Published online Feb 26, 2019. doi: 10.12998/wjcc.v7.i4.431
Peer-review started: November 27, 2018
First decision: December 15, 2018
Revised: December 25, 2018
Accepted: December 29, 2018
Article in press: December 30, 2018
Published online: February 26, 2019
Processing time: 92 Days and 1.7 Hours
Currently, it is difficult to predict the complications of children at the early stage of sepsis. Brighton pediatric early warning score (PEWS) is a disease risk assessment system that is simple and easy to operate, which has good sensitivity and specificity in disease recognition among children. Because detection indicators vary widely in children, a single indicator is difficult to assess the post-treatment status of children with sepsis.
To investigate the relationship between serological markers, Brighton PEWS, and death in children with sepsis after treatment.
A total of 205 children diagnosed with sepsis at our hospital were enrolled. The baseline data, serum scores, and PEWS scores were recorded. In the nested case-control study, children who died during the study period were included in an observation group. According to the matching principle, the children who were not dead in the same cohort were included in a control group. The influencing factors of death in children with sepsis after treatment and the value of each evaluation index in predicting the prognosis of children were analyzed.
A total of 96 children were enrolled in the study, including 48 each in the observation group and the control group. Multivariate logistic regression analysis indicated that antibacterial treatments within 1 h (P = 0.017), shock (P = 0.044), multiple organ dysfunction syndrome (MODS) (P = 0.027), serum procalcitonin (PCT) (P = 0.047), serum albumin (ALB) (P = 0.024), and PEWS (P = 0.012) were independent risk factors for the death of children with sepsis. The area under the curve of the combination of ALB, PCT, and PEWS to predict the death in children with sepsis was the highest (0.908).
Antibacterial treatments within 1 h, shock, MODS, PCT, ALB, and PEWS are independent risk factors for the death of children with sepsis. The predictive accuracy of the combination of PCT, ALB, and PEWS for the prognosis of children with sepsis is the best.
Core tip: Currently, it is difficult to predict the complications of children at the early stage of sepsis. Brighton pediatric early warning score (PEWS) is a risk assessment system that is simple and easy to operate, which has good sensitivity and specificity in disease recognition among children. In this study, the nested case-control study found that the use of antimicrobial agents within 1 h, shock, number of organs with dysfunction, serum procalcitonin (PCT), serum albumin (ALB), and PEWS were independent risk factors for death of children with sepsis. The combination of ALB, PCT, and PEWS can predict the prognosis of children with sepsis with good accuracy and can improve the sensitivity of prediction.
- Citation: Xie X, Li M, Xiong TT, Wang R, Xiao L. Nested case-control study of multiple serological indexes and Brighton pediatric early warming score in predicting death of children with sepsis. World J Clin Cases 2019; 7(4): 431-440
- URL: https://www.wjgnet.com/2307-8960/full/v7/i4/431.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i4.431
Sepsis is a complicated systemic inflammatory reaction syndrome and also the main cause of death in children's intensive care unit. Sepsis can be caused by a variety of pathogenic bacteria and can cause severe complications such as shock and multiple organ dysfunction syndrome (MODS), resulting in higher mortality in children[1-4]. Some non-specific inflammatory markers such as serum procalcitonin (PCT), C-reactive protein (CRP), and serum albumin (ALB) have certain value in the evaluation of post-treatment complications in children with sepsis, but they are inaccurate. Currently, it is difficult to make more accurate predictions of complications in children with sepsis at an early stage[5-7]. Therefore, the assessment of death in children with sepsis is still a difficult problem to be solved clinically. Brighton pediatric early warning score (Brighton PEWS) is a simple and easy-to-use disease risk assessment system based on adult early warning scores[8,9]. It has good sensitivity and specificity in condition recognition among children. Because detection indicators vary widely in children, a single indicator is difficult to assess the post-treatment status of children with sepsis. Therefore, the present study intended to assess the value of serological markers combined with Brighton PEWS in predicting the prognosis of children with sepsis by performing a nested case-control study.
A total of 205 children with sepsis/severe sepsis were enrolled as the study subjects (sepsis: n = 135, severe sepsis: n = 70). They were all diagnosed at Gezhouba Group Central Hospital from October 2015 to December 2017. Among them, 143 were male and 62 were female. They were aged from 6 mo to 9 years and the average age was 5.5 ± 3.3 years. The diagnostic criteria for sepsis/severe sepsis in children were based on the definition of 2005 international pediatric sepsis[10]. The inclusion criteria and exclusion criteria are shown in Table 1. All patients and their families signed an informed consent form and the study was approved by the Ethics Committee of Gezhouba Group Central Hospital.
| Inclusion criteria | Exclusion criteria |
| Data completed within 24 h after admission | Previously diagnosed with tumor disease |
| Age > 28 d | Previously diagnosed with autoimmune disease |
After admission, the children began to accept broad-spectrum antibiotic treatment and retained bacterial culture. The central venous catheter was left in place and fluid was given early to prevent shock. Endotracheal intubation for mechanical ventilation was given when severe sepsis occurs. Once hypotension or lactic acidosis was detected, fluid resuscitation was given, which made the central venous pressure reach 8 to 10 mmHg within 6 h, the mean arterial pressure > 65 mmHg, the urine volume ≥ 0.5 mL/kg per hour, and the central venous mixed oxygen saturation ≥ 70%. Vasopressors or inotropes such as dopamine may be used when volume expansion was poorly treated.
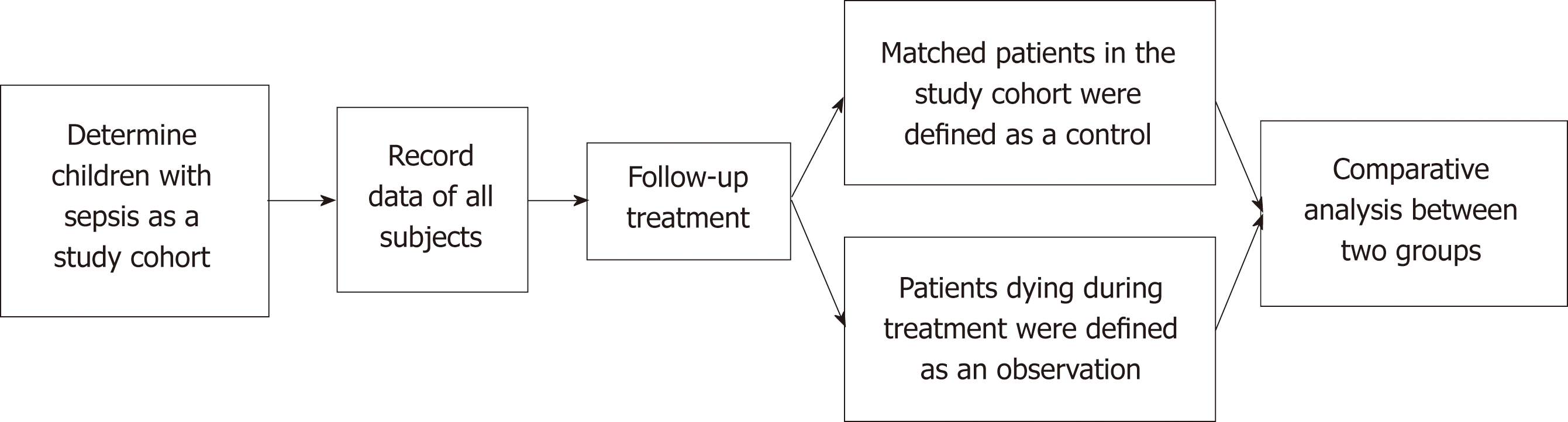
This study began with patients admitted to hospital for sepsis/severe sepsis and ended with their death or discharge. The nested case-control study was used to define the child who died during the study period as an observation group. In addition, whenever a child died in the cohort, one child with similar or identical conditions such as age, sex, and infection site was enrolled in a control group by 1:1 matching in the cohort (Figure 1)[11].
Blood sample collection: At 8 to 12 h after admission, three tubes of fasting venous blood were taken from the subjects in the early morning using vacuum blood collection tubes. There was 3 mL of blood in each tube. One of the tubes was centrifuged (3000 r/min) for 10 min to obtain the supernatant, which was frozen in a refrigerator at -20 °C.
Indicator determination: White blood cell (WBC), platelet (PLT), and hemoglobin (Hb) counts were measured using a XE-2100 automatic hematology analyzer. PCT was measured with a Roche E170 Electrochemiluminometer and supporting PCT kit. CRP was measured by the rate scattering turbidimetric method using an IMMAGE800 Automated Immunoassay Analyzer (Beckman, USA). ALB was measured using a BECK MAN LX20 automatic biochemical analyzer. Hemoglobin scavenger receptor (sCD163) was measured by enzyme-linked immunosorbent assay; the kit was supplied by THERMO of Finland and the instrument was a DENLEY DRAGON Wellscan MK3 microplate reader. Serum D-dimer (DD) was determined using a fully automated coagulation analyzer; the kits and associated control products were manufactured by SIEMENS. Serum lactic acid value (Lac) was measured using a Hitachi 7600-120 fully automated biochemical analyzer; the reagent was provided by Beijing Lederman Biochemical Co., Ltd. Creatinine (Cr) was measured using a Bio-Rad automatic biochemical analyzer.
Scoring criteria: Brighton PEWS consists of behavioral, respiratory, and circulatory parameters. The higher the score, the heavier the condition (Table 2).
| Project | 0 Points | 1 Point | 2 Points | 3 Points |
| Behavior | Normal | Somnolence | Irritability | Lethargy/coma |
| Reduced pain response | ||||
| Cardiovascular system | Pink skin | Pale skin | Gray skin | Cold skin, clammy skin |
| CRT 1-2 s | CRT 3 s | CRT 4 s | CRT ≥ 5 s | |
| Heart rate increased 20 times/min | Heart rate increased 20 times/min or bradycardia | |||
| Respiratory system | Normal | Normal respiratory frequency | Normal respiratory frequency | Normal respiratory frequency |
| Air-free depression | Increased 10 times/min, FiO2 0.3, or oxygen inhalation flow 4 L/min | Increased 20 times/min, inspiratory depression, FiO2 0.4, or oxygen inhalation flow 4 L/min | Reduced 5 times/min, with sternal inspiratory depression, moaning, FiO2 0.5, or oxygen inhalation flow 8 L/min |
Baseline data of the two groups were collected and the treatment status of the children was recorded, including the number of antibacterial treatments within 1 h, duration of mechanical ventilation, and duration of vasoactive drug maintenance. The patient's complications were recorded, including acute respiratory distress syndrome (ARDS), number of organs with dysfunction, and shock. Laboratory diagnostic indicators and PEWS scores were recorded for both groups.
Statistical analyses were performed using SPSS 19.0 software. The measurement data are expressed as the mean ± SD, and the count data are expressed as numbers (percentages). The t-test was used to compare the measurement data between the two groups. The comparison of the count data was performed by the χ2 test. Conditional logistic regression was used to further screen out the independent influencing factors of the death of children. A receiver operating characteristic (ROC) curve was established to analyze the ability of potential indicators to assess death in children with sepsis. The difference was considered statistically significant at P < 0.05.
According to the nested case-control study design, a total of 48 children died during the study period, with a total case fatality rate of 23.4%. They were defined as the observation group. A total of 48 children with similar conditions were recruited as the control group. Among the 96 children with sepsis, 64 were male and 32 were female, aged 9 mo to 5 years, with an average age of (2.5 ± 1.8) years. The underlying diseases in the 96 patients included: 65 cases of blood system diseases (67.7%), 19 cases of congenital heart disease (19.8%), 7 cases of nephrotic syndrome (7.3%), and 5 cases of neuromuscular diseases (5.2%). The location of the primary infection included: 54 cases of pulmonary infection (56.3%), 28 cases of digestive system infection (29.2%), and 14 cases of blood system infection (14.5%).
There was no significant difference between the observation group and the control group in the baseline data, primary infection site, underlying disease, duration of vasoactive drug maintenance, ARDS, WBC, Hb, CRP, or Cr (P > 0.05). The duration of mechanical ventilation, shock, MODS, PCT, sCD163, DD, Lac, and PEWS in the observation group were significantly higher than those in the control group (P < 0.05), and the number of antibacterial treatments within 1 h, PLT, and ALB were significantly lower than those in the control group (P < 0.05) (Table 3).
| Control group (n = 48) | Observation group (n = 48) | t/χ2 | P-value | |
| Baseline data | ||||
| Age (yr) | 2.48 ± 1.71 | 2.51 ± 1.79 | 0.084 | 0.933 |
| Gender (male/female) | 32/16 | 32/16 | 0.000 | 1.000 |
| Temperature (°C) | 37.58 ± 1.03 | 38.26 ± 1.45 | 1.597 | 0.114 |
| Weight (kg) | 15.24 ± 2.68 | 14.39 ± 1.47 | 1.927 | 0.057 |
| Primary infection site | ||||
| Pulmonary infection | 28 (58.3) | 31 (64.6) | 0.396 | 0.529 |
| Digestive system infections | 17 (35.4) | 16 (33.3) | 0.046 | 0.830 |
| Blood system infection | 10 (20.8) | 7 (14.6) | 0.643 | 0.423 |
| Basic disease | ||||
| Blood system diseases | 33 (70.2) | 32 (66.7) | 0.048 | 0.827 |
| Congenital heart disease | 11 (22.9) | 8 (16.7) | 0.591 | 0.442 |
| Treatment measures | ||||
| Antibacterial treatments within 1 h | 40 (83.3) | 33 (68.8) | 2.802 | 0.094 |
| Duration of mechanical ventilation (h) | 83.41 ± 35.17 | 144.36 ± 52.55 | 6.678 | 0.000 |
| Duration of vasoactive drug maintenance (h) | 74.02 ± 31.90 | 78.16 ± 42.56 | 0.539 | 0.591 |
| Complication | ||||
| ARDS | 13 (27.1) | 15 (31.3) | 0.202 | 0.653 |
| Shock | 21 (43.8) | 35 (72.9) | 8.400 | 0.004 |
| Number of organs with dysfunction | 1.76 ± 0.78 | 3.42 ± 1.51 | 6.767 | 0.000 |
| Laboratory examination | ||||
| WBC (×109/L) | 13.96 ± 2.85 | 12.74 ± 3.21 | 1.969 | 0.052 |
| PLT (×109/L) | 107.38 ± 33.70 | 85.26 ± 45.35 | 2.712 | 0.008 |
| Hb (g/L) | 105.03 ± 20.25 | 104.42 ± 22.19 | 0.141 | 0.888 |
| PCT (ng/mL) | 7.48 ± 4.51 | 19.97 ± 6.38 | 11.177 | 0.000 |
| CRP (ng/mL) | 115.67 ± 66.23 | 135.14 ± 77.62 | 1.324 | 0.189 |
| ALB (g/L) | 40.11 ± 2.57 | 33.57 ± 4.97 | 8.098 | 0.000 |
| sCD163 (mg/L) | 165.31 ± 175.26 | 253.66 ± 216.81 | 2.196 | 0.031 |
| DD (μg/L) | 802.16 ± 269.08 | 1648.93 ± 502.55 | 10.282 | 0.000 |
| Lac (mmol/L) | 3.22 ± 2.13 | 7.69 ± 5.24 | 5.475 | 0.000 |
| Cr (μmol/L) | 452.56 ± 348.72 | 336.15 ± 356.80 | 1.617 | 0.109 |
| Children's score | ||||
| PEWS | 5.35 ± 1.89 | 7.22 ± 1.43 | 5.467 | 0.000 |
Further multivariate logistic regression analysis showed that antibacterial treatments within 1 h (P = 0.017), shock (P = 0.044), number of organs with dysfunction (P = 0.027), PCT (P = 0.047), ALB (P = 0.024) and PEWS (P = 0.012) were independent influencing factors for the death of children with sepsis (Table 4).
| Assignment | P | OR | 95%CI | |
| Antibacterial treatments within 1 h | Yes = 0, No = 1 | 0.017 | 1.654 | 1.620-1.892 |
| Duration of mechanical ventilation | - | 0.182 | 1.679 | 0.848-2.675 |
| Shock | No = 0, Yes = 1 | 0.044 | 1.574 | 1.388-3.043 |
| Number of organs with dysfunction | - | 0.027 | 2.101 | 1.615-2.013 |
| PLT (109/L) | - | 0.126 | 1.086 | 0.821-1.678 |
| PCT (ng/mL) | - | 0.047 | 1.283 | 1.434-1.792 |
| ALB (g/L) | - | 0.024 | 0.842 | 0.323-2.806 |
| sCD163 (mg/L) | - | 0.563 | 1.558 | 0.466-3.850 |
| DD (μg/L) | - | 0.180 | 1.149 | 0.709-1.251 |
| Lac (mmol/L) | - | 0.115 | 1.435 | 0.562-1.785 |
| PEWS | - | 0.012 | 2.476 | 1.153-2.617 |
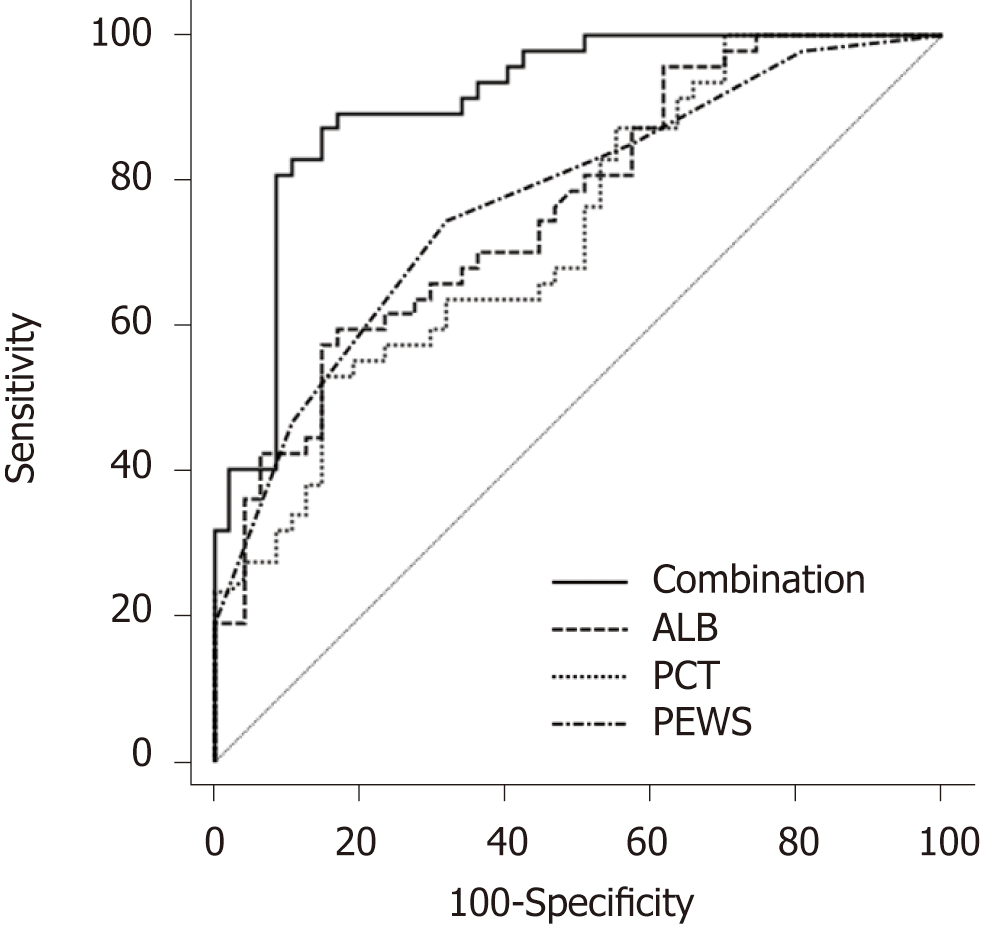
The ROC curve was used to further analyze the predictive value of PCT, ALB, and PEWS for the death of children with sepsis, as shown in Figure 2. The area under the curve (AUC) of ALB was 0.761, and the best diagnostic cutoff value was 35.20 g/L. The sensitivity was 57.45%, and the specificity was 85.11%. The AUC of PCT was 0.730, and the best diagnostic cutoff value was 59.65 μg/L. The sensitivity was 53.20%, and the specificity was 85.10%. The AUC of PEWS was 0.771, and the best diagnosis cutoff value was 6.5 points. The sensitivity was 74.50%, and the specificity was 68.10%. The AUC of the combination of ALB, PCT, and PEWS predicting the death in children with sepsis was 0.908. The sensitivity was 87.23%, and the specificity was 85.11%.
Sepsis is an inflammatory reaction caused by infections, which are mainly caused by bacteria in the clinic[12]. Early detection, diagnosis, and treatment have significant effects on post-treatment complications in children[13,14]. In the early stage of the disease, the clinical manifestations in children are nonspecific. However, once the inflammatory cascade reaction is triggered, the condition of children will decrease sharply. Even if the treatment is delayed for several hours, the mortality rate of children will be significantly increased[15]. Therefore, early accurate assessment of the prognosis of children with sepsis is the most effective way to improve the complications in children with sepsis. However, there is no specific laboratory test to predict the prognosis of children with sepsis. In addition, some clinical symptoms of sepsis in children are often similar to other diseases, which increases the misdiagnosis rate of sepsis undoubtedly. Current studies show that PLT, CRP, PCT, ALB, sCD163, and PEWS can be used to assess the recovery of children with sepsis after treatment[16-18]. However, throughout the disease cycle, children's indicators vary widely by individual and environmental influences. Current assessment methods are not sufficient to accurately assess the degree of recovery after treatment in children with sepsis. In order to find a more accurate method of assessing the prognosis of children with sepsis, this nested case-control study was conducted to evaluate serologic markers in combination with PEWS in predicting death in children with sepsis.
Epidemiological data from the United States showed that the mortality rate of children with sepsis was 10.3%. The mortality rate of children with underlying diseases increased to 12.8%. In the present study, 48 of 205 children died and the mortality rate was 23.4%, which was relatively high. The potential reason might be related to the late diagnosis. As the clinical symptoms of sepsis were mainly fever, the disease was difficult to identify early so that it was easy to misdiagnose and mistreat. Because the clinical symptoms of sepsis are mainly caused by fever, it is difficult to pay attention to the early stage of the disease. Hence, sepsis in children is easy to be misdiagnosed or mistreated.
Sepsis in children develops rapidly after infection occurs because children have weaker immune systems. Studies have revealed that immediate antibacterial treatment within 1 h could clear the lesion promptly, which preventing further deterioration[19]. According to the pathogenesis of sepsis, sepsis is most often associated with insufficient tissue perfusion pressure and tissue hypoxia[20]. Therefore, correcting shock and appropriate ventilation should also be stressed in the early treatment. When the infection control of children with sepsis is not ideal or the condition deteriorates, it may appears as shock and MODS[21], which often indicate a poor prognosis of sepsis, especially when the number of organs with dysfunction increases. Serological indicators can objectively indicate changes in the sepsis condition and provide a direct basis for assessing the outcome of the disease after treatment. For example, CRP and PCT are clinically widely used indicators of inflammation[22,23]. They have a high specificity in the diagnosis of the degree of inflammation[24]. In addition, blood system disease is the most common underlying disease in children with sepsis. Therefore, the degree of thrombocytopenia and changes in DD can reflect the disorders of the blood system and abnormal coagulation function, suggesting the regression of the disease[25].
Based on the analysis of existing studies on the death of children with sepsis, this study analyzed WBC, PLT, Hb, CRP, PCT, ALB, sCD163, DD, Lar, Cr, and PEWS between two groups and performed a multivariate logistic regression analysis for screening the impact factors of the prognosis of sepsis. It is shown that the antibacterial treatments within 1 h, shock, MODS, PCT, ALB, and PEWS were independent influencing factors of death in children. The reason why PEWS is an independent factor for the death of children may be because the scoring system is easy to operate and can monitor children's condition continuously and dynamically without using special equipment[26]. PEWS is not affected by age and is suitable for general wards. It can identify children who need intervention in the early stage, and there is good repeatability between different operators. Studies have shown that it has a sensitivity of 70% and a specificity of 90% in critically ill children who need to be transferred to the ICU for emergency care[27]. This allows doctors to assess children's condition more objectively and accurately, and avoids empirical subjective judgment. Therefore, early PEWS scores on admission can achieve early intervention, thereby improving the complications of death in children. PCT is a calcitonin precursor protein secreted by thyroid C cells[28]. Inflammatory mediators such as bacterial endotoxin and interleukin can stimulate the secretion of PCT by neuroendocrine cells of the liver, kidney, and spleen. When the level is raised, the degree of inflammatory reaction of the body can be more accurately indicated. During the process of sepsis, capillary vascular endothelial cells are destroyed due to the release of a large number of inflammatory factors in the blood, resulting in increased capillary permeability of the whole body, and hypoproteinemia caused by leakage of intravascular albumin. Hypoproteinemia can cause a decrease in plasma osmotic pressure, a reduction in effective circulating blood volume, and multiple organ dysfunction[29]. Therefore, a decrease in ALB levels can indirectly indicate the extent of inflammatory infections[30].
The detection indicators vary widely among children and the children’s compensation mechanism is more complicated than that of adults, which will affect the accuracy of PCT, ALB, and PEWS in predicting the prognosis of children[31]. Therefore, the accuracy of a single indicator in predicting the death of children with sepsis is poor, and the AUC is lower. The results of this study showed that the AUC was the largest when PCT and ALB were combined with PEWS, and the sensitivity was significantly higher compared to any of the single indicators. Therefore, the combined diagnosis of the three indicators can reduce the impact of differences in organization and body compensation and improve the accuracy.
In summary, the present nested case-control study demonstrates that antibacterial treatments within 1 h, shock, MODS, PCT, ALB, and PEWS are independent risk factors for death in children with sepsis. The combined of PCT, ALB, and PEWS for predicting the prognosis of children with sepsis is better than any of the single detection indicators.
Sepsis is an inflammatory reaction caused by infection, and the microorganisms that cause infection are mainly bacteria. In the early stage of the disease, the clinical manifestations in children are nonspecific. However, once the inflammatory reaction is stimulated, even if the treatment is delayed several hours, the mortality of children can be significantly increased. Therefore, accurate early assessment of the prognosis of children with sepsis is the most effective way to improve the complications of children with sepsis.
There are currently no specific laboratory tests or markers that can early predict the prognosis of children with sepsis. Moreover, some clinical symptoms of children with sepsis are often similar to those of other diseases, which increases the difficulty in diagnosing sepsis. Currently, studies have shown that platelet, C-reactive protein, serum procalcitonin (PCT), serum albumin (ALB), hemoglobin scavenger receptor, and pediatric early warning score (PEWS) can assess the recovery of children with sepsis after treatment, but the children's various indicators are affected by individual and environmental changes. Therefore, the clinic needs a method to make a more accurate prediction of the complications of children with sepsis at an early stage.
The present study intended to conduct a nested case-control study to assess the value of serum markers in combination with Brighton PEWS in predicting the prognosis of children with sepsis, in order to explore whether it is a more accurate means of assessing the prognosis of children with sepsis or not.
A total of 205 children diagnosed with sepsis were enrolled. After admission, the patient began broad-spectrum antibiotic treatment and retained bacterial culture. The central venous catheter was indwelled and early rehydration was given to prevent shock. In the nested case-control study, patients who died during the study cohort were included in a study group, and children who did not die in the same cohort were defined as a control group. Baseline data, serological markers, and PEWS scores were recorded for the subjects. Conditional logistic regression was used to analyze the influencing factors of death in children with sepsis after treatment. Receiver operating characteristic (ROC) curves were established to evaluate the value of the indicators to predict the prognosis of the children.
There were 48 children each in the experimental group and the control group. Multivariate logistic regression analysis indicated that antibacterial treatments within 1 h, shock, multiple organ dysfunction syndromes (MODS), PCT, ALB, and PEWS were independent influencing factors of death in children. ROC curve analysis found that the area under the curve of the combination of ALB, PCT, and PEWS in predicting the death in children with sepsis was the highest, and the sensitivity was significantly higher than those of the three individuals. Therefore, the combination of the three indicators can reduce the impact of differences in organizational and physical compensation on the assessment results and improve the accuracy.
The present study found that antibacterial treatments within 1 h, shock, MODS, PCT, ALB, and PEWS were independent risk factors for the death of children with sepsis. The combination of PCT, ALB, and PEWS for predicting the prognosis of children with sepsis can improve the accuracy of the prediction.
The results of the study need further evaluation because the sample size was limited. However, the results suggest that PEWS score combined with serological indicators can improve the accuracy of prognosis prediction in children with sepsis, which provides useful information for the treatment of children with sepsis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kantsevoy S, Snyder J, Tokunaga Y S- Editor: Wang JL L- Editor: Wang TQ E- Editor: Tan WW
| 1. | Zonneveld R, Martinelli R, Shapiro NI, Kuijpers TW, Plötz FB, Carman CV. Soluble adhesion molecules as markers for sepsis and the potential pathophysiological discrepancy in neonates, children and adults. Crit Care. 2014;18:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 2. | Cuenca AG, Joiner DN, Gentile LF, Cuenca AL, Wynn JL, Kelly-Scumpia KM, Scumpia PO, Behrns KE, Efron PA, Nacionales D, Lui C, Wallet SM, Reeves WH, Mathews CE, Moldawer LL. TRIF-dependent innate immune activation is critical for survival to neonatal gram-negative sepsis. J Immunol. 2015;194:1169-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Bogale TN, Worku AG, Bikis GA, Kebede ZT. Why gone too soon? Examining social determinants of neonatal deaths in northwest Ethiopia using the three delay model approach. BMC Pediatr. 2017;17:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Sankar MJ, Natarajan CK, Das RR, Agarwal R, Chandrasekaran A, Paul VK. When do newborns die? A systematic review of timing of overall and cause-specific neonatal deaths in developing countries. J Perinatol. 2016;36 Suppl 1:S1-S11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | van Paridon BM, Sheppard C, G GG, Joffe AR; Alberta Sepsis Network. Timing of antibiotics, volume, and vasoactive infusions in children with sepsis admitted to intensive care. Crit Care. 2015;19:293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Hahn WH, Song JH, Kim H, Park S. Is procalcitonin to C-reactive protein ratio useful for the detection of late onset neonatal sepsis? J Matern Fetal Neonatal Med. 2018;31:822-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Yang AP, Liu J, Yue LH, Wang HQ, Yang WJ, Yang GH. Neutrophil CD64 combined with PCT, CRP and WBC improves the sensitivity for the early diagnosis of neonatal sepsis. Clin Chem Lab Med. 2016;54:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Agulnik A, Méndez Aceituno A, Mora Robles LN, Forbes PW, Soberanis Vasquez DJ, Mack R, Antillon-Klussmann F, Kleinman M, Rodriguez-Galindo C. Validation of a pediatric early warning system for hospitalized pediatric oncology patients in a resource-limited setting. Cancer. 2017;123:4903-4913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Miranda JOF, Camargo CL, Nascimento CL Sobrinho, Portela DS, Monaghan A. Accuracy of a pediatric early warning score in the recognition of clinical deterioration. Rev Lat Am Enfermagem. 2017;25:e2912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Brilli RJ, Goldstein B. Pediatric sepsis definitions: past, present, and future. Pediatr Crit Care Med. 2005;6:S6-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Ernster VL. Nested case-control studies. Prev Med. 1994;23:587-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 192] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | DeAngelo AJ, Bell DG, Quinn MW, Long DE, Ouellette DR. Erythropoietin response in critically ill mechanically ventilated patients: a prospective observational study. Crit Care. 2005;9:R172-R176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Chun K, Syndergaard C, Damas C, Trubey R, Mukindaraj A, Qian S, Jin X, Breslow S, Niemz A. Sepsis Pathogen Identification. J Lab Autom. 2015;20:539-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Plunkett A, Tong J. Sepsis in children. BMJ. 2015;350:h3017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016;353:i1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 662] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 16. | Weiss SL, Fitzgerald JC, Balamuth F, Alpern ER, Lavelle J, Chilutti M, Grundmeier R, Nadkarni VM, Thomas NJ. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med. 2014;42:2409-2417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 334] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 17. | Sapa A, Rak A, Wybieralska M, Machoń J, Krzywonos-Zawadzka A, Zawadzki K, Wełna M, Woźniak M. Diagnostic usefulness of sCD163, procalcitonin and neopterin for sepsis risk assessment in critically ill patients. Adv Clin Exp Med. 2017;26:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Lu Q, Duan H, Yu J, Yao Y. Are Global Coagulation and Platelet Parameters Useful Markers for Predicting Late-Onset Neonatal Sepsis? Clin Lab. 2016;62:73-79. [PubMed] |
| 19. | Vincent JL, De Backer D, Wiedermann CJ. Fluid management in sepsis: The potential beneficial effects of albumin. J Crit Care. 2016;35:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 20. | Schlapbach LJ, Straney L, Alexander J, MacLaren G, Festa M, Schibler A, Slater A; ANZICS Paediatric Study Group. Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002-13: a multicentre retrospective cohort study. Lancet Infect Dis. 2015;15:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 229] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 21. | Castillo L. High elevated ferritin levels and the diagnosis of HLH/Sepsis/SIRS/MODS/MAS. Pediatr Blood Cancer. 2008;51:710; author reply 710-710; author reply 711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Carcillo JA, Simon DW, Podd BS. How We Manage Hyperferritinemic Sepsis-Related Multiple Organ Dysfunction Syndrome/Macrophage Activation Syndrome/Secondary Hemophagocytic Lymphohistiocytosis Histiocytosis. Pediatr Crit Care Med. 2015;16:598-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Garnacho-Montero J, Huici-Moreno MJ, Gutiérrez-Pizarraya A, López I, Márquez-Vácaro JA, Macher H, Guerrero JM, Puppo-Moreno A. Prognostic and diagnostic value of eosinopenia, C-reactive protein, procalcitonin, and circulating cell-free DNA in critically ill patients admitted with suspicion of sepsis. Crit Care. 2014;18:R116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 24. | Hedegaard SS, Wisborg K, Hvas AM. Diagnostic utility of biomarkers for neonatal sepsis--a systematic review. Infect Dis (Lond). 2015;47:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Meisner M, Tschaikowsky K, Palmaers T, Schmidt J. Comparison of procalcitonin (PCT) and C-reactive protein (CRP) plasma concentrations at different SOFA scores during the course of sepsis and MODS. Crit Care. 1999;3:45-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 225] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Larkin CM, Santos-Martinez MJ, Ryan T, Radomski MW. Sepsis-associated thrombocytopenia. Thromb Res. 2016;141:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Fuijkschot J, Vernhout B, Lemson J, Draaisma JM, Loeffen JL. Validation of a Paediatric Early Warning Score: first results and implications of usage. Eur J Pediatr. 2015;174:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Rivero-Martín MJ, Prieto-Martínez S, García-Solano M, Montilla-Pérez M, Tena-Martín E, Ballesteros-García MM. [Results of applying a paediatric early warning score system as a healthcare quality improvement plan]. Rev Calid Asist. 2016;31 Suppl 1:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Liu Y, Yang W, Wei J. Guiding Effect of Serum Procalcitonin (PCT) on the Antibiotic Application to Patients with Sepsis. Iran J Public Health. 2017;46:1535-1539. [PubMed] |
| 30. | Conner BJ. Treating Hypoalbuminemia. Vet Clin North Am Small Anim Pract. 2017;47:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Seo MH, Choa M, You JS, Lee HS, Hong JH, Park YS, Chung SP, Park I. Hypoalbuminemia, Low Base Excess Values, and Tachypnea Predict 28-Day Mortality in Severe Sepsis and Septic Shock Patients in the Emergency Department. Yonsei Med J. 2016;57:1361-1369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |