Published online Feb 6, 2019. doi: 10.12998/wjcc.v7.i3.270
Peer-review started: October 31, 2018
First decision: November 27, 2018
Revised: December 8, 2018
Accepted: December 12, 2018
Article in press: December 12, 2018
Published online: February 6, 2019
Processing time: 92 Days and 11.6 Hours
Polyomavirus-associated nephropathy is a leading cause of kidney allograft failure. Therapeutic options are limited and prompt reduction of the net state of immunosuppression represents the mainstay of treatment. More recent application of aggressive screening and management protocols for BK-virus infection after renal transplantation has shown encouraging results. Nevertheless, long-term outcome for patients with BK-viremia and nephropathy remains obscure. Risk factors for BK-virus infection are also unclear.
To investigate incidence, risk factors, and outcome of BK-virus infection after kidney transplantation.
This single-centre observational study with a median follow up of 57 (31-80) mo comprises 629 consecutive adult patients who underwent kidney transplantation between 2007 and 2013. Data were prospectively recorded and annually reviewed until 2016. Recipients were periodically screened for BK-virus by plasma quantitative polymerized chain reaction. Patients with BK viral load ≥ 1000 copies/mL were diagnosed BK-viremia and underwent histological assessment to rule out nephropathy. In case of BK-viremia, immunosuppression was minimized according to a prespecified protocol. The following outcomes were evaluated: patient survival, overall graft survival, graft failure considering death as a competing risk, 30-d-event-censored graft failure, response to treatment, rejection, renal function, urologic complications, opportunistic infections, new-onset diabetes after transplantation, and malignancies. We used a multivariable model to analyse risk factors for BK-viremia and nephropathy.
BK-viremia was detected in 9.5% recipients. Initial viral load was high (≥ 10000 copies/mL) in 66.7% and low (< 10000 copies/mL) in 33.3% of these patients. Polyomavirus-associated nephropathy was diagnosed in 6.5% of the study population. Patients with high initial viral load were more likely to experience sustained viremia (95% vs 25%, P < 0.00001), nephropathy (92.5% vs 15%, P < 0.00001), and polyomavirus-related graft loss (27.5% vs 0%, P = 0.0108) than recipients with low initial viral load. Comparison between recipients with or without BK-viremia showed that the proportion of patients with Afro-Caribbean ethnicity (33.3% vs 16.5%, P = 0.0024), panel-reactive antibody ≥ 50% (30% vs 14.6%, P = 0.0047), human leukocyte antigen (HLA) mismatching > 4 (26.7% vs 13.4%, P = 0.0110), and rejection within thirty days of transplant (21.7% vs 9.5%; P = 0.0073) was higher in the viremic group. Five-year patient and overall graft survival rates for patients with or without BK-viremia were similar. However, viremic recipients showed higher 5-year crude cumulative (22.5% vs 12.2%, P = 0.0270) and 30-d-event-censored (22.5% vs 7.1%, P = 0.001) incidences of graft failure than control. In the viremic group we also observed higher proportions of recipients with 5-year estimated glomerular filtration rate < 30 mL/min than the group without viremia: 45% vs 27% (P = 0.0064). Urologic complications were comparable between the two groups. Response to treatment was complete in 55%, partial in 26.7%, and absent in 18.3% patients. The nephropathy group showed higher 5-year crude cumulative and 30-d-event-censored incidences of graft failure than control: 29.1% vs 12.1% (P = 0.008) and 29.1% vs 7.2% (P < 0.001), respectively. Our multivariable model demonstrated that Afro-Caribbean ethnicity, panel-reactive antibody > 50%, HLA mismatching > 4, and rejection were independent risk factors for BK-virus viremia whereas cytomegalovirus prophylaxis was protective.
Current treatment of BK-virus infection offers sub-optimal results. Initial viremia is a valuable parameter to detect patients at increased risk of nephropathy. Panel-reactive antibody > 50% and Afro-Caribbean ethnicity are independent predictors of BK-virus infection whereas cytomegalovirus prophylaxis has a protective effect.
Core tip: Polyomavirus-associated nephropathy is a leading cause of kidney transplant failure. A systematic screening and treatment protocol in line with current international guideline was evaluated. Our results showed that despite early diagnosis and prompt intervention, graft-related outcomes for patients with BK-virus infection remain substantially inferior to control. We confirmed that initial viral load ≥ 10000 copies/mL is highly predictive of nephropathy. New putative risk factors for BK-viremia and nephropathy were also identified. Properly designed large multi-centre studies are warranted to further investigate individual susceptibility to BK-virus infection and validate alternative antiviral therapies.
- Citation: Favi E, Puliatti C, Sivaprakasam R, Ferraresso M, Ambrogi F, Delbue S, Gervasi F, Salzillo I, Raison N, Cacciola R. Incidence, risk factors, and outcome of BK polyomavirus infection after kidney transplantation. World J Clin Cases 2019; 7(3): 270-290
- URL: https://www.wjgnet.com/2307-8960/full/v7/i3/270.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i3.270
Kidney transplantation is the best treatment option for patients with end-stage renal disease[1]. However, due to chronic exposure to powerful immunosuppressive agents, transplant recipients have a greater risk of cardiovascular disease, malignancies, and infections than the general population[2]. Polyomavirus infection is serious complication of immunosuppression as it is recognized as a leading cause of impaired graft function and premature transplant loss[3].
There are three main members of the polyomavirus family infecting humans: BK-virus (BKV), JC-virus, and Merkel cell polyomavirus. The majority of the infections following kidney transplantation are attributed to BKV. An ubiquitous double-stranded DNA pathogen, it is usually contracted during childhood[4] and is detectable in up to 80% of adults undergoing serological screening[5]. Following contact with the host, the virus reaches the kidney via haematogenous spread where it establishes a latent or sub-lytic infection in the tubular cells[6]. In immunocompromised individuals, the virus can undergo uncontrolled replication causing cell lysis and necrosis through direct and indirect mechanisms[7]. In the allograft, this process may eventually lead to irreversible tissue damage and progressive loss of function[8].
Immunosuppression is considered the most important risk factor for post-transplant BKV infection[9]. Nevertheless, more recent evidence shows that interactions between immunosuppressive drugs and BKV are not as simple as initially postulated[10-12]. Clinical practice has also evolved and current data may not reflect previous reports in the literature.
There is still no consensus in the transplant community regarding the best screening and treatment strategies for BKV infection or polyomavirus-associated nephropathy (PVAN). Most authors recommend periodic assessment by quantitative polymerized chain reaction (qPCR) analysis of blood samples but target population, exact timing, and length of the screening program remain debated[13-15]. Reducing the net state of immunosuppression is the mainstay of treatment[16]. Dose adjustment, drug withdrawal, or substituting agents have been proposed with mixed results[13]. Proliferation signal inhibitors (i.e. sirolimus and everolimus) have shown encouraging results but solid evidence is missing[17]. Unfortunately, benefits arising from antiviral therapies such as cidofovir, quinolones, leflunomide or intravenous immunoglobulin remain controversial. Sub-optimal results have been reported and efficacy is overall limited by drug-induced nephrotoxicity and side effects[18].
Since 2007, we have been enforcing a strict surveillance policy and a step by step management strategy for BKV infection in all kidney transplant recipients followed up at our centre. This experience is herein reported.
In this single-centre observational cohort study we enrolled adult patients who had undergone kidney transplantation at The Royal London Hospital (London, UK), between April 2007 and March 2013. Considering previous studies on the same topic, a minimum sample size of 400 subjects was established. Inclusion criteria were: living or deceased donor kidney transplant and recipient age ≥ 18 years. Recipients of other solid organ transplants or the second kidney transplant for those transplanted twice during the study period were excluded. Donor data, organ details, recipient characteristics, and transplant outcomes were prospectively recorded on a central database as per standard practice at our institution by dedicated staff and annually reviewed by the authors until June 2016. Patients were all consented at the time of activation on the national transplant waiting list. According to Barts Health NHS Trust General Data Protection Act, General Data Protection and Regulation 2016, and Data Protection Act 2018, they were aware that their anonymized data including viral status as well as other biomedical parameters would have been used for research purpose. Patients who did not want their personal information to be used for planning and research were given the possibility to express their preference under the National Data Opt-out Programme. Based on the observational nature of the study and given the fact that no off-label medications were used to treat BKV-related complications, the protocol was not reviewed by our Ethical Committee. The study was conducted according to the World Medical Association Declaration of Helsinki and applicable regulatory requirements.
After transplantation, all patients were screened for BKV replication by plasma qPCR. The test was performed every two weeks during the first three months, monthly from month three to month six, every two months from month six to month twelve, and during investigation of worsening graft function (i.e. serum creatinine concentration increase ≥ 30% from nadir) thereafter. Recipients with BKV plasma qPCR ≥ 1000 copies/mL were diagnosed BKV viremia and were further assessed by allograft histology (ultrasound-guided biopsy). Initial viral load was classified as high (≥ 10000 copies/mL) or low (< 10000 copies/mL). Episodes of viremia were defined as early (< 180 d from transplant) or late (≥ 180 d from transplant) and as transient (< 3 consecutive weeks) or sustained (≥ 3 consecutive weeks). Patients with viremia and positive histology were diagnosed PVAN. PVAN was always confirmed by SV40 immunohistochemistry[19].
Recipients with BKV viremia (regardless of symptoms or histology) had their immunosuppressive therapy progressively modified according to the following scheme: (1) 50% reduction of mycophenolate mofetil (MMF) or azathioprine; (2) withdrawal of MMF or azathioprine; (3) 30% reduction of cyclosporine or tacrolimus; (4) 50% reduction of cyclosporine or tacrolimus; and (5) switch from tacrolimus to cyclosporine. Sequential changes were made every two weeks according to clinical findings. Response to treatment was defined as: (a) Complete (no viremia with restored graft function); (b) partial (no viremia with permanently impaired graft function); and (c) absent (persistent viremia with progressive graft failure due to PVAN).
Aim of the present study was to assess incidence, risk factors, and outcome of BKV viremia and PVAN in a cohort of kidney transplant recipients managed using a systematic anti-BKV protocol. The following outcome measures were evaluated: patient survival, overall graft survival, graft failure considering death without graft failure as a competing risk, 30-d-event-censored graft failure (excluding any graft losses recorded within thirty days of transplant), biopsy-proven rejection (BPR), proportion of patients with 5-year Modification of Diet in Renal Disease (MDRD)[20] estimated glomerular filtration rate (eGFR) < 30 mL/min per 1.73 m2, response to reduction of the net state of immunosuppression, urologic complications (ureteral leakage or stenosis), opportunistic infections, new-onset diabetes after transplantation (NODAT), and malignancies. Results were compared between groups.
Relationships between BKV-related clinical findings (i.e. onset of viremia, initial viral load, duration of viremia or allograft histology) and course of the infection were also investigated.
Risk factors analysis for BKV viremia and PVAN included: donor age, donor gender, donor-recipient human leukocyte antigen (HLA) mismatching (cumulative, HLA-A, HLA-B, and HLA-DR), panel-reactive antibody (PRA) test (≤ 50% vs > 50%), cold ischemia time, recipient age, recipient gender, donor-recipient gender mismatching, recipient ethnicity (Caucasian vs Afro-Caribbean), recipient body mass index, dialysis modality, pre-transplant diabetes, cytomegalovirus (CMV) donor-recipient immunization, CMV prophylaxis, delayed graft function (DGF), and BPR within thirty days of transplant.
Primary non-function (PNF) was defined as graft function unable to prevent continued renal replacement therapy or requiring re-transplantation when surgical causes of renal failure or rejection were excluded (by imaging, exploration or histology). DGF was defined as the need for dialysis during the first week after surgery. Diagnosis of rejection was based on serum creatinine concentration increase ≥ 30% from nadir and always confirmed by histology (BPR)[21]. Renal function was measured by serum creatinine concentration (mmol/L) and MDRD eGFR formula. NODAT was diagnosed using the American Diabetes Association criteria (updated in 2003) for the diagnosis of diabetes mellitus[22].
As induction treatment, patients received intravenous (IV) methylprednisolone (500 mg on day 0, 250 mg on day 1, and 125 mg on day 2) in association with one of the following: (1) IV rabbit anti-thymocyte globulin (Thymoglobulin®, Genzyme, Cambridge, MA) 1 mg/kg/day from day 0 to day 4; (2) IV basiliximab (Simulect®, Novartis, Basel, Switzerland) 20 mg on days 0 and day 4; (3) IV daclizumab (Zenapax®, Roche, Basel, Switzerland) 1 mg/kg on days 0, 14, 28, 42, and 56; (4) IV rituximab (Rituxan®, Genentech, San Francisco, CA) 375 mg/m2 three weeks before transplant plus intravenous basiliximab 20 mg on days 0 and day 4; (5) IV alemtuzumab (Campath®, Millennium and ILEX Partners, Cambridge, MA) 30 mg on day 0; or (6) IV muromonab-CD3 (Orthoclone OKT®3, Centocor Ortho Biotech Products, Raritan, NJ) 5 mg/kg/day from day 0 to day 7.
As maintenance immunosuppression, we administered one of the following combinations: (1) cyclosporine, MMF, and steroids; (2) cyclosporine, azathioprine, and steroids; (3) tacrolimus, MMF, and steroids; d) tacrolimus, azathioprine, and steroids; (4) cyclosporine and MMF; or (5) tacrolimus and azathioprine. Cyclosporine (Neoral®, Novartis, Basel, Switzerland) was orally given 5 mg/kg twice a day from day 0 and the dose was adjusted to achieve a target trough level of 200 ng/mL during the first month and 150-100 ng/mL thereafter. Tacrolimus (Prograf®, Astellas Pharma, Deerfield, IL) was administered orally 0.1 mg/kg twice daily from day 0 and the dose was adjusted to achieve a target trough level of 8-12 ng/mL during the first month and 5-8 ng/mL thereafter. MMF (Myfenax®, Teva, Petach Tikva, Israel) was orally given 1000 mg twice a day from day 0. In Afro-Caribbean patients MMF daily dose was increased to 3000 mg. Azathioprine (Imuran®, Prometheus Laboratories, San Diego, CA) was orally administered 5 mg/kg per day from day 0. Prednisone was orally given 20 mg/d starting on day 3 and progressively tapered to 5 mg a day after one month of follow up.
Patients were all given prophylaxis for pneumocystis jirovecii pneumonia with oral trimethoprim/sulfamethoxazole 160-800 mg every other day for three months. Recipients at increased risk of CMV disease (i.e. donor CMV positive/recipient CMV negative immunization, rabbit anti-thymocyte globulin induction or anti-rejection treatment) also received antiviral prophylaxis with oral valganciclovir (dose titrated according to renal function) for six months.
Categorical and ordinal outcomes were described using proportions, medians, 1st-3rd interquartile range and were compared using Fisher’s exact, Chi-square or Mann-Whitney U test as appropriate. Patient survival and overall graft survival were analyzed with the Kaplan-Meier method. Survival curves were compared with log-rank. Graft failure was also analysed considering death without graft failure as a competing risk. The same analysis was conducted excluding any graft failures within thirty days of transplant (30-d-event-censored graft failure). Gray’s test was used to compare the crude cumulative incidence curves. Competing risks were also used to estimate the crude cumulative incidence of BKV viremia and PVAN. We assessed the association of a pool of variables with the risk of BKV viremia and PVAN. According to standard guidelines on the number of variables that can be considered in relation to the dependent variables (at least 5 events per variable), a maximum of 12 variables for BKV viremia and of 4 variables for PVAN could be considered. According to this requirement, a mild univariate screening for covariates having a P-value < 0.8 for viremia and < 0.5 for PVAN was used for building the multivariable models. Primary renal disease, renal replacement therapy, donor type, induction, and maintenance immunosuppression were not considered. Continuous variables were dichotomized according to generally accepted cut-off values. Moreover, only HLA mismatching > 4 was considered among the HLA-related variables. We chose a backward method based on AIC criterion to select significant independent covariates. Bootstrap was used to evaluate the stability of the results by counting the percentage of bootstrap samples for which each covariate was selected. Bootstrap with variable selection was also used to estimate a C-index corrected for optimism. Odds ratios, and 95%CI were reported for variables in the final model. The same strategy was used in PVAN analysis, excluding viral load. Penalized regression (Firth) was used to estimate the association with viral load, as almost perfect separation was present. We performed analyses with R software[23]. The statistical methods of this study were reviewed by a senior biomedical statistician (Federico Ambrogi, Associate Professor from the Laboratory of Medical Statistics, Biometrics and Epidemiology of the Department of Clinical Sciences and Community Health of the University of Milan).
From April 2007 to March 2013, we performed 639 consecutive kidney transplants in 633 patients. According to our inclusion/exclusion criteria, 629/633 (99.4%) subjects were enrolled into the study. Reasons for exclusion were recipient age < 18 years (n = 2) and previous liver transplant (n = 2).
Three-hundred-ninety-five/629 (62.8%) patients received a kidney from a deceased donor (263/395, 66.6% DBD; 132/395, 33.4% DCD) and 234/629 (37.2%) from a living donor (153/234, 65.4% related; 81/234, 34.6% unrelated). Median recipient and donor age were 47 (36-55) and 48 (39-57) years whereas median HLA mismatching and cold ischemia time were 3 (2-4) and 12 (4-16) h, respectively. Baseline recipient, donor, and transplant-related data of the study cohort are detailed in Table 1.
| Variables | Median (IQR) or n (%) |
| Patients | 629 |
| Recipient Male : Female | 372:257 |
| Caucasian ethnicity | 279/629 (44.3) |
| Afro-Caribbean ethnicity | 114/629 (18.1) |
| Recipient age (yr) | 47 (36-55) |
| Recipient body mass index (kg/m²) | 25.2 (22.2-27.5) |
| Pre-transplant diabetes | 83/629 (13.2) |
| Pre-transplant cardiovascular disease | 62/629 (9.9) |
| Recipient cytomegalovirus IgG positive | 431/629 (68.5) |
| Glomerular disease | 185/629 (29.4) |
| Polycystic kidney disease | 71/629 (11.3) |
| Tubular-interstitial nephropathy | 81/629 (12.9) |
| Diabetic nephropathy | 49/629 (7.8) |
| Hypertension | 55/629 (8.7) |
| Unknown renal disease | 141/629 (22.4) |
| Other renal disease | 47/629 (7.5) |
| Haemodialysis | 295/629 (46.9) |
| Peritoneal dialysis | 136/629 (21.6) |
| Peritoneal dialysis and haemodialysis | 118/629 (18.8) |
| Pre-emptive transplant | 80/629 (12.7) |
| Panel-reactive antibody > 50% | 101/629 (16.1) |
| Primary transplant | 550/629 (87.4) |
| Deceased donor | 395/629 (62.8) |
| Donation after brain death donor | 263/629 (41.8) |
| ABO-incompatible transplant | 25/629 (4.0) |
| Donor age (yr) | 48 (39-57) |
| Donor-recipient gender mismatch | 340/629 (54.1) |
| Donor cytomegalovirus IgG positive | 354/629 (56.3) |
| Cytomegalovirus D+R- immunization | 83/629 (13.2) |
| Cold ischemia time (hours) | 12 (4-16) |
| Cold ischemia time >12 h | 282/629 (44.8) |
| Cumulative HLA mismatch | 3 (2-4) |
| HLA mismatch > 4 | 92/629 (14.6) |
| Induction treatment | |
| Anti-IL2-receptor antagonist | 431/629 (68.5) |
| Rabbit anti-thymocyte globulin | 169/629 (26.9) |
| Rituximab | 25/629 (4.0) |
| Alemtuzumab | 3/629 (0.5) |
| Muromonab-CD3 | 1/629 (0.2) |
| Maintenance immunosuppression | |
| CyA-MMF-steroid | 422/629 (67.1) |
| CyA-AZA-steroid | 3/629 (0.5) |
| Tacrolimus-MMF-steroid | 109/629 (17.3) |
| Tacrolimus-AZA-steroid | 79/629 (12.6) |
| Tacrolimus-MMF | 12/629 (1.9) |
| Tacrolimus-AZA | 2/629 (0.3) |
| AZA-MMF-steroid | 1/629 (0.2) |
| AZA-steroid | 1/629 (0.2) |
| CyA-based scheme | 425/629 (67.6) |
| Tacrolimus-based scheme | 202/629 (32.1) |
| CNI-free scheme | 2/629 (0.3) |
| MMF-containing scheme | 544/629 (86.5) |
| AZA-containing scheme | 86/629 (13.7) |
| Steroid-free scheme | 14/629 (2.2) |
| Cytomegalovirus prophylaxis | 241/629 (38.3) |
Seventy-three/629 (11.6%) recipients were transferred to other institutions before study completion (38/73, 52% within one year of transplant). Median follow up was 57 (31-80) mo. No patients were excluded from the analysis (intention to treat).
One-year and 5-year patient survival rates were 93.7% (95%CI: 91.5%-95.3%) and 88.5% (95%CI: 85.5%-90.9%), respectively. During the follow up, 68/629 (10.8%) recipients died. The following causes of death were recorded: sepsis (n = 32), sudden cardiac death (n = 14), malignancy (n = 5), post-operative surgical complication (n = 3), sclerosing peritonitis (n = 3), myocardial infarction (n = 3), oesophageal variceal bleeding (n = 2), stroke (n = 2), heart failure (n = 1), ruptured abdominal aortic aneurysm (n = 1), and unknown (n = 2).
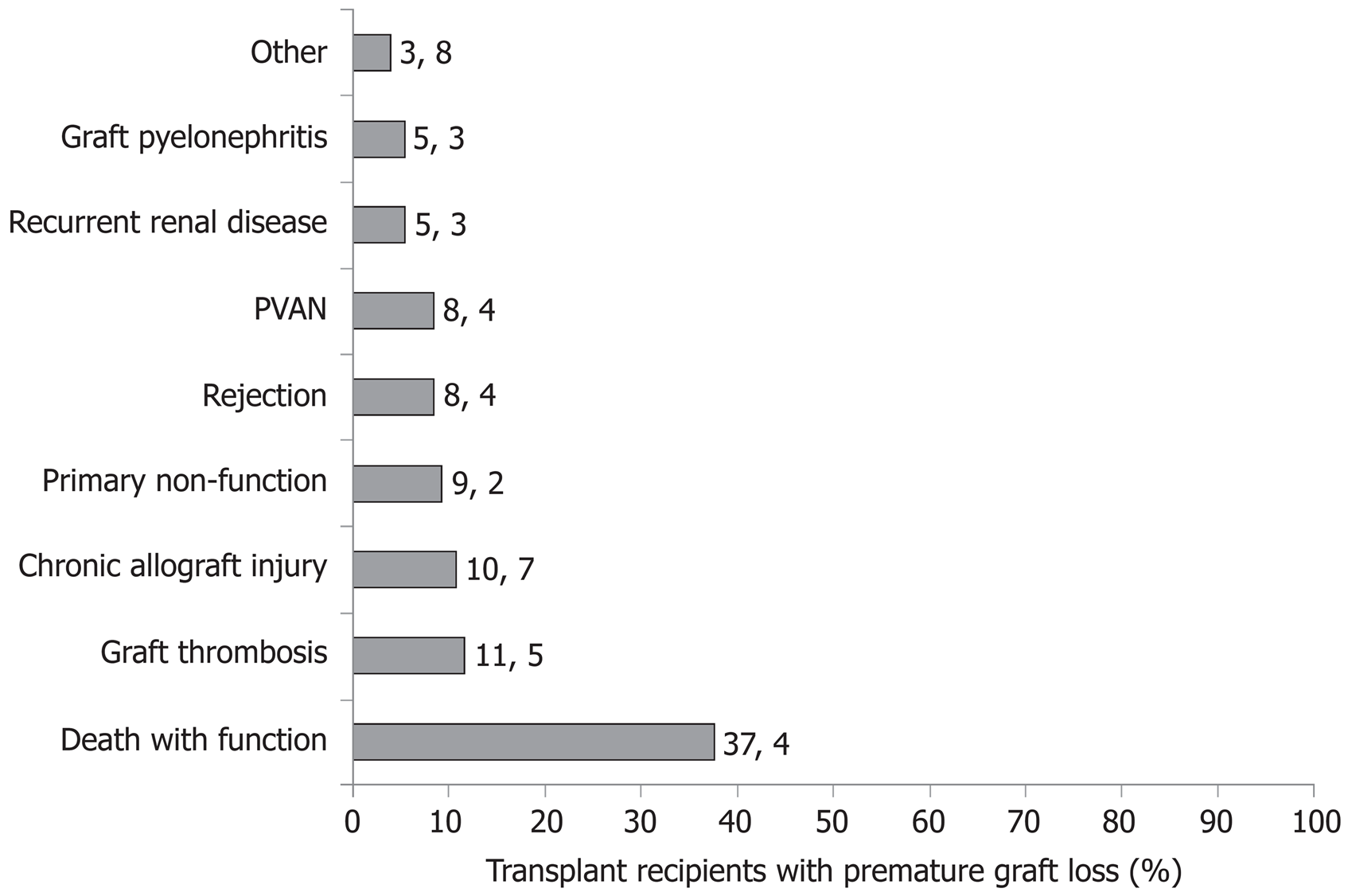
One-year and 5-year crude cumulative incidences of graft failure were 7.1% (95%CI: 5.0%-9.8%) and 13.3% (95%CI: 10.5%-16.0%), respectively. During the study, 131/629 (20.8%) transplant losses were observed. As shown in Figure 1, reasons for graft loss were: death with function (n = 49), chronic allograft injury (n = 14), PNF (n = 12), renal vein thrombosis (n = 11), PVAN (n = 11), BPR (n = 11), recurrent primary renal disease (n = 7), allograft pyelonephritis (n = 7), renal artery thrombosis (n = 4), atypical haemolytic uremic syndrome (n = 1), calcineurin inhibitor-induced haemolytic uremic syndrome (n = 1), septic shock (n = 1), post-operative bleeding (n = 1), and prophylactic allograft nephrectomy due to donor malignancy (n = 1).
PNF and DGF rates were 12/629 (1.9%) and 169/629 (26.9%), respectively. One-year BPR rate was 151/629 (24%) with a median time from transplant to BPR (considering patients with rejection only) equal to 25 (7-93) d. Donor-specific antibody were detected in 98/629 (15.6%) recipients.
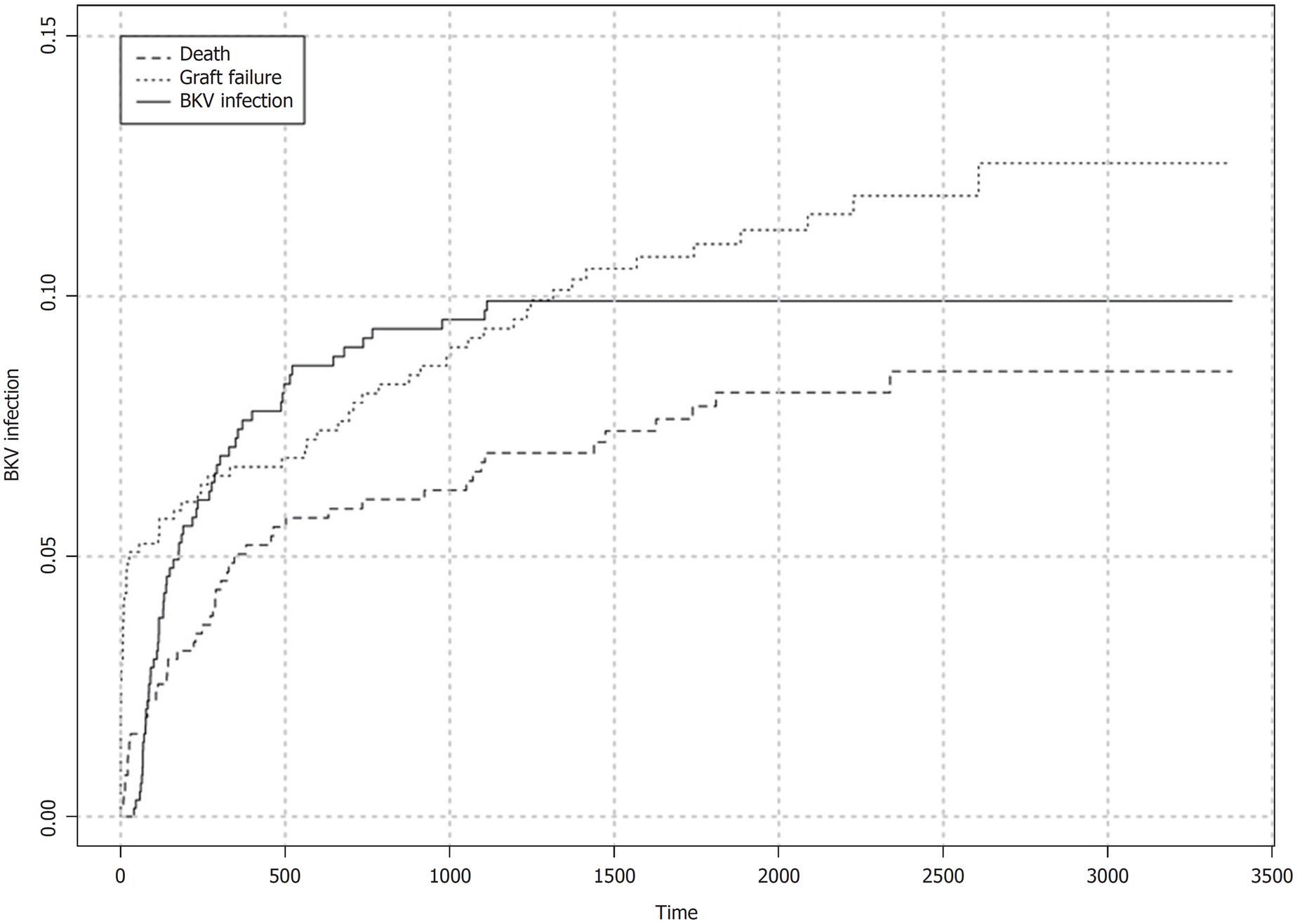
BKV viremia (BKV plasma qPCR ≥ 1000 copies/mL) was detected in 60/629 (9.5%) patients. Median time from transplant to first diagnosis of viremia (considering the infected patients only) was 152 (84-346) d. Within 180 d of transplant, crude cumulative incidence of BKV viremia was 5.0% (95%CI: 3.5%-7.0%) with 33 episodes recorded. Crude cumulative incidence of BKV viremia during the follow up is shown in Figure 2. At the time of diagnosis, graft function was normal in 10/60 (16.7%) recipients and impaired in 50/60 (83.3%). Initial viral load was high (≥ 10000 copies/mL) in 40/60 (66.7%) cases and low (< 10000 copies/mL) in 20/60 (33.3%). Viral replication was transient (< 3 wk) in 17/60 (28.3%) patients whereas it was sustained (≥ 3 wk) in 43/60 (71.7%). PVAN (allograft biopsy positive to SV40 immunostaining) was diagnosed in 40/60 (66.7%) recipients with viremia. Crude cumulative incidence of PVAN in the study cohort was 6.5% (95%CI: 4.6%-8.5%) with 40 events recorded.
We could not demonstrate any relationships between onset of viremia (< 180 d vs ≥ 180 d after transplant) and initial viral load (low vs high), duration of viremic phase (transient vs. sustained), PVAN or BKV-related graft failure. Nevertheless, we observed that patients with high initial viral load were more likely to experience sustained viremia (38/40, 95% vs 5/20, 25%, P < 0.00001), PVAN (37/40, 92.5% vs 3/20, 15%, P < 0.00001), and BKV-related graft loss (11/40, 27.5% vs 0/20, 0%, P = 0.0108) than recipients with low initial viral load. Accordingly, comparison between patients with transient or sustained viremia showed that the proportion of patients with initial viral load ≥ 10000 copies/mL was significantly higher in the group with prolonged viral replication (38/43, 88.4% vs 2/17, 11.8%, P < 0.00001). Sustained viremia was also associated with higher rate of PVAN (38/43, 88.4% vs 2/17, 11.8%, P < 0.00001) and BKV-related graft failure (11/43, 25.6% vs 0/17, 0%, P = 0.0247).
Comparison of characteristics of the recipients experiencing BKV viremia or not (Table 2) showed that the proportion of patients with Afro-Caribbean ethnicity (20/60, 33.3% vs 94/569, 16.5%, P = 0.0024), PRA test ≥ 50% (18/60, 30% vs 83/569, 14.6%, P = 0.0047), HLA mismatch > 4 (16/60, 26.7% vs 76/569, 13.4%, P = 0.0110), and BPR within thirty days of transplant (13/60, 21.7% vs 54/569, 9.5%, P = 0.0073) was significantly higher in the viremic group than control. We also observed a significant difference in median donor age (No BKV: 47, 38-57 vs BKV: 51.5, 40.75-60.25 years, P = 0.0483) and HLA-DR mismatch (No BKV: 1, 0-1 vs BKV: 0-1.25, P = 0.0482). Distributions of induction and maintenance immunosuppression therapies were similar. Subanalysis of baseline characteristics of viremic patients with or without PVAN were not significantly different (Table 3).
| Variables | Median (IQR) or n (%) | P | |
| No BKV group | BKV group | ||
| Patients | 569 | 60 | - |
| Recipient Male : Female | 333 : 236 | 39 : 21 | 0.4076 |
| Caucasian ethnicity | 255/569 (44.8) | 24/60 (40) | 0.4977 |
| Afro-Caribbean ethnicity | 94/569 (16.5) | 20/60 (33.3) | 0.0024 |
| Recipient age (years) | 47 (36-55) | 48.5 (37-53.25) | 0.7263 |
| Recipient age ≥ 60 yr | 74/569 (13) | 10/60 (16.7) | 0.4255 |
| Recipient BMI ≥ 30 kg/m2 | 79/569 (13.9) | 8/60 (13.3) | 1.0000 |
| Pre-transplant diabetes | 77/569 (13.5) | 6/60 (10) | 0.5499 |
| Pre-transplant cardiovascular disease | 55/569 (9.7) | 7/60 (11.7) | 0.5473 |
| Recipient CMV IgG positive | 391/569 (68.7) | 40/60 (66.7) | 0.7707 |
| Primary renal disease | |||
| Glomerular disease | 167/569 (29.4) | 18/60 (30) | |
| Polycystic kidney disease | 62/569 (10.9) | 9/60 (15) | |
| Tubular-interstitial nephropathy | 77/569 (13.5) | 4/60 (6.7) | |
| Diabetic nephropathy | 45/569 (7.9) | 4/60 (6.7) | |
| Hypertension | 47/569 (8.3) | 8/60 (13.3) | |
| Unknown renal disease | 130/569 (22.8) | 11/60 (18.3) | |
| Other renal disease | 41/569 (7.2) | 6/60 (10) | |
| Haemodialysis | 266/569 (46.7) | 28/60 (46.7) | 1.0000 |
| Peritoneal dialysis | 123/569 (21.6) | 13/60 (21.7) | 1.0000 |
| Pre-emptive transplant | 72/569 (12.7) | 8/60 (13.3) | 0.8395 |
| Panel-reactive antibody test > 50% | 83/569 (14.6) | 18/60 (30) | 0.0047 |
| Primary transplant | 497/569 (87.3) | 53/60 (88.3) | 1.0000 |
| Deceased donor | 357/569 (62.7) | 38/60 (63.3) | 1.0000 |
| Donation after brain death donor | 235/569 (41.3) | 27/60 (45) | 0.5848 |
| ABO-incompatible transplant | 21/569 (3.7) | 4/60 (6.7) | 0.2856 |
| Donor age (yr) | 47 (38-57) | 51.5 (40.75-60.25) | 0.0483 |
| Donor age ≥ 60 yr | 115/569 (20.2) | 17/60 (28.3) | 0.1808 |
| Donor-recipient gender mismatch | 308/569 (54.1) | 32/60 (53.3) | 1.0000 |
| Donor CMV IgG positive | 323/569 (56.8) | 31/60 (51.7) | 0.4947 |
| Cytomegalovirus D+R- immunization | 79/569 (13.9) | 4/60 (6.7) | 0.1582 |
| Cold ischemia time (h) | 12 (4-16) | 12 (4-17.25) | 0.9920 |
| Cumulative HLA mismatch | 3 (2-4) | 3 (2-5) | 0.0759 |
| HLA mismatch > 4 | 76/569 (13.4) | 16/60 (26.7) | 0.0110 |
| HLA-A mismatch | 1 (1-2) | 1 (1-2) | 0.6079 |
| HLA-B mismatch | 1 (1-1) | 1 (1-2) | 0.1896 |
| HLA-DR mismatch | 1(0-1) | 1 (0-1.25) | 0.0482 |
| Induction treatment | |||
| Anti-IL2-receptor antagonist | 388/569 (68.2) | 43/60 (71.7) | |
| Rabbit anti-thymocyte globulin | 156/569 (27.4) | 13/60 (21.7) | |
| Rituximab | 21/569 (3.7) | 4/60 (6.7) | |
| Alemtuzumab | 3/569 (0.5) | 0/60 (0) | |
| Muromonab-CD3 | 1/569 (0.2) | 0/60 (0) | |
| Maintenance immunosuppression | |||
| CyA-based scheme | 385/569 (67.7) | 40/60 (66.7) | |
| Tacrolimus-based scheme | 182/569 (32.0) | 20/60 (33.3) | |
| MMF-containing scheme | 490/569 (86.1) | 54/60 (90) | |
| AZA-containing scheme | 80/569 (14.1) | 6/60 (10) | |
| CNI-free scheme | 2/569 (0.4) | 0/60 (0) | |
| Steroid-free scheme | 13/569 (2.3) | 1/60 (1.7) | |
| Cytomegalovirus prophylaxis | 224/569 (39.4) | 17/60 (28.3) | 0.1239 |
| DGF | 152/569 (26.7) | 15/60 (25) | 0.8783 |
| BPR within 30 d of transplantation | 54/569 (9.5) | 13/60 (21.7) | 0.0073 |
| Variables | Median (IQR) or n (%) | P | |
| No PVAN group | PVAN group | ||
| Patients | 20 | 40 | |
| Recipient Male : Female | 13 : 7 | 26 : 14 | 1.0000 |
| Caucasian ethnicity | 9/20 (45) | 15/40 (37.5) | 0.5896 |
| Afro-Caribbean ethnicity | 4/20 (20) | 16/40 (40) | 0.1536 |
| Recipient age (yr) | 49 (33.5-58) | 48.5 (37.75-53) | 0.8887 |
| Pre-transplant diabetes | 1/20 (5) | 5/40 (12.5) | 0.6532 |
| Pre-transplant CVD | 4/20 (20) | 3/40 (7.5) | 0.2077 |
| Recipient CMV IgG positive | 13/20 (65) | 27/40 (67.5) | 1.0000 |
| Haemodialysis | 9/20 (45) | 19/40 (47.5) | 1.0000 |
| Peritoneal dialysis | 6/20 (30) | 7/40 (17.5) | 0.3258 |
| Pre-emptive transplant | 2/20 (10) | 6/40 (15) | 0.7068 |
| PRA test > 50% | 6/20 (30) | 12/40 (30) | 1.0000 |
| Primary transplant | 17/20 (85) | 36/40 (90) | 0.6763 |
| Deceased donor | 13/20 (65) | 25/40 (62.5) | 1.0000 |
| ABO-incompatible transplant | 1/20 (5) | 3/40 (7.5) | 1.0000 |
| Donor age (yr) | 53.5 (42.25-62.75) | 51 (40.75-57) | 0.30302 |
| Donor age ≥ 60 yr | 8/20 (40) | 9/40 (22.5) | 0.2246 |
| Donor CMV IgG positive | 7/20 (35) | 24/40 (60) | 0.1004 |
| CMV D+R- immunization | 1/20 (5) | 3/40 (7.5) | 1.0000 |
| Cold ischemia time (h) | 14.25 (4.3-21.5) | 12 (4-15) | 0.4413 |
| Cumulative HLA mismatch | 3 (3-4.25) | 3 (2-5) | 0.4654 |
| HLA mismatch > 4 | 5/20 (25) | 11/40 (27.5) | 1.0000 |
| HLA-A mismatch | 1 (1-1.25) | 1 (1-2) | 0.78716 |
| HLA-B mismatch | 1 (1-2) | 1 (1-2) | 0.25014 |
| HLA-DR mismatch | 1 (0-1.25) | 1 (0-1.25) | 0.8181 |
| Induction immunosuppression | |||
| Anti-IL2-receptor antagonist | 15/20 (75) | 28/40 (70) | |
| Anti-thymocyte globulin | 4/20 (20) | 9/40 (22.5) | |
| Rituximab | 1/20 (5) | 3/40 (7.5) | |
| Maintenance immunosuppression | |||
| CyA-based scheme | 14/20 (70) | 26/40 (65) | |
| Tacrolimus-based scheme | 6/20 (30) | 14/40 (35) | |
| MMF-containing scheme | 19/20 (95) | 35/40 (87.5) | |
| AZA-containing scheme | 1/20 (5) | 5/40 (12.5) | |
| CNI-free scheme | 0/20 (0) | 0/40 (0) | |
| Steroid-free scheme | 0/20 (0) | 1/40 (2.5) | |
| CMV prophylaxis | 4/20 (20) | 13/40 (32.5) | 0.3752 |
| DGF | 6/20 (30) | 9/40 (22.5) | 0.5424 |
| BPR within 30 d of transplant | 4/20 (20) | 9/40 (22.5) | 1.0000 |
| Initial viremia ≥ 10000 copies/mL | 3/20 (15) | 37/40 (92.5) | < 0.00001 |
| Initial viral load (copies/mL) | 6000 (2975-7550) | 134800 (28750-425000) | < 0.00001 |
| BKV viremia ≥ 3 wk | 5/20 (25) | 38/40 (95) | < 0.00001 |
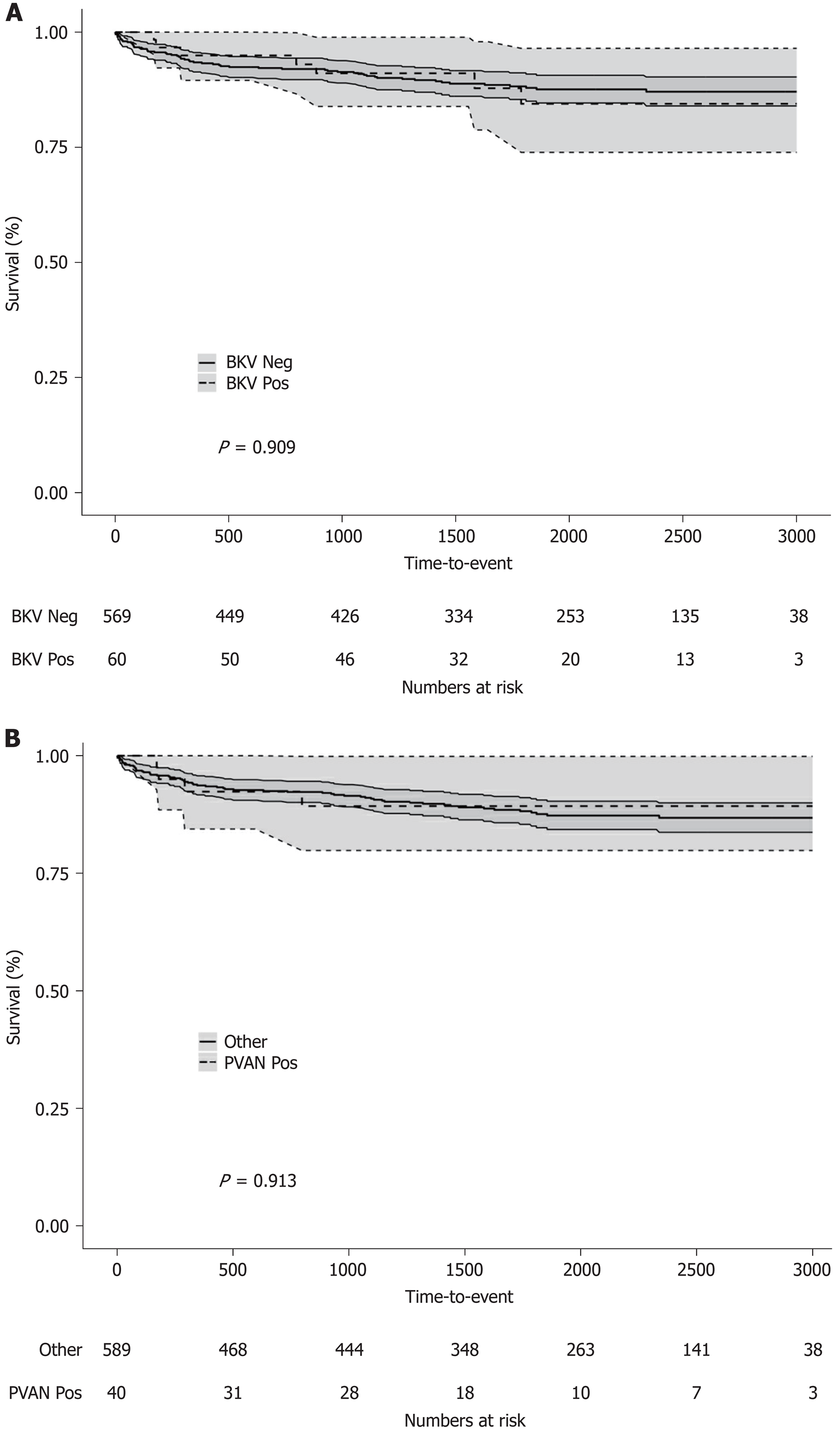
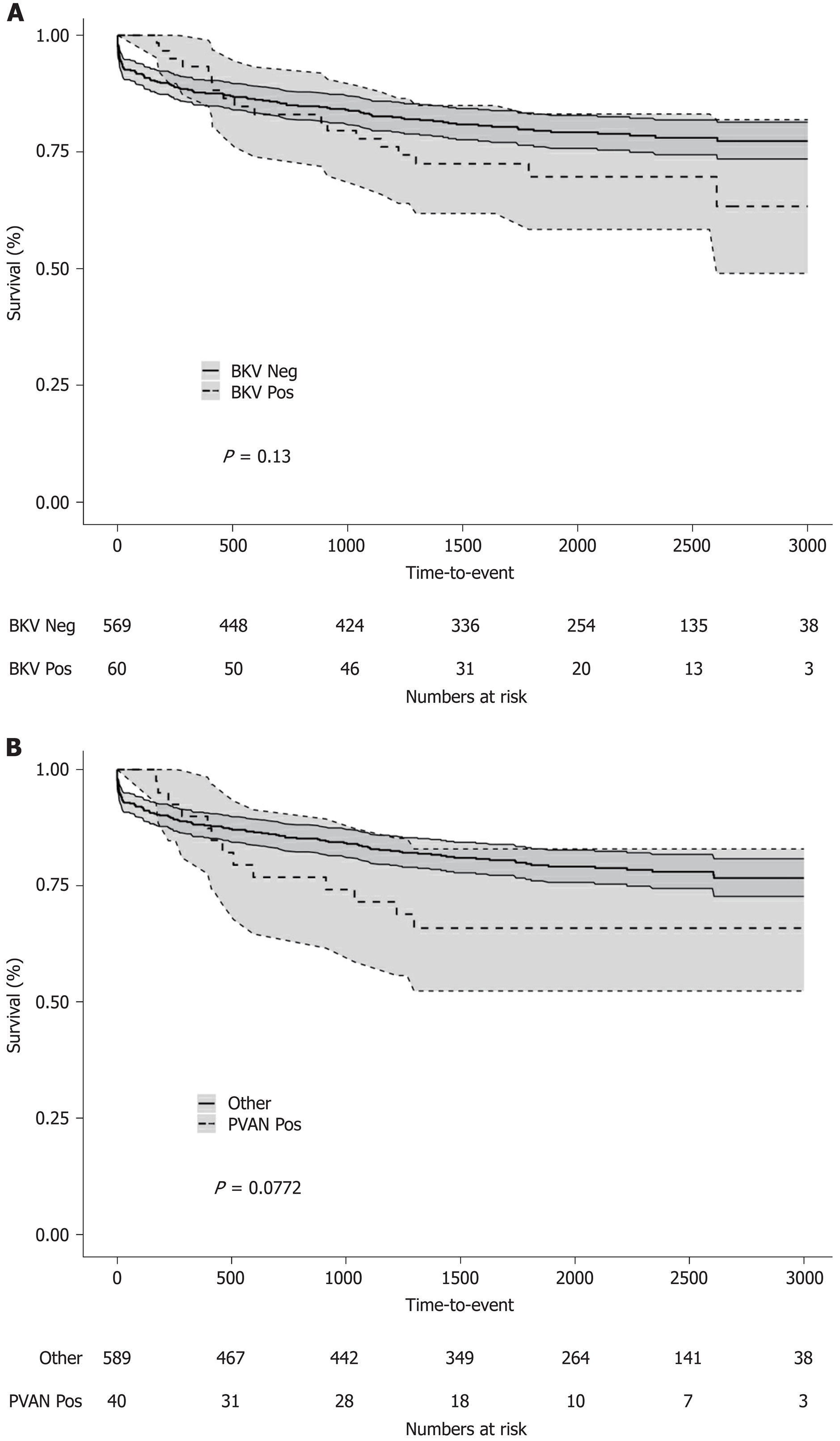
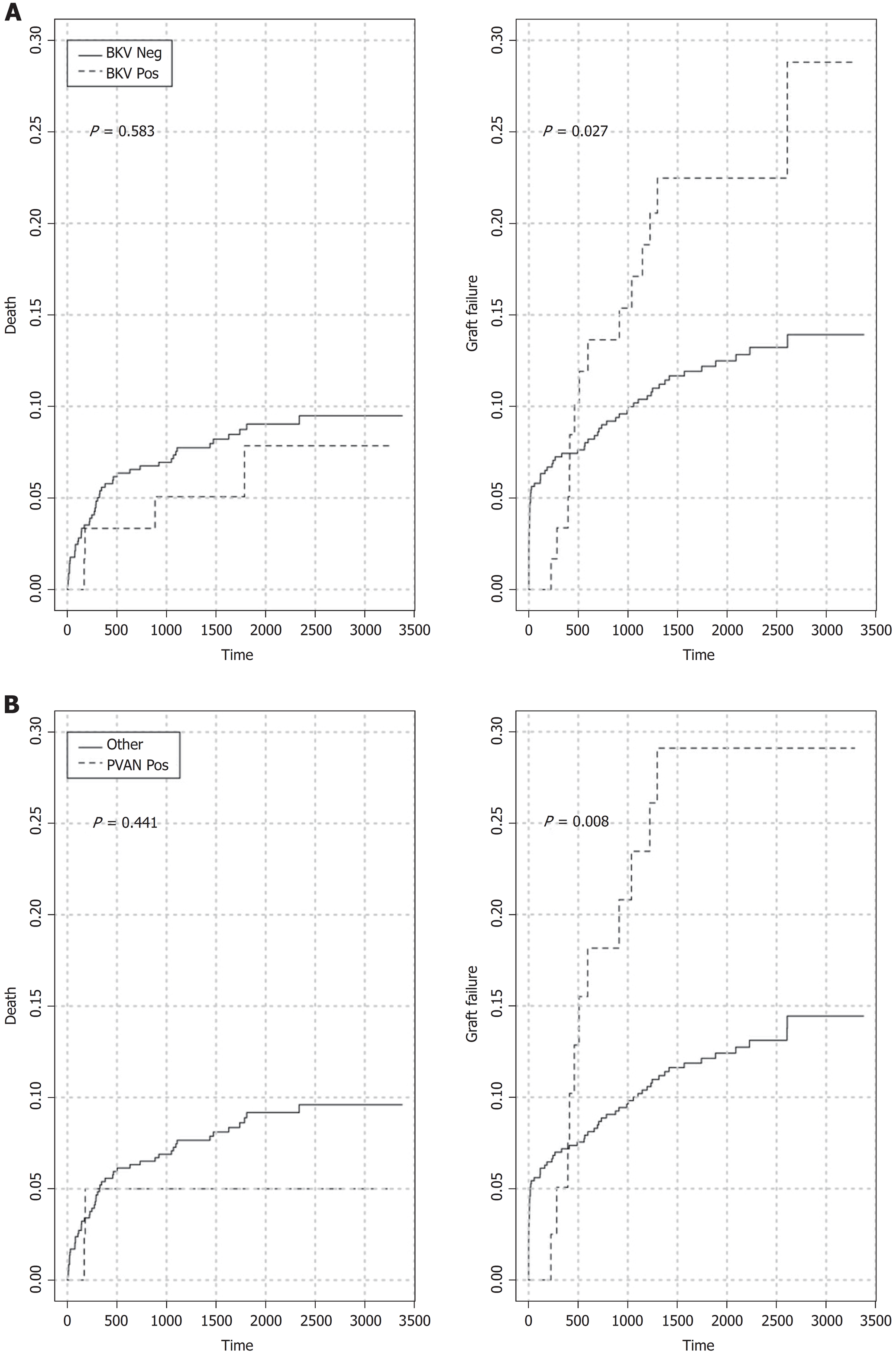
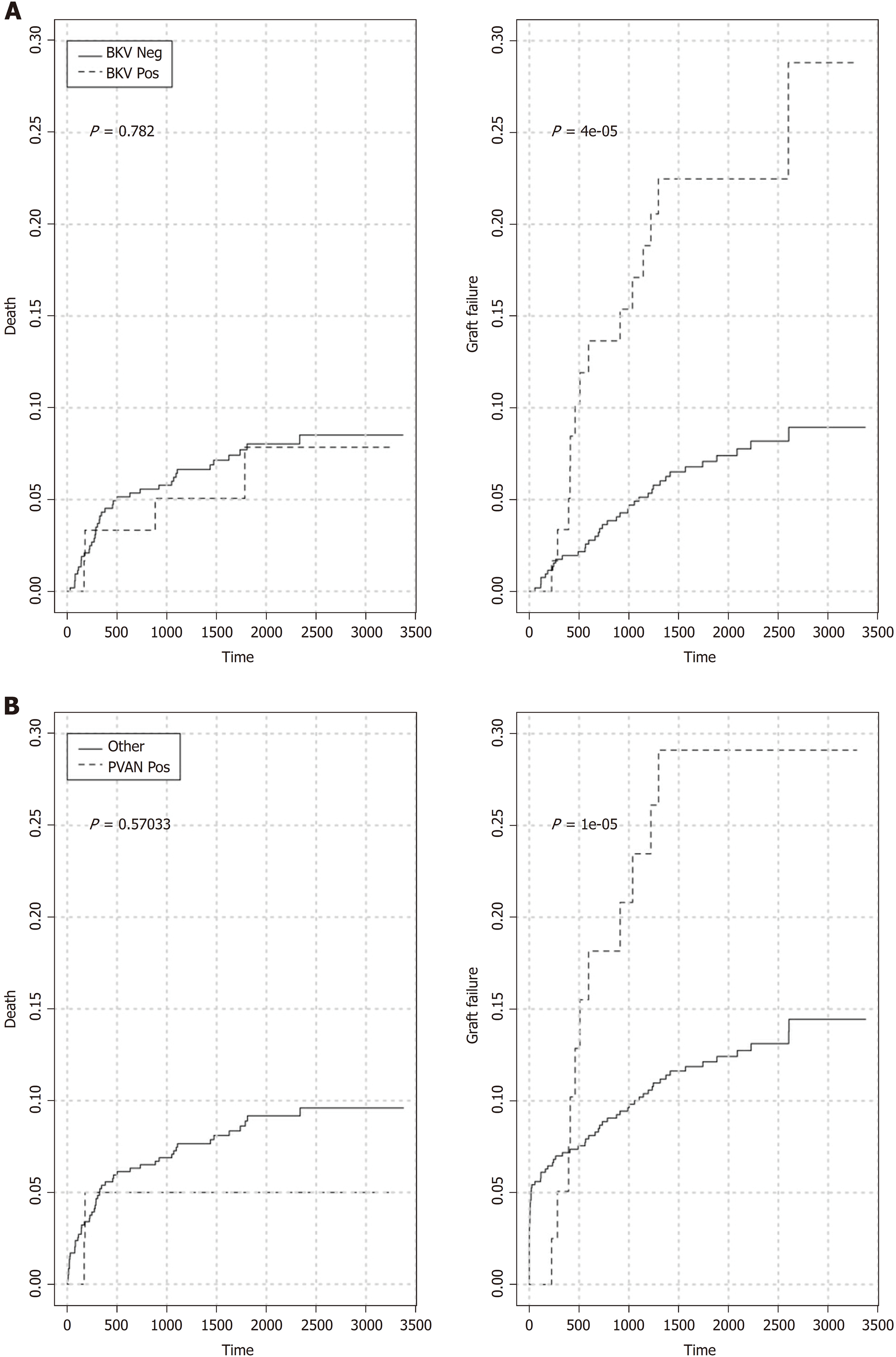
Five-year patient (86.6% vs 88.7%, log-rank P = 0.8736) and overall graft (70.6% vs 80.2%, log-rank P = 0.1201) survival rates for patients with or without BKV viremia were similar (Figure 3A and Figure 4A). However, the viremic group showed higher 5-year crude cumulative (22.5% vs 12.2%, Gray’s test P = 0.0270) and 30-d-event-censored (22.5% vs 7.1%, Gray’s test P = 0.001) incidences of graft failure than control (Figure 5A and Figure 6A).
In the viremic group, higher proportions of recipients also experienced BPR or impaired graft function (5-year MDRD eGFR < 30 mL/min) than the group without viremia: 26/60, 43.3% vs 125/575, 21.7% (P = 0.0004) and 27/60, 45% vs 155/575, 27% (P = 0.0064), respectively.
We could not find any significant differences between the two groups in the proportions of recipients with urinary leakage (viremia: 1/60, 1.7% vs no viremia: 7/575, 1.2%, P = 0.5501) or ureteral stenosis (viremia: 2/60, 3.3% vs no viremia: 11/575, 1.9%, P = 0.3520). Cumulative incidences of opportunistic infection, CMV disease, varicella-zoster virus-associated disease (chickenpox or shingles), malignancy, and NODAT were also comparable (data not shown).
Recipients with BKV viremia had their immunosuppressive therapy modified according to the scheme described above. Response to treatment was complete in 33/60 (55%), partial in 16/60 (26.7%), and absent in 11/60 (18.3%) patients. Following reduction of the net state of immunosuppression, 7/60 (11.7%) recipients developed BPR.
To further investigate the specific impact of PVAN on transplant outcome, we compared results of patients with or without nephropathy. Five-year patient survival distributions for the two groups were equivalent (PVAN: 89.9% vs No PVAN: 88.4%, log-rank P = 0.913; Figure 3B). Five-year overall graft survival rate was better for patients without PVAN but the difference did not reach statistical significance (80.1% vs 66.5%, log-rank P = 0.077; Figure 4B). As depicted in Figure 5B and Figure 6B, the PVAN group showed higher 5-year crude cumulative and 30-d-event-censored incidences of graft failures than control: 29.1% vs 12.1% (Gray’s test P = 0.008) and 29.1% vs 7.2% (Gray’s test P < 0.001), respectively.
PVAN was the leading cause of transplant loss in patients with BKV viremia (PVAN: 11/18, 61.1% vs death with function: 4/18, 22.2% vs chronic allograft injury: 1/18, 5.6%) and the fourth in the entire study cohort (Figure 1).
Univariate analysis, based on chi-square or Fisher exact test for recipients with or without BKV viremia are reported in Table 2. Variables with a P-value greater than 0.8 were not considered in the multivariable model building. This left 16 variables to be studied. Among the 16 variables, the selected variables according to the backward procedure were: Afro-Caribbean ethnicity, pre-transplant diabetes, PRA value > 50%, CMV prophylaxis, HLA mismatching > 4, BPR within thirty days of transplant. The final model is reported in Table 4. By repeating the backward variable selection in 500 bootstrap samples, it is worth noticing that the selected variables were also selected in at least 60% of the bootstrap samples. Moreover recipient CMV IgG positive was selected in 68% of the bootstrap samples and recipient cytomegalovirus D+R- immunization in 60% of the samples. The C-index is 0.64 after correction for optimism.
| Variable | OR | 95%CI | P |
| Afro-Caribbean ethnicity | 2.882 | 1.549; 5.259 | 0.001 |
| Panel-reactive antibody > 50% | 3.352 | 1.737; 6.338 | < 0.001 |
| HLA mismatch > 4 | 2.585 | 1.303; 4.955 | 0.005 |
| CMV Prophylaxis | 0.467 | 0.244; 0.854 | 0.017 |
| BPR within 30 d of transplant | 2.342 | 1.117; 4.664 | 0.019 |
Univariate analysis, based on Chi-square or Fisher exact test for 60 recipients with BKV viremia, classified as PVAN positive or negative, are reported in Table 3. Variables with a P-value greater than 0.5 were not considered in the multivariable model building. This left 8 variables to be studied. It is worth noticing that initial viremia ≥ 10000 copies/mL and sustained vs. transient viremia almost perfectly discriminated between patients with or without PVAN. The odds ratio contrasting initial viremia ≥ 10000 vs < 10000 copies/mL is 54 (95%CI: 13-477) calculated using Firth penalized logistic regression with a C-index equal to 0.89. Considering the remaining variables, a backward selection procedure was performed. The selected variables were: Afro-Caribbean ethnicity, cardiovascular disease, donor CMV IgG positive, donor age ≥ 60 years. The final model is reported in Table 5. By repeating the backward variable selection in 500 bootstrap samples, only the 4 selected variables were also selected in at least 50% of the bootstrap samples. The C-index is 0.62 after correction for optimism.
| Variable | OR | 95%CI | P |
| Afro-Caribbean ethnicity | 2.717 | 0.705-12.458 | 0.164 |
| Cardiovascular disease | 0.235 | 0.034-1.42 | 0.117 |
| Donor Age ≥ 60 yr | 0.243 | 0.055-0.949 | 0.048 |
| Recipient CMV IgG positive | 4.371 | 1.261-17.968 | 0.027 |
The development of new and more powerful immunosuppressive agents has led to a significant reduction of acute rejection rates after kidney transplantation[24]. As a consequence, we have observed an impressive improvement in short- and mid-term graft survival rates. However, long-term outcomes have only marginally improved[25]. Death with function, chronic allograft injury, and PVAN have now emerged as leading causes of premature transplant loss[25]. Viral infections are a well known complication of chronic immunosuppression[26]. CMV and BKV are the most common opportunistic infections in renal transplant recipients[27]. Due to a widespread application of systematic protocols for the prevention and treatment of CMV in immunocompromised hosts, remarkable results have been obtained[28]. Management of BKV infection has been also evolving[14]. Nevertheless, clinical outcome of kidney transplant recipients with PVAN remains sub-optimal[29].
In the present study we investigated incidence, risk factors, and long-term outcome of BKV infection in a cohort of kidney transplant recipients managed according to an aggressive screening and diagnostic protocol for PVAN. Effectiveness of a treatment strategy based on a step by step reduction of the net state of immunosuppression was also evaluated.
KDIGO guideline suggests screening all kidney transplant recipients for BKV replication with plasma qPCR at least monthly for the first six months and then every three months until the end of the first post-transplant year[14]. As described above, we performed a more intensive screening, especially during the first three months after transplant. We found an incidence of BKV viremia and PVAN of 9.5% and 6.5%, respectively. More than half (55%) of the episodes of viremia were recorded within 180 d of transplant; none in the first thirty days. These results are in line with previously published data[30-32] and support the recommendations of current clinical guidelines. Indeed, our experience suggests that a more aggressive screening strategy, at least in the very early post-transplant phase, may not be beneficial.
Despite a previous report demonstrating an association between onset of viremia and histological evidence for nephropathy[32], we could not find any relationships between the time viremia was first diagnosed and PVAN. We did observe that patients with an initial viral load ≥ 10000 copies/mL were more likely to develop nephropathy and lose their graft due to BKV than recipients with lower viral loads. An association between duration of the viremic phase and risk of PVAN or PVAN-related graft loss was also demonstrated. Interestingly, no graft failures due to BKV were recorded in patients with an initial viral load < 10000 copies/mL or with a viral replication < 3 wk. Plasma qPCR is considered the most reliable test to monitor viral replication and assess response to treatment[13]. Even though there is no established viral load cut-off associated with PVAN, some authors have suggested that a viral load > 4 log copies/mL may be predictive of nephropathy[33]. KDIGO guidelines also recommend prompt reduction of immunosuppression in patients with a BKV viral load > 10000 copies/mL[14]. Our data confirm the predictive ability of initial viral load for the risk of PVAN and strongly support the use of 10000 copies/mL as a cut-off to discriminate between low- and high-risk kidney transplant recipients. At the same time, as a few cases of nephropathy have been observed in patients with an initial viral load < 10000 copies/mL, we suggest other prognostic information such as the duration of viremia to be considered along with initial viral load in the evaluation of this specific subgroup of recipients.
Allograft histology is accepted as the gold standard for the diagnosis of PVAN[13]. Several grading systems are also available[34]. Most centres reserve transplant biopsy for patients with high viral loads or unexplained deterioration of function. Such a policy, whilst reasonable, does not take into account the aforementioned proportion of patients with a low viral load and histological evidence of PVAN (15% in our series). Since early diagnosis of nephropathy and reduction of immunosuppression have been recognized as key factors for the prevention of BKV-related graft loss, we believe that every patient with BKV viremia should be offered histological evaluation. For low-risk recipients (initial viral load < 10000 copies/mL and duration of viral replication < 3 wk) or for patients more likely to develop biopsy-related complications, a non-invasive test may represent a reasonable option. In this regard, interesting results have been obtained using electron microscopy[35] and urine mRNA profiles[36].
Our treatment strategy for BKV infection followed recommendations by current international guidelines[13,14]. Response to progressive reductions in the net state of immunosuppression was observed in 82% of the patients. These results are overall satisfactory but it is worth noting that among recipients with BKV infection, 27% experienced permanent deterioration of function (5-year MDRD eGFR < 30 mL/min/1.73 m2) and 18% eventually lost their graft due to PVAN. Moreover, in line with Park and colleagues[37], analyses of death-censored and 30-d-event-censored graft survival rates showed inferior long-term outcome for patients with BKV infection (regardless of nephropathy) than control. Compared with other reports[30,38,39], these results are less encouraging and strongly advocate for further research on specific anti-BKV treatment strategies.
Risk factors for BKV infection after kidney transplantation have been extensively studied but results remain inconsistent. The net state of immunosuppression is currently considered the most important determinant of BKV infection and nephropathy[13]. Our multivariable model showed that Afro-Caribbean ethnicity, donor-recipient HLA mismatch > 4, PRA test > 50%, and BPR within thirty days of transplant are significant predictor of BKV infection. It also showed that CMV prophylaxis reduces the risk of BKV infection.
Previous rejection is a well recognized risk factor for PVAN[40]. Renal injury arising from cellular or humoral allograft-specific immune response and increased immunosuppression can reasonably account for such an association.
The relationship between degree of HLA mismatching and BKV infection is less clear[34]. The accepted explanation is that recipients with a higher mismatch are at increased risk of rejection and therefore generally receive more intensive immunosuppressive protocols than patients with more favourable donor-recipient matching[34]. BKV-specific immune response inability to control viral replication and progression to nephropathy due an impaired HLA-restricted recognition of viral antigens or protective effect of specific HLA antigens may also play a role[41].
We found that a pre-transplant PRA value > 50% increases the risk of BKV infection. Pre-immunization is widely considered a strong determinant of rejection[42] and transplant candidates with preformed anti-HLA antibodies are more likely to receive antibody-removal therapies, lymphocyte-depleting agents, and high-dose calcineurin inhibitors than non-sensitized patients. Therefore, the increased susceptibility to BKV infection observed in this group of recipients may be due to over immunosuppression.
It has been reported that Caucasian recipients are at increased risk of PVAN[34]. In contrast with a previous study[43] we found that Afro-Caribbean recipients were more likely to experience BKV viremia than Caucasian. Moreover, our multivariable model showed that Afro-Caribbean ethnicity was an independent risk factor for both BKV viremia and PVAN. Comparisons between different ethnic groups are difficult to explain given the various confounding factors such as seroprevalence of BKV infection, HLA phenotype, risk of rejection, and access to care. Recent data also demonstrate that transplant outcomes for patients with similar heritage may not be translatable between different countries[44]. Taking into account all these possible limitations, it is plausible to speculate that the increased risk of BKV infection and PVAN observed in our Afro-Caribbean recipients may be at least partially related to the increased amount of immunosuppression they generally receive compared to other ethnicities[45].
CMV is a major cause of post-transplant morbidity and mortality[46]. Similarly to BKV, immunosuppression is considered the strongest risk factor for CMV reactivation. A possible interaction between CMV and BKV has been already postulated. However, the existence of an epidemiological association remains debated[47,48]. It is also still unclear whether CMV infection has a protective effect on BKV reactivation[48]. As was recently shown by Blazquez-Navarro and colleagues[27], our data indirectly support the hypothesis that CMV infection may rather favour BKV replication. CMV prophylaxis has been demonstrated to reduce the incidence of CMV infection during the first six-twelve months after transplant[46]. Therefore, we can reasonably speculate that the lower risk of BKV infection observed in patients receiving CMV prophylaxis may be due to a lower incidence of sub-clinical CMV reactivations.
This is one of the largest single-centre observational study investigating incidence, risk factors, and outcomes of BKV infection after kidney transplantation. We confirm previously reported epidemiological data and support systematic screening, early histological evaluation, and prompt reduction of the net state of immunosuppression for the prevention of PVAN and BKV-related graft failure. At the same time, we highlight the limitations of current treatment modalities and the need for proper antiviral therapies. An initial plasma viral load ≥ 10000 copies/mL is the strongest determinant of sustained viral replication, PVAN, and graft loss. In addition to other well recognized risk factors, we identified a PRA test > 50% and Afro-Caribbean ethnicity as independent predictors of BKV infection. A protective effect of CMV prophylaxis has been also suggested. Properly designed large multi-centre studies are warranted to further investigate specific risk factors for PVAN and alternative anti-BKV strategies.
Polyomavirus-associated nephropathy (PVAN) is recognized as a leading cause of kidney allograft loss. Current antiviral therapies offer limited results. Clinical guideline suggests periodic screening for BK-virus replication in blood and recommends prompt reduction of the net state of immunosuppression in case of viremia. However, long-term outcome of kidney transplant recipients with BK-virus infection remains sub-optimal.
The development of new and more powerful immunosuppressive agents has led to a significant reduction of acute rejection rates after kidney transplantation. As a consequence, we have observed an impressive improvement in short- and mid-term graft survival rates but long-term results have only marginally improved. BK-virus is one of the most common opportunistic infection in renal transplant recipients and has been demonstrated to have a deleterious impact on allograft function and survival. Management of BK-virus infection has significantly changed and encouraging results have been obtained. Nevertheless, long-term data are scarce and previously published reports may not reflect current clinical practice. Therefore, we performed an observational study to investigate incidence, risk factors, and long-term outcome of BK-virus infection in a cohort of kidney transplant recipients managed according to an aggressive screening and diagnostic protocol for PVAN. Effectiveness of a treatment strategy based on a step by step reduction of the net state of immunosuppression was also evaluated.
The aim of the present study was to evaluate incidence, risk factors, and outcome of BK-virus infection after kidney transplantation.
This single-centre observational study with a median follow up of 5 years was conducted in a National Health Service hospital in UK and comprises 629 consecutive adult patients who underwent kidney transplantation between 2007 and 2013. Data were prospectively recorded and annually reviewed until 2016. Recipients were periodically screened for BK-virus by plasma quantitative polymerized chain reaction. Patients with BK plasma viral load ≥ 1000 copies/mL were diagnosed BK-viremia and underwent histological assessment to rule out nephropathy. In case of BK-viremia, immunosuppression was minimized according to a prespecified protocol. The following outcomes were evaluated: patient survival, overall graft survival, graft failure considering death as a competing risk, 30-d-event-censored graft failure, response to treatment, rejection, renal function, urologic complications, opportunistic infections, new-onset diabetes after transplantation, and malignancies. We used a multivariable model to analyse risk factors for BK-viremia and nephropathy.
BK-viremia and PVAN were detected in 9.5% and 6.5% of the study population, rspectively. Patients with initial plasma viral load ≥ 10000 copies/mL were more likely to experience sustained viremia (95% vs 25%, P < 0.00001), nephropathy (92.5% vs 15%, P < 0.00001), and polyomavirus-related graft loss (27.5% vs. 0%, P = 0.0108) than recipients with low initial plasma viral load. Recipients with viremia showed higher 5-year crude cumulative (22.5% vs 12.2%, P = 0.0270) and 30-d-event-censored (22.5% vs 7.1%, P = 0.001) incidences of graft failure than control. In the viremic group we also observed higher proportions of recipients with 5-year estimated glomerular filtration rate < 30 mL/min than the group without viremia: 45% vs 27% (P = 0.0064). Response to treatment was complete in 55%, partial in 26.7%, and absent in 18.3% patients. The PVAN group showed higher 5-year crude cumulative and 30-d-event-censored incidences of graft failure than control: 29.1% vs 12.1% (P = 0.008) and 29.1% vs 7.2% (P < 0.001), respectively. Afro-Caribbean ethnicity, panel-reactive antibody > 50%, human leukocyte antigen mismatching > 4, and rejection were independent risk factors for BK-virus viremia whereas cytomegalovirus prophylaxis was protective.
As recommended by most recent international clinical guideline, our study supports systematic screening, early histological evaluation, and prompt reduction of the net state of immunosuppression for the prevention of PVAN and BK-related graft failure after kidney transplantation. Nevertheless, we also demonstrated the limitations of current treatment strategies and the need for more specific antiviral therapies. In particular, we showed that both BK-viremia and nephropathy negatively affect long-term graft function and survival. We confirmed that an initial plasma viral load ≥ 10000 copies/mL is the strongest determinant of sustained viral replication, PVAN, and graft loss. We identified a PRA test > 50% and Afro-Caribbean ethnicity as independent predictors of BK-virus infection. Our data also suggest a protective effect of cytomegalovirus prophylaxis on BK-virus replication.
Our study represents one of the largest observational study on BK-virus infection in kidney transplant recipients. We managed to confirm our preliminary hypothesis and demonstrated that despite aggressive screening and treatment strategies, long-term outcome for patients with BK-virus infection remain consistently inferior than control. We identified new risk factors for BK-viremia but larger populations are needed to further investigate risk factors for PVAN. In order to improve results, future research should be focusing on alternative prognostic markers, non-invasive diagnostic tests, and novel antiviral therapies. Properly designed multi-centre prospective randomized clinical trials are warranted.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Choi MR, Ramsay MA, Stavroulopoulos A, Nechifor G S- Editor: Wang JL L- Editor: A E- Editor: Song H
| 1. | Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3684] [Cited by in RCA: 3857] [Article Influence: 148.3] [Reference Citation Analysis (1)] |
| 2. | Ashton-Chess J, Giral M, Soulillou JP, Brouard S. Can immune monitoring help to minimize immunosuppression in kidney transplantation? Transpl Int. 2009;22:110-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Hirsch HH, Drachenberg CB, Steiger J, Ramos E. Polyomavirus-associated nephropathy in renal transplantation: critical issues of screening and management. Adv Exp Med Biol. 2006;577:160-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Randhawa P, Uhrmacher J, Pasculle W, Vats A, Shapiro R, Eghtsead B, Weck K. A comparative study of BK and JC virus infections in organ transplant recipients. J Med Virol. 2005;77:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Knowles WA. Discovery and epidemiology of the human polyomaviruses BK virus (BKV) and JC virus (JCV). Adv Exp Med Biol. 2006;577:19-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 220] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 6. | Ambalathingal GR, Francis RS, Smyth MJ, Smith C, Khanna R. BK Polyomavirus: Clinical Aspects, Immune Regulation, and Emerging Therapies. Clin Microbiol Rev. 2017;30:503-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 7. | Bennett JE, Dolin R, Blaser M. Mandell, Douglas, and Bennett’s Principle and practice of infectious diseases. 8th ed. Amsterdam: Elsevier 2015; 1794-1807. |
| 8. | Sawinski D, Trofe-Clark J. BK Virus Nephropathy. Clin J Am Soc Nephrol. 2018;13:1893-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Wong AS, Chan KH, Cheng VC, Yuen KY, Kwong YL, Leung AY. Relationship of pretransplantation polyoma BK virus serologic findings and BK viral reactivation after hematopoietic stem cell transplantation. Clin Infect Dis. 2007;44:830-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Binet I, Nickeleit V, Hirsch HH, Prince O, Dalquen P, Gudat F, Mihatsch MJ, Thiel G. Polyomavirus disease under new immunosuppressive drugs: a cause of renal graft dysfunction and graft loss. Transplantation. 1999;67:918-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 344] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 11. | Mengel M, Marwedel M, Radermacher J, Eden G, Schwarz A, Haller H, Kreipe H. Incidence of polyomavirus-nephropathy in renal allografts: influence of modern immunosuppressive drugs. Nephrol Dial Transplant. 2003;18:1190-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Vasudev B, Hariharan S, Hussain SA, Zhu YR, Bresnahan BA, Cohen EP. BK virus nephritis: risk factors, timing, and outcome in renal transplant recipients. Kidney Int. 2005;68:1834-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Sawinski D, Goral S. BK virus infection: an update on diagnosis and treatment. Nephrol Dial Transplant. 2015;30:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 14. | Kasiske BL, Zeier MG, Chapman JR, Craig JC, Ekberg H, Garvey CA, Green MD, Jha V, Josephson MA, Kiberd BA, Kreis HA, McDonald RA, Newmann JM, Obrador GT, Vincenti FG, Cheung M, Earley A, Raman G, Abariga S, Wagner M, Balk EM; Kidney Disease: Improving Global Outcomes. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 2010;77:299-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 509] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 15. | Bechert CJ, Schnadig VJ, Payne DA, Dong J. Monitoring of BK viral load in renal allograft recipients by real-time PCR assays. Am J Clin Pathol. 2010;133:242-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis. 2003;3:611-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 569] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 17. | Mallat SG, Tanios BY, Itani HS, Lotfi T, McMullan C, Gabardi S, Akl EA, Azzi JR. CMV and BKPyV Infections in Renal Transplant Recipients Receiving an mTOR Inhibitor-Based Regimen Versus a CNI-Based Regimen: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. Clin J Am Soc Nephrol. 2017;12:1321-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 18. | Johnston O, Jaswal D, Gill JS, Doucette S, Fergusson DA, Knoll GA. Treatment of polyomavirus infection in kidney transplant recipients: a systematic review. Transplantation. 2010;89:1057-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 19. | Drachenberg CB, Hirsch HH, Ramos E, Papadimitriou JC. Polyomavirus disease in renal transplantation: review of pathological findings and diagnostic methods. Hum Pathol. 2005;36:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11183] [Cited by in RCA: 11824] [Article Influence: 454.8] [Reference Citation Analysis (0)] |
| 21. | Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1514] [Cited by in RCA: 1499] [Article Influence: 88.2] [Reference Citation Analysis (0)] |
| 22. | Pham PT, Pham PM, Pham SV, Pham PA, Pham PC. New onset diabetes after transplantation (NODAT): an overview. Diabetes Metab Syndr Obes. 2011;4:175-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | Dean CB, Nielsen JD. Generalized linear mixed models: a review and some extensions. Lifetime Data Anal. 2007;13:497-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 24. | Gaston RS. Current and evolving immunosuppressive regimens in kidney transplantation. Am J Kidney Dis. 2006;47:S3-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011;11:450-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 697] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 26. | Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 518] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 27. | Blazquez-Navarro A, Dang-Heine C, Wittenbrink N, Bauer C, Wolk K, Sabat R, Westhoff TH, Sawitzki B, Reinke P, Thomusch O, Hugo C, Or-Guil M, Babel N. BKV, CMV, and EBV Interactions and their Effect on Graft Function One Year Post-Renal Transplantation: Results from a Large Multi-Centre Study. EBioMedicine. 2018;34:113-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | De Keyzer K, Van Laecke S, Peeters P, Vanholder R. Human cytomegalovirus and kidney transplantation: a clinician's update. Am J Kidney Dis. 2011;58:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 29. | Randhawa P, Brennan DC. BK virus infection in transplant recipients: an overview and update. Am J Transplant. 2006;6:2000-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, Mihatsch MJ, Steiger J. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 919] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 31. | Koukoulaki M, Grispou E, Pistolas D, Balaska K, Apostolou T, Anagnostopoulou M, Tseleni-Kotsovili A, Hadjiconstantinou V, Paniara O, Saroglou G, Legakis N, Drakopoulos S. Prospective monitoring of BK virus replication in renal transplant recipients. Transpl Infect Dis. 2009;11:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Jacobi J, Prignitz A, Büttner M, Korn K, Weidemann A, Hilgers KF, Heller K, Velden J, Knöll A, Wullich B, May C, Eckardt KU, Amann KU. BK viremia and polyomavirus nephropathy in 352 kidney transplants; risk factors and potential role of mTOR inhibition. BMC Nephrol. 2013;14:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Hirsch HH, Brennan DC, Drachenberg CB, Ginevri F, Gordon J, Limaye AP, Mihatsch MJ, Nickeleit V, Ramos E, Randhawa P, Shapiro R, Steiger J, Suthanthiran M, Trofe J. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation. 2005;79:1277-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 743] [Cited by in RCA: 707] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 34. | Hirsch HH, Randhawa P; AST Infectious Diseases Community of Practice. BK polyomavirus in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 407] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 35. | Singh HK, Andreoni KA, Madden V, True K, Detwiler R, Weck K, Nickeleit V. Presence of urinary Haufen accurately predicts polyomavirus nephropathy. J Am Soc Nephrol. 2009;20:416-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 36. | Dadhania D, Snopkowski C, Ding R, Muthukumar T, Lee J, Bang H, Sharma VK, Seshan S, August P, Kapur S, Suthanthiran M. Validation of noninvasive diagnosis of BK virus nephropathy and identification of prognostic biomarkers. Transplantation. 2010;90:189-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Park WY, Kang SS, Jin K, Park SB, Choe M, Han S. Long-term prognosis of BK virus-associated nephropathy in kidney transplant recipients. Kidney Res Clin Pract. 2018;37:167-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Alméras C, Foulongne V, Garrigue V, Szwarc I, Vetromile F, Segondy M, Mourad G. Does reduction in immunosuppression in viremic patients prevent BK virus nephropathy in de novo renal transplant recipients? A prospective study. Transplantation. 2008;85:1099-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Weiss AS, Gralla J, Chan L, Klem P, Wiseman AC. Aggressive immunosuppression minimization reduces graft loss following diagnosis of BK virus-associated nephropathy: a comparison of two reduction strategies. Clin J Am Soc Nephrol. 2008;3:1812-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Prince O, Savic S, Dickenmann M, Steiger J, Bubendorf L, Mihatsch MJ. Risk factors for polyoma virus nephropathy. Nephrol Dial Transplant. 2009;24:1024-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Bohl DL, Storch GA, Ryschkewitsch C, Gaudreault-Keener M, Schnitzler MA, Major EO, Brennan DC. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. Am J Transplant. 2005;5:2213-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 226] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 42. | Ekberg H, Tedesco-Silva H, Demirbas A, Vitko S, Nashan B, Gürkan A, Margreiter R, Hugo C, Grinyo JM, Frei U, Vanrenterghem Y, Daloze P, Halloran PF; ELITE-Symphony Study. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1362] [Cited by in RCA: 1381] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 43. | Sood P, Senanayake S, Sujeet K, Medipalli R, Saad E, Vasudev B, Bresnahan BA, Johnson CP, Hariharan S. Lower prevalence of BK virus infection in African American renal transplant recipients: a prospective study. Transplantation. 2012;93:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Tahir S, Gillott H, Jackson-Spence F, Nath J, Mytton J, Evison F, Sharif A. Do outcomes after kidney transplantation differ for black patients in England versus New York State? A comparative, population-cohort analysis. BMJ Open. 2017;7:e014069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Patel SJ, Suki WN, Loucks-DeVos J, Graviss EA, Nguyen DT, Knight RJ, Kuten SA, Moore LW, Teeter LD, Gaber LW, Gaber AO. Disparate rates of acute rejection and donor-specific antibodies among high-immunologic risk renal transplant subgroups receiving antithymocyte globulin induction. Transpl Int. 2016;29:897-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | Fehr T, Cippà PE, Mueller NJ. Cytomegalovirus post kidney transplantation: prophylaxis versus pre-emptive therapy? Transpl Int. 2015;28:1351-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 47. | Schachtner T, Babel N, Reinke P. Different risk factor profiles distinguish early-onset from late-onset BKV-replication. Transpl Int. 2015;28:1081-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Elfadawy N, Flechner SM, Liu X, Schold J, Srinivas TR, Poggio E, Fatica R, Avery R, Mossad SB. CMV Viremia is associated with a decreased incidence of BKV reactivation after kidney and kidney-pancreas transplantation. Transplantation. 2013;96:1097-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |