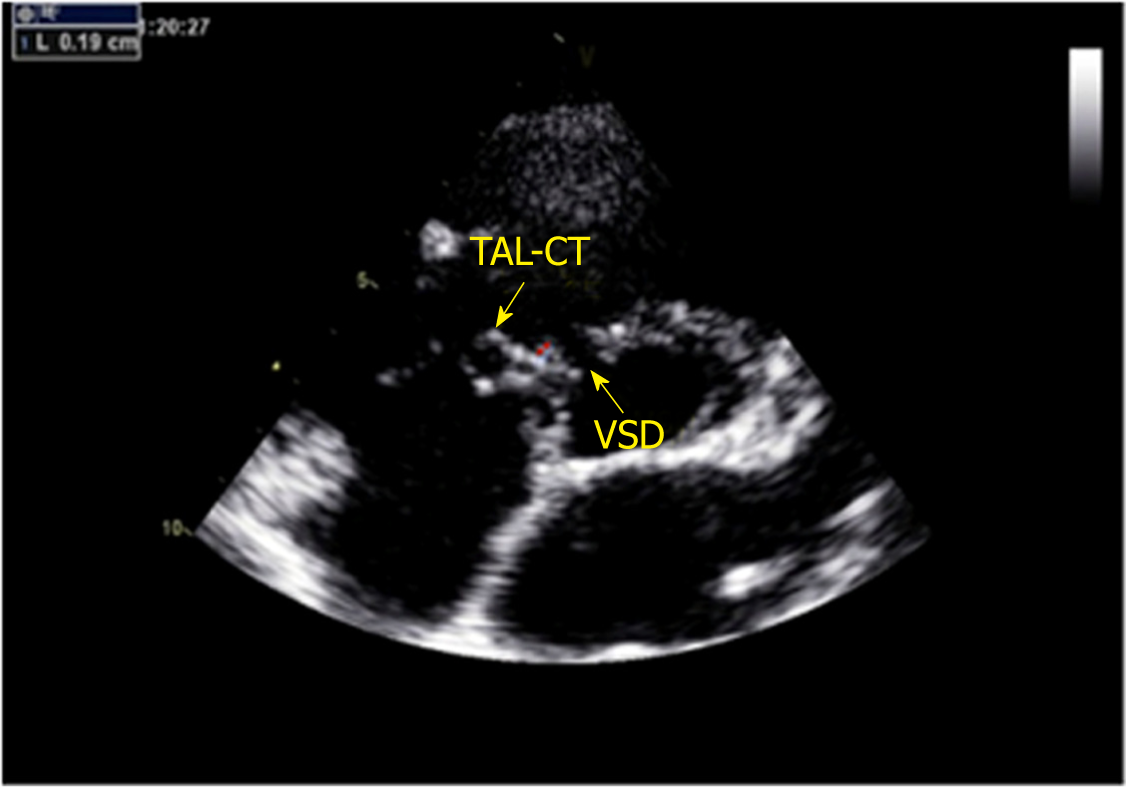
Between January 2012 and January 2016, 360 cases of PmVSD received interventional therapy at the First Affiliated Hospital of Xi’an Jiaotong University. Among these cases, 20 with abnormally attached tricuspid chordae tendineae were selected for inclusion in a retrospective analysis based on transthoracic echocardiography (TTE). According to TTE findings, abnormally attached tricuspid chordae tendineae was defined by a distance of the attachment point of the tricuspid chordae tendineae to the defect on the RV side of 1-2 mm. This study was approved by the Regional Ethics Committee of our hospital, all patients or their parents signed informed consent, and patient anonymity was preserved.
A GE-ViVid-E9 color Doppler ultrasound system (General Electric Corporation, Norfolk, Virginia) equipped with a 2-4 MHz transducer was used to perform TTE. All patients underwent TTE before the procedure. TTE was performed in the apical 5-chamber, long-axis, and short-axis parasternal view. The VSD sizes measured by TTE were 5.0-10.0 mm on the left ventricular (LV) side and 2.5-5.5 mm on the RV side. The ventricular septal rim below the aortic valve was 3.0-5.0 mm, and the distance of the attachment point of the tricuspid chordae tendineae to the defect on the RV side was 1.0-2.0 mm (Figures 1 and 2). Among the 20 patients, the attachment point of the tricuspid chordae tendineae was close to the inferior edge of the defect in 13 (65.0%) patients and close to the superior edge of the defect in 7 (35.0%).
The grading of TR has been well described previously[15]. The majority of parameters are qualitative or semiquantitative. Minor TR is defined as a small, central jet (usually < 4 cm2 or < 20% of the right atrial area), moderate TR defined as a moderate central jet (usually 4-10 cm2 or 20%-40% of the right atrial area), and severe TR as a large central jet (usually > 10 cm2 or > 40% of the right atrial area).
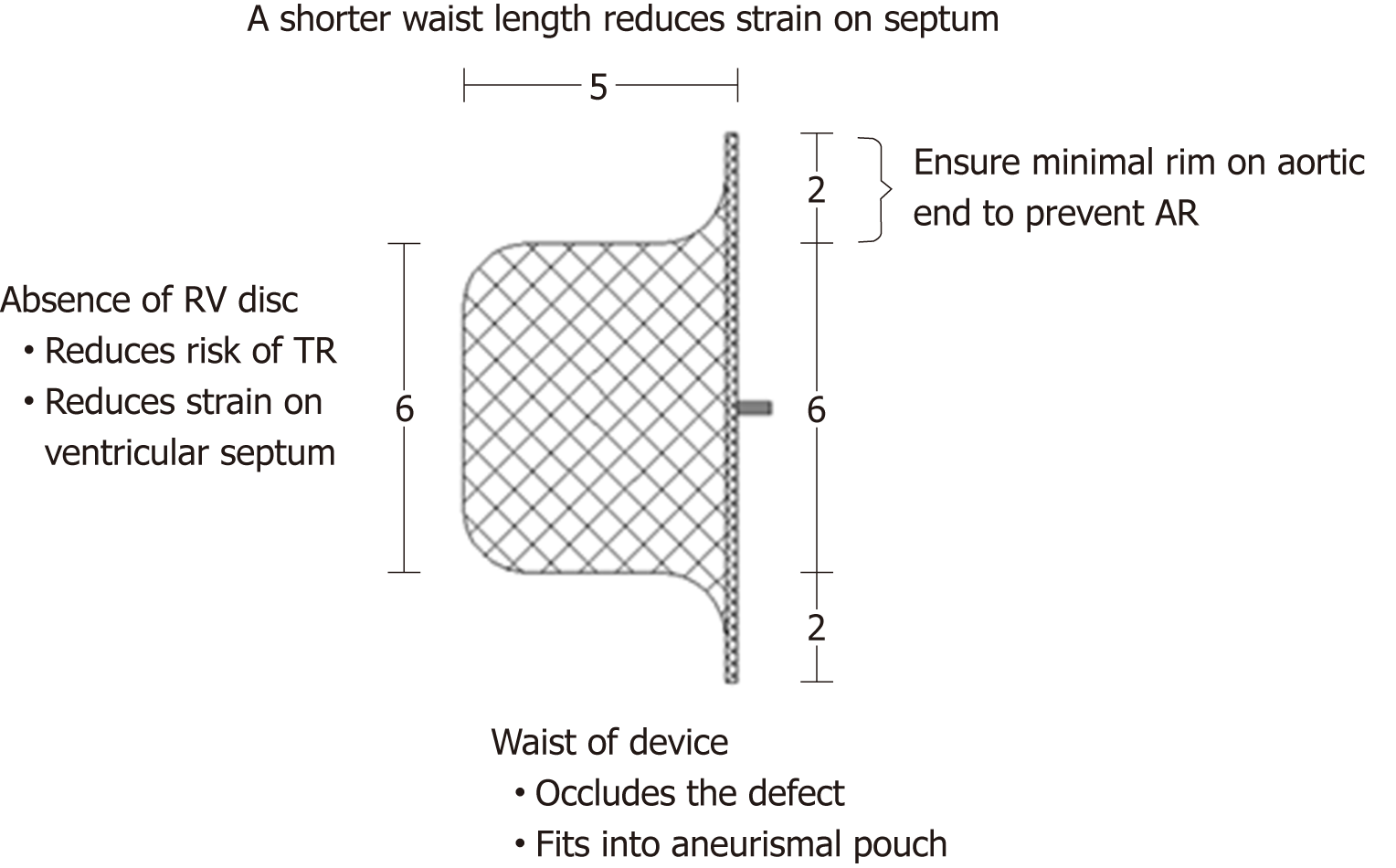
This study used an improved PDA occluder (Starway Medical Technology, Inc., Beijing, China), which is based on the ADO. The improved PDA occluder has no RV disc; the edge of the LV disc is 2 mm, and the waist is 5 mm. The shape of the occluder is similar to that of the ADO, but the waist is shorter, consistent with the designation of this device as the “improved PDA occlude”. The size of the device is ranging from 6 to 12 mm. Figure 3 shows a schematic diagram of the improved PDA occluder and its advantages for the interventional therapy of PmVSD with abnormally attached tricuspid chordae tendineae.
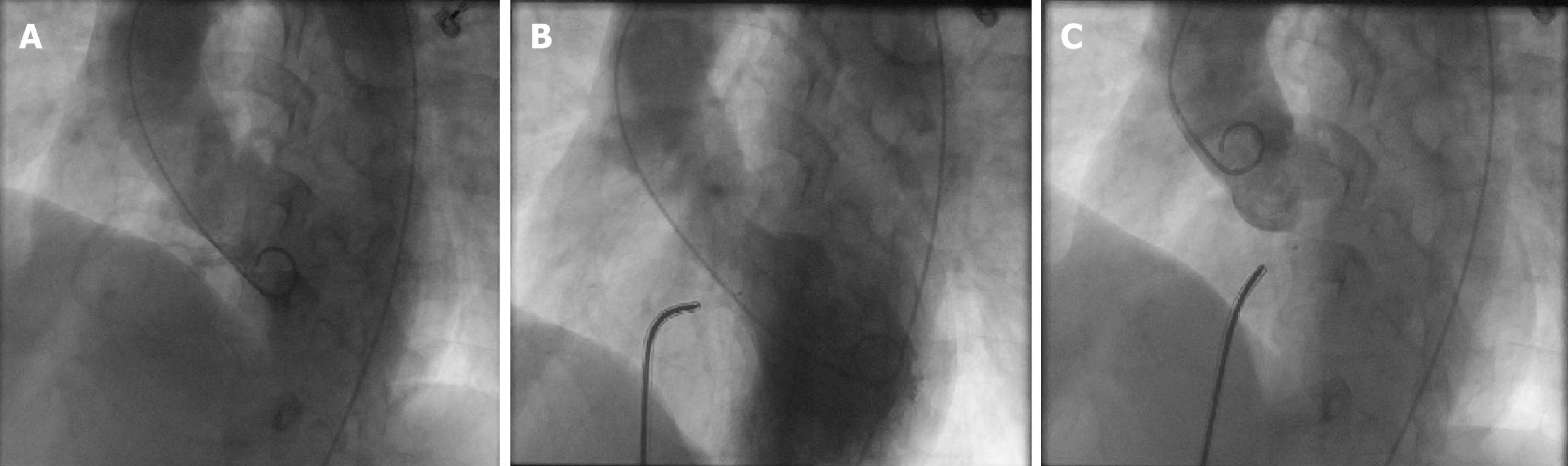
All patients received antibiotics 1 h before the procedure. The procedure was performed under 2% lidocaine local anesthesia for patients over 7 years of age, whereas general anesthesia with ketamine was used for patients under 7 years of age. Fluoroscopy was used to guide the device implantation only. The right femoral vein and artery were accessed, and heparin was administered according to the patient’s body weight (80-100 IU/kg). Right and left cardiac catheterization was performed to evaluate the pulmonary vascular resistance. Hemodynamic parameters, including pulmonary arterial, aortic, atrial, and ventricular pressures, were recorded. Oximetric values were measured in the cardiac chambers and vessels and used to calculate Qp/Qs. Angiography in a single plane (45°-60° left anterior oblique/10°-25° cranial view) was performed to define the location and size of the VSD. Figure 4 shows left ventricle angiography performed before and after the procedure. Angiography of the ascending aorta was also performed in a 50° left atrial oblique view to check for aortic regurgitation (AR).
The technique of PmVSD closure was described in detail elsewhere[9]. An appropriately sized device (> 1-3 mm based on the defect size determined by TTE or angiography) was selected. The device was released when its proper position was obtained (left ventriculography was performed to confirm the proper position of the occluder) and interference with the aortic and tricuspid valve had been excluded (TTE confirmed the absence of AR and TR).
After the procedure, one dose of antibiotic therapy was administered intravenously to prevent infective endocarditis. Holter monitoring was performed for one day, followed by electrocardiography performed daily before discharge. Patients were typically discharged 5 d after the procedure. Aspirin (3-5 mg/kg daily) was administered to all patients for 6 mo after the procedure. Clinical examination, TTE, and chest X-ray were performed before discharge, 1, 3, 6, and 12 mo post-procedure, and yearly thereafter.
The data analyses were performed using SPSS version 19.0. Summary statistics for normally distributed quantitative variables are expressed as the mean ± SD. For non-normally distributed variables, we use median and interquartile range (IQR). Categorical data are summarized as count and percentages. The objective of this article was to present the early experience with the improved PDA occluder for the interventional treatment of PmVSD with abnormally attached tricuspid chordae tendineae in a single-arm, single-center study; therefore, statistical comparisons between groups were not performed.
From January 2012 to January 2016, 360 patients diagnosed with PmVSD received interventional therapy at our center. We performed a retrospective analysis of 20 patients with abnormally attached tricuspid chordae tendineae selected by TTE.
The 20 patients included 12 males and 8 females with an age range of 4 to 52 (20.7 ± 12.3) years and a weight range of 15.5 to 83 (50.1 ± 17.5) kg. Among the 20 patients, 5 (25.0%) had a membranous VSD. The VSD size measured by TTE was 5.0-10.0 (7.3 ± 1.5) mm on the LV side and 2.5-5.5 (3.9 ± 0.8) mm on the RV side. The VSD size measured by angiography was 4.0-10.0 (6.7 ± 1.5) mm on the LV side and 2.5-4.5 (3.5 ± 0.6) mm on the RV side. The ventricular septal rim below the aortic valve was 3.0-5.0 (median 4.0) mm, and the attachment point of the tricuspid chordae tendineae to the defect on the RV side was 1.0-2.0 (median 1.8) mm. The attachment point of the tricuspid chordae tendineae was close to the inferior edge of the defect in 13 (65.0%) patients but was close to the superior edge of the defect in 7 (35.0%). One (5.0%) patient had minor AR, and one (5.0%) had minor TR. The average follow-up was 2.4 years. The baseline parameters and TTE data evaluated are listed in Table 1.
| Patients, n | 20 |
| Sex (M/F), n (%) | 11 (55)/9 (45) |
| Age, yr, mean ± SD | 20.7 ± 12.3 |
| Weight, kg, mean ± SD | 50.1 ± 17.5 |
| Chest radiography | |
| Cardiomegaly, n (%) | 8 (40.0) |
| Increased vascularity, n (%) | 16 (80.0) |
| Echocardiography | |
| Defect size on LV side, mm, mean ± SD | 7.3 ± 1.5 |
| Defect size on RV side, mm, mean ± SD | 3.9 ± 0.8 |
| MVSA, n (%) | 5 (25.0) |
| Ventricular septal rim below the aortic valve, mm, median (IQR) | 4.0 (1.4) |
| Attachment point of the tricuspid chordae tendineae to the defect on RV side, mm, median (IQR) | 1.8 (0.5) |
| Minor AR, n (%) | 1 (5.0) |
| Minor TR, n (%) | 1 (5.0) |
| Follow-up, yr, mean ± SD | 2.4 ± 1.0 |
Procedure success, defined as the ability to release the device without embolization or heart block during the procedure, was achieved in all 20 patients (100.0%). The 6 mm improved PDA occluder was implanted in 3 (15.0%) patients, 7 mm in 3 (15.0%), 8 mm in 5 (25.0%), 9 mm in 5 (25.0%), 10 mm in 3 (15.0%) and, 12 mm in 1 (5.0%). The average operation time was 49.3 ± 6.0 min, and the average fluoroscopy time was 24.3 ± 2.8 min.
No serious arrhythmia occurred during the procedure. One (5.0%) case of complete right bundle branch block occurred within 48 h after the procedure and reverted to normal rhythm after intravenous injections of dexamethasone for 3 d. At follow-ups 1, 3, 6, and 12 mo after the procedure and yearly thereafter, no serious new arrhythmia was found in any of the patients.
TTE was performed 48 h after occluder implantation. For all 20 patients, no residual shunt was observed by TTE post-procedure. Following the procedure, TR was increased in one (5.0%) patient (minor regurgitation). At follow-ups 1, 3, 6, and 12 mo after the procedure and yearly thereafter, one patient with aggravated TR exhibited no obvious change. No deaths occurred after the procedure. No AR was observed. The procedural and follow-up data are listed in Table 2.
| Angiography | |
| Defect size on LV side, mm, mean ± SD | 6.7 ± 1.5 |
| Defect size on RV side, mm, mean ± SD | 3.5 ± 0.6 |
| Routine right and left heart catheterization | |
| sPAP, mmHg, mean ± SD | 31.4 ± 4.4 |
| mPAP, mmHg, mean ± SD | 19.6 ± 3.6 |
| Qp/Qs, mean ± SD | 2.3 ± 0.4 |
| Occluder size, n (%) | |
| 6 mm | 3 (15.0) |
| 7 mm | 3 (15.0) |
| 8 mm | 5 (25.0) |
| 9 mm | 5 (25.0) |
| 10 mm | 3 (15.0) |
| 12 mm | 1 (5.0) |
| Average operative time, min, mean ± SD | 49.3 ± 6.0 |
| Average fluoroscopy time, min, mean ± SD | 24.3 ± 2.8 |
| Post-procedural complications, n (%) | |
| CRBBB (transient) | 1 (5.0) |
| Increased TR, n (%) | 1 (5.0) |
| Sheath, Fr, median (IQR) | 8.0 (2.0) |
Although the off-label use of a number of different devices, such as ADO, ADO II, and Amplatzer Vascular Plug I[10,14,16], has been described for interventional therapy of PmVSD, there is still controversy regarding whether PmVSD with abnormally attached tricuspid chordae tendineae is suitable for interventional therapy.
The most remarkable discovery from our study includes the high success rate of the procedure (100%), the ability to close moderately large defects up to 10 mm, and the absence of severe TR during or after the procedure. There was only one case of complete right bundle branch block within 48 h after the procedure, which reverted to normal rhythm after intravenous injections of dexamethasone for 3 d. Late-onset complete heart block was not observed for any of the 20 patients on routine follow-up through 2 years after the procedure.
Combined with initial 2 years of our experience with the closure of certain PmVSD, we tended to use ADO. Based on several heartening reports from other centers and our own experience, we put this change into practice[12,14,17,18]. However, during follow-up, moderate-to-severe TR occurred in some patients in the immediate or early postoperative period. We propose that when the attachment point of the tricuspid chordae tendineae is close to the defect on the RV side or when the occluder rim is adjacent to the chordae tendineae, the sharp rim of the RV disc can wear the chordae tendineae and increase the likelihood of iatrogenic TR. Additionally, the strain produced by the septum to the waist of the occluder may have an impact on the tricuspid valve and potentially cause TR or tricuspid valve rupture.
The bilateral disc rim of the symmetric PmVSD occluder is 2 mm longer than the waist; when an appropriately sized device (> 1-3 mm, based on the defect size measured by TTE or angiography) is selected, the actual condition of the occluder is in compression by the surrounding tissue of the defect. Therefore, the rims of the RV and LV discs are larger than 2 mm. Thus, the RV disc is longer, and the sharp rim of the RV disc can wear the chordae tendineae and increase the likelihood of iatrogenic TR. To prevent such damage, Lee et al[12] used ADO to treat 20 cases of PmVSD in South Korea. The ADO was successfully implanted in all patients without any significant complications[12]. Because the ADO has no RV disc, extrusion or abrasion of the tricuspid chordae tendineae is less likely to happen. However, the waist of the ADO is 6-8 mm, which will increase after occluder implantation due to extrusion of the surrounding tissue. The increased length of the waist may have an impact on the tricuspid valve and potentially cause severe TR or tricuspid valve rupture.
The risk of waist compression or erosion of the tricuspid valve is even greater for PmVSD with abnormally attached tricuspid chordae tendineae. Based on previous research results, the special improved PDA occluder, which has no RV disc and a shorter waist, was used in the present study (its advantages for the closure of PmVSD with abnormally attached tricuspid chordae tendineae are shown in Figure 1). After occluder implantation, the tissue surrounding the defect compresses the occluder. The RV disc of the occluder can then form a smooth edge, and the stretched length of the waist is far shorter than the waist of the ADO. Therefore, abrasion of the RV disc and an excessively long waist on the chordae tendineae are avoided.
In this study, procedure success was achieved in all 20 patients diagnosed with PmVSD with abnormally attached tricuspid chordae tendineae who underwent interventional treatment using the improved PDA occluder. No residual shunt was found by left ventriculography, and no AR was found by ascending aortic angiography immediately after the procedure. These outcomes may be attributable to the use of the improved PDA occluder, which has a short rim (2 mm) in the distal part. This rim is intended to be implanted in the space between the right coronary cusp and non-coronary cusp of the aortic valve and thus is unlikely to touch the aortic valve, protecting it from AR. No severe arrhythmia occurred in any patient during or after the procedure. Complete right bundle branch block occurred in one patient and reverted to normal rhythm. Complete right bundle branch block may have occurred because there was less contact with the left bundle branch than with the VSD occluder due to the shorter rim. The other crucial advantage of the improved PDA occluder is that it does not have a proximal disc and thus does not squeeze the His bundle.
Following the procedure, TR was increased in one patient (minor regurgitation) after the procedure. At the follow-up visits 1, 3, 6, and 12 mo after device implantation and yearly thereafter, one patient with aggravated TR exhibited no obvious change. This patient was 4 years old, and the defect was relatively large. Angiography showed a defect size of 9 mm on the LV side and 3.5 mm on the RV side, and an improved 10 mm PDA occluder was placed. The postoperative TTE examination revealed that the occluder had no cutting action on the tricuspid chordae tendineae because it had no RV disc. However, the occluder was relatively large and had a longer waist, so the RV disc compressed the chordae tendineae, thus causing TR. This result suggests that for large defects, especially for the relatively weak endocardial tissue and abnormally attached tricuspid chordae tendineae of pediatric patients, interventional treatment should be performed with caution. In these cases, the use of the improved PDA occluder with the shorter waist should be considered, which highlights the fact that the interventional therapy of PmVSD should follow the principle of individualization. The choice of occluder should depend on the defect size, morphology, and clinical features.
The results after an average follow-up of 2.4 years showed that the improved PDA device can be used in the interventional therapy of PmVSD with abnormally attached tricuspid chordae tendineae. The immediate and short-term curative effect was reliable, and no serious TR or other complications were observed.
In conclusion, although there is a concern that the improved PDA occluder is not designed for the interventional therapy of PmVSD, the improved PDA occluder might provide a valid and secure option in selected patients with abnormally attached tricuspid chordae tendineae. The availability of the improved PDA occluder might allow a wider range of interventional therapy of PmVSD. However, longer follow-up in a large number of population is still warranted.
Whether certain defects such as perimembranous ventricular septal defects (PmVSD) with abnormally attached tricuspid chordae tendineae are fit for interventional treatment is still disputable. We explored the feasibility and safety of transcatheter closure of PmVSD with abnormally attached tricuspid chordae tendineae using an improved patent ductus arteriosus (PDA) occluder through an observational, single-center study.
The off-label use of various devices has been reported for the transcatheter closure of PmVSD because of serious complications, such as heart block and tricuspid regurgitation, associated with conventional ventricular septal defect devices. However, whether PmVSD with abnormally attached tricuspid chordae tendineae are suitable for interventional treatment has rarely been reported. Therefore, this study hopes to provide guidance for the choice of occluder which can be used in such defects. That might allow a wider range of interventional therapy of PmVSD.
The research objective of this study was to explore the feasibility and safety of transcatheter closure of PmVSD with abnormally attached tricuspid chordae tendineae using an improved PDA occluder.
We retrospectively analyzed 20 patients diagnosed with PmVSD with abnormally attached tricuspid chordae tendineae who underwent interventional treatment using an improved PDA occluder at our center from January 2012 to January 2016. Baseline characteristics and procedural and follow-up data were analyzed. This study is a single-center, non-randomized, observational study.
Our research found that the improved PDA occluder might provide an valid and secure option in selected patients diagnosed with PmVSD with abnormally attached tricuspid chordae tendineae. Given its nature, the present study shares all of the limitations of observational studies. In our study, the mean follow-up time was only 2.4 years. Although the major complications of PmVSD closure usually appears within this time frame, the results may have been affected. The small sample size is another limitation of this study.
In this single-center, non-randomized, observational study cohort, despite its small sample size, our study suggests that using of the improved PDA occluder for the transcatheter closure of PmVSD with abnormally attached tricuspid chordae tendineae is a safe and promising treatment option. The availability of the improved PDA occluder might allow a wider range of interventional therapy of PmVSD.
We used the improved PDA occluder for transcatheter closure of PmVSD with abnormally attached tricuspid chordae tendineae as a breakthrough point, and concluded that using of the improved PDA occluder for the transcatheter closure of PmVSD with abnormally attached tricuspid chordae tendineae is a safe and promising treatment option. However, the number of patients is too small, and the single-center observational study still has its own shortcomings. So we still believe that the highest level of evidence in clinical studies is a multi-center, prospective, randomized controlled study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Arroyo-Martinez Q, Fekaj E S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Bian YN
| 1. | Zhou T, Shen XQ, Zhou SH, Fang ZF, Hu XQ, Zhao YS, Qi SS, Zhou Z, Li J, Lv XL. Atrioventricular block: a serious complication in and after transcatheter closure of perimembranous ventricular septal defects. Clin Cardiol. 2008;31:368-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Wei Y, Wang X, Zhang S, Hou L, Wang Y, Xu Y, Sun Q, Zhao H. Transcatheter closure of perimembranous ventricular septal defects (VSD) with VSD occluder: early and mid-term results. Heart Vessels. 2012;27:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Pedra CA, Pedra SR, Esteves CA, Pontes SC, Braga SL, Arrieta SR, Santana MV, Fontes VF, Masura J. Percutaneous closure of perimembranous ventricular septal defects with the Amplatzer device: technical and morphological considerations. Catheter Cardiovasc Interv. 2004;61:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Butera G, Carminati M, Chessa M, Piazza L, Micheletti A, Negura DG, Abella R, Giamberti A, Frigiola A. Transcatheter closure of perimembranous ventricular septal defects: early and long-term results. J Am Coll Cardiol. 2007;50:1189-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 208] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 5. | Carminati M, Butera G, Chessa M, De Giovanni J, Fisher G, Gewillig M, Peuster M, Piechaud JF, Santoro G, Sievert H, Spadoni I, Walsh K; Investigators of the European VSD Registry. Transcatheter closure of congenital ventricular septal defects: results of the European Registry. Eur Heart J. 2007;28:2361-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 243] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 6. | Xu XD, Liu SX, Bai Y, Zhang M, Zhao XX, Qin YW. Decreased tricuspid regurgitation following percutaneous closure of congenital perimembranous ventricular septal defect: immediate and 6-month echocardiographic assessment. Heart Vessels. 2015;30:611-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Anderson RH, Wilcox BR. The surgical anatomy of ventricular septal defect. J Card Surg. 1992;7:17-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Mertens L, Meyns B, Gewillig M. Device fracture and severe tricuspid regurgitation after percutaneous closure of perimembranous ventricular septal defect: a case report. Catheter Cardiovasc Interv. 2007;70:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Fu YC, Bass J, Amin Z, Radtke W, Cheatham JP, Hellenbrand WE, Balzer D, Cao QL, Hijazi ZM. Transcatheter closure of perimembranous ventricular septal defects using the new Amplatzer membranous VSD occluder: results of the U.S. phase I trial. J Am Coll Cardiol. 2006;47:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Arora R, Trehan V, Kumar A, Kalra GS, Nigam M. Transcatheter closure of congenital ventricular septal defects: experience with various devices. J Interv Cardiol. 2003;16:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Christiani LA, Bergman F, Tress JC, Vanzillotta PP, Pedra CA. Severe tricuspid stenosis during percutaneous occlusion of perimembranous ventricular septal defect with the new Amplatzer device. Congenit Heart Dis. 2006;1:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Lee SM, Song JY, Choi JY, Lee SY, Paik JS, Chang SI, Shim WS, Kim SH. Transcatheter closure of perimembranous ventricular septal defect using Amplatzer ductal occluder. Catheter Cardiovasc Interv. 2013;82:1141-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Mahimarangaiah J, Subramanian A, Kikkeri Hemannasetty S, Chandra S, Karur S, Mandikal Kodandaramasastry U, Cholenahally Nanjappa M. Transcatheter closure of perimembranous ventricular septal defects with ductal occluders. Cardiol Young. 2015;25:918-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | El Said HG, Bratincsak A, Gordon BM, Moore JW. Closure of perimembranous ventricular septal defects with aneurysmal tissue using the Amplazter Duct Occluder I: lessons learned and medium term follow up. Catheter Cardiovasc Interv. 2012;80:895-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ; American Society of Echocardiography. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3183] [Cited by in RCA: 3086] [Article Influence: 140.3] [Reference Citation Analysis (0)] |
| 16. | Michel-Behnke I, Le TP, Waldecker B, Akintuerk H, Valeske K, Schranz D. Percutaneous closure of congenital and acquired ventricular septal defects--considerations on selection of the occlusion device. J Interv Cardiol. 2005;18:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Dilawar M, Numan M, El-Sisi A, Gendi SM, Ahmad Z. Percutaneous closure of ventricular septal defect associated with tunnel-shaped aneurysm using the Amplatzer duct occluder. Pediatr Cardiol. 2008;29:366-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Tan CA, Levi DS, Moore JW. Percutaneous closure of perimembranous ventricular septal defect associated with a ventricular septal aneurysm using the Amplatzer ductal occluder. Catheter Cardiovasc Interv. 2005;66:427-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |