Published online Dec 6, 2019. doi: 10.12998/wjcc.v7.i23.4119
Peer-review started: August 22, 2019
First decision: September 23, 2019
Revised: October 14, 2019
Accepted: November 15, 2019
Article in press: November 15, 2019
Published online: December 6, 2019
Processing time: 106 Days and 5.2 Hours
Pancreatic mixed serous-neuroendocrine neoplasms (MSNNs) are mixed tumors containing two components with different pathologies, namely, pancreatic serous cystic neoplasm (PSCN) and pancreatic neuroendocrine tumor (PanNET). For MSNNs, diffuse PSCN involving the whole pancreas is extremely rare, with only eight previous case reports.
A 45-year-old Chinese woman, with a free previous medical history and no obvious symptoms, was found to have a pancreatic neoplasm and admitted to our hospital for further diagnosis in March 2018. Abdominal palpation revealed a painless, mobile mass in the epigastrium, and no abnormalities were observed in an examination of the nervous system and ocular system. A computed tomography scan showed multiple cystic lesions involving the whole pancreas ranging in diameter from 0.4 to 2 cm and also revealed an enhanced mass, 2.2 cm in diameter, in the head of the pancreas. Moreover, multiple cysts were found in the kidneys bilaterally, and the right lobe of the liver contained a small cyst. A Whipple operation with total pancreatectomy and splenectomy was performed. A diagnosis of pancreatic MSNN was established, consisting of diffuse serous microcystic cystadenoma with a concomitant grade 2 PanNET. Of note, the patient had no personal or family history of Von Hippel-Lindau syndrome or other disease.
We report the first case of MSNN with a diffuse PSCN component involving the entire pancreas in a Chinese woman. It is important to be aware of its relationship with VHL syndrome, and close clinical follow-up is recommended.
Core tip: Mixed serous-neuroendocrine neoplasm (MSNN). MSNNs are a mixed tumor containing two components with different pathologies, namely pancreatic serous cystic neoplasm (PSCN) and pancreatic neuroendocrine tumor (PanNET). For MSNNs, diffuse PSCN involving the whole pancreas is extremely rare with only eight previous case reports. Here, we report a novel case of diffuse PSCN admixture with an isolated PanNET in a Chinese woman, and also review the previous literature. Due to its close relationship with Von Hippel-Lindau syndrome, and the malignant potential of neuroendocrine component, patients with MSNN should undergo thorough systemic examinations, including molecular genetic analysis, and close clinical follow-up is also recommended.
- Citation: Xu YM, Li ZW, Wu HY, Fan XS, Sun Q. Mixed serous-neuroendocrine neoplasm of the pancreas: A case report and review of the literature. World J Clin Cases 2019; 7(23): 4119-4129
- URL: https://www.wjgnet.com/2307-8960/full/v7/i23/4119.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i23.4119
Pancreatic serous cystic neoplasms (PSCNs) account for only 1%-2% of all pancreatic tumors[1]. In the 2010 World Health Organization (WHO) Digestive System Tumor Classification, PSCN was classified into five subtypes[2], including serous microcystic cystadenoma (SMA), serous oligocystic cystadenoma (SOA), solid serous adenoma, Von Hippel-Lindau (VHL) syndrome-associated serous cystic neoplasm, and mixed serous-neuroendocrine neoplasm (MSNN). MSNNs are termed mixed tumors containing two components with different pathologies, namely, PSCN and pancreatic neuroendocrine tumor (PanNET). For MSNNs, PSCN diffuse involvement of the whole pancreas is extremely rare, with only eight cases reported in the literature[3-10]. Here, we report a novel case of diffuse PSCN admixture with an isolated PanNET in a Chinese woman and review the previous literature. The timeline is shown in Table 1.
| Time line | |
| 2019.03-2019.04 | Collecting the data and follow-up |
| 2019.04-2019.05 | Reading the literature |
| 2019.05-2019.06 | Writing |
| 2019.06-2019.07 | Revising and plotting |
| 2019.08- | Submitting |
A 45-year-old Chinese woman, with no obvious symptoms, was found to have a pancreatic neoplasm in the physical examination and admitted to our hospital for further diagnosis.
The patient had a free previous medical history.
She denied any special personal or family history of VHL syndrome or other disease.
Abdominal palpation revealed a painless, mobile mass in the epigastrium, and no abnormalities were observed in an examination of the nervous system and ocular system.
Laboratory tests for blood glucose, amylase, and tumor markers, including carcinoembryonic antigen (0.51 ng/mL, normal 0-10 ng/mL), cancer antigen-19-9 (11.11 U/mL, normal 0-27 U/mL), and cancer antigen-242 (8.54 U/mL, normal 0-10 U/mL) were within normal limits.
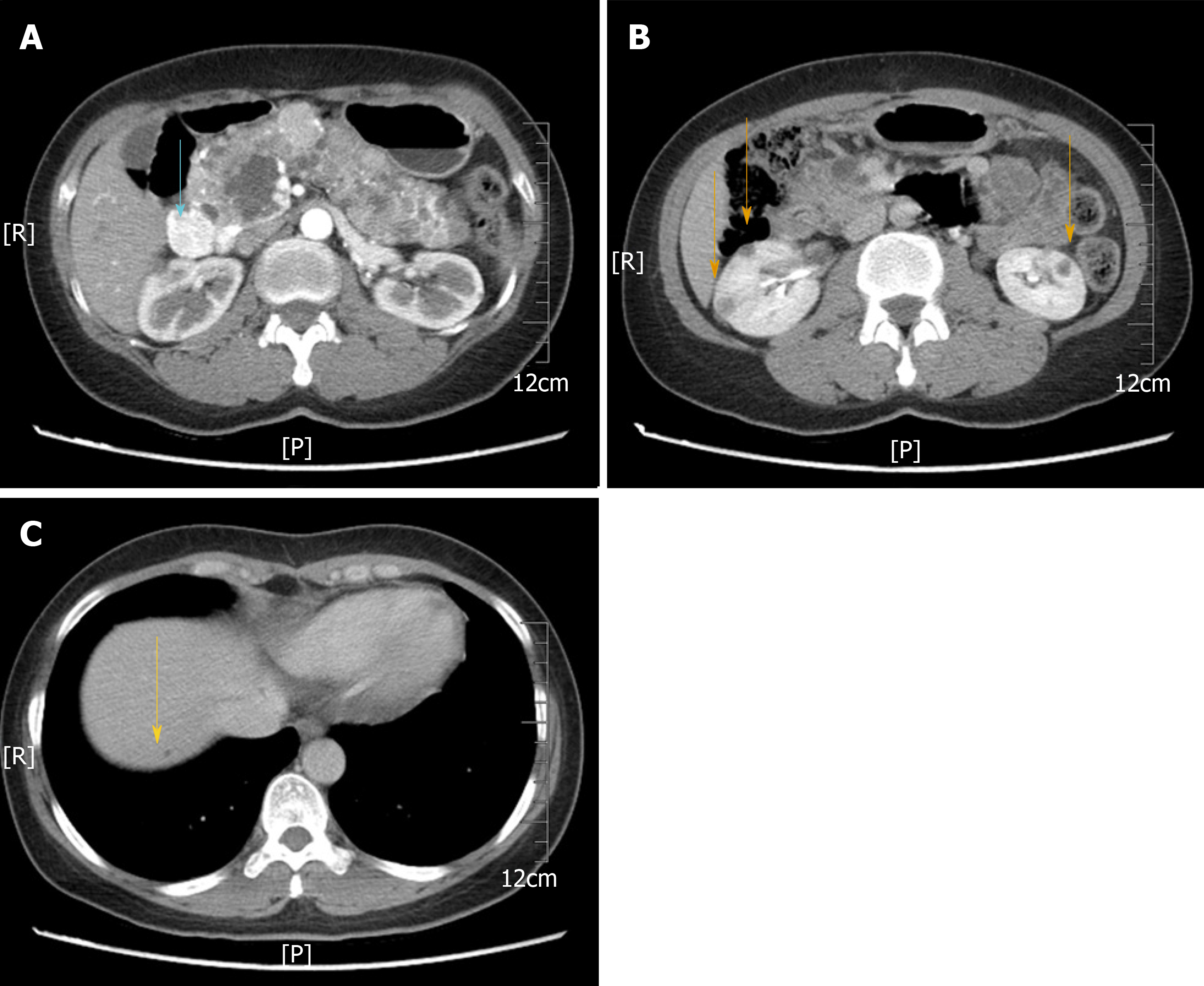
A computed tomography scan showed multiple cystic lesions involving the whole pancreas ranging in diameter from 0.4 to 2 cm and also revealed an enhanced mass, 2.2 cm in diameter, in the head of the pancreas (Figure 1A). Moreover, multiple cysts were found in the kidneys bilaterally (Figure 1B), and the right lobe of the liver contained a small cyst (Figure 1C). No abnormalities were noted on brain magnetic resonance imaging.
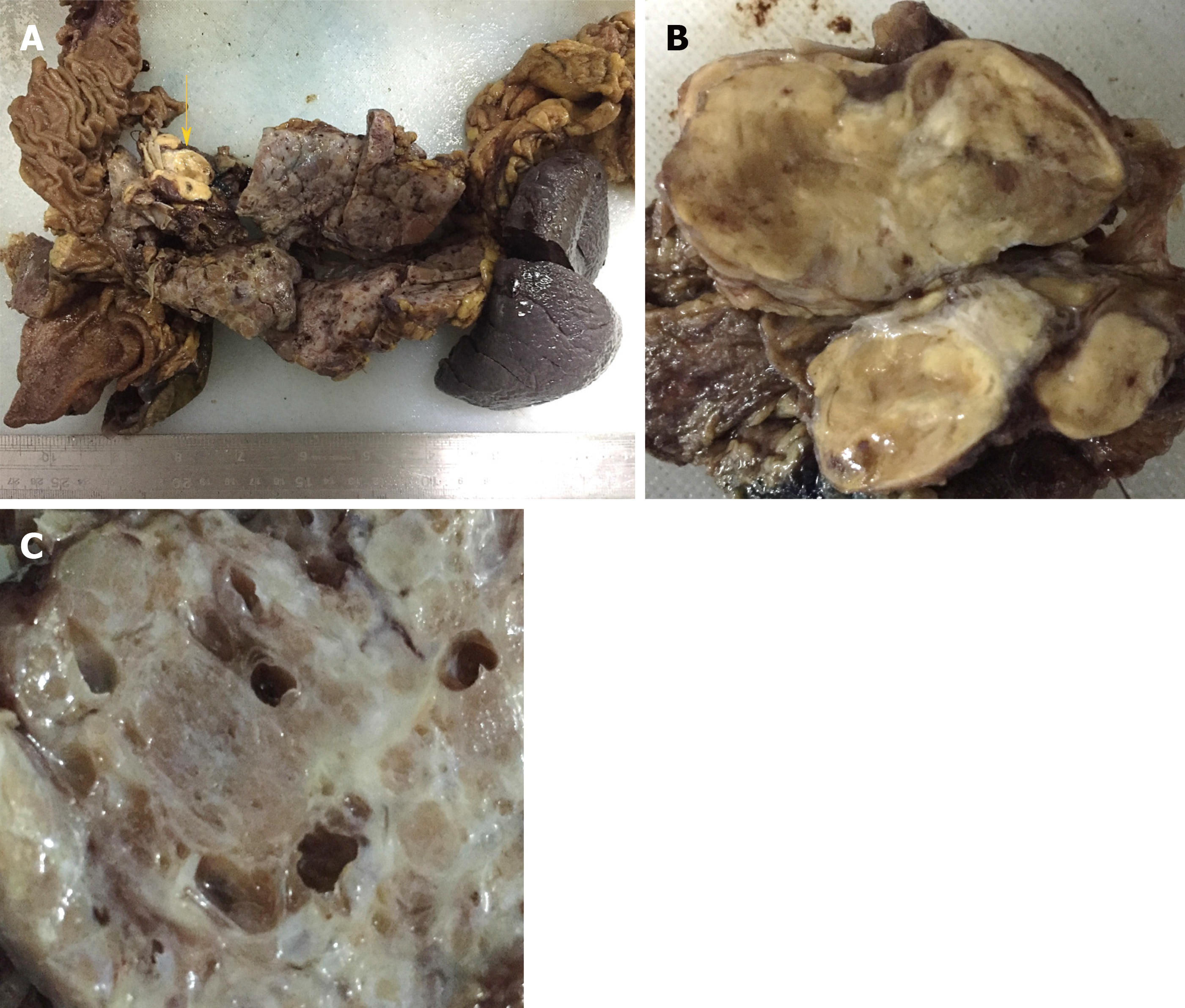
Grossly, the pancreatic parenchyma was replaced with numerous discrete and confluent cysts. The pancreatic parenchyma displayed spongy-like changes on histological section, which was composed of cystic, honeycomb-like, and cystic-nodular areas. The cysts varied in size, ranging from 0.1 to 1.1 cm in diameter. The nodular area was demarcated by grayish-white fibrous septa, and a radial scar was observed in the center of some nodules. Clear fluid was found in the cystic cavity. A grayish-yellow solid mass, which was 3.5 cm × 2 cm × 2 cm in size, was in the pancreatic head with a clear boundary separated from the cystic area (Figure 2).
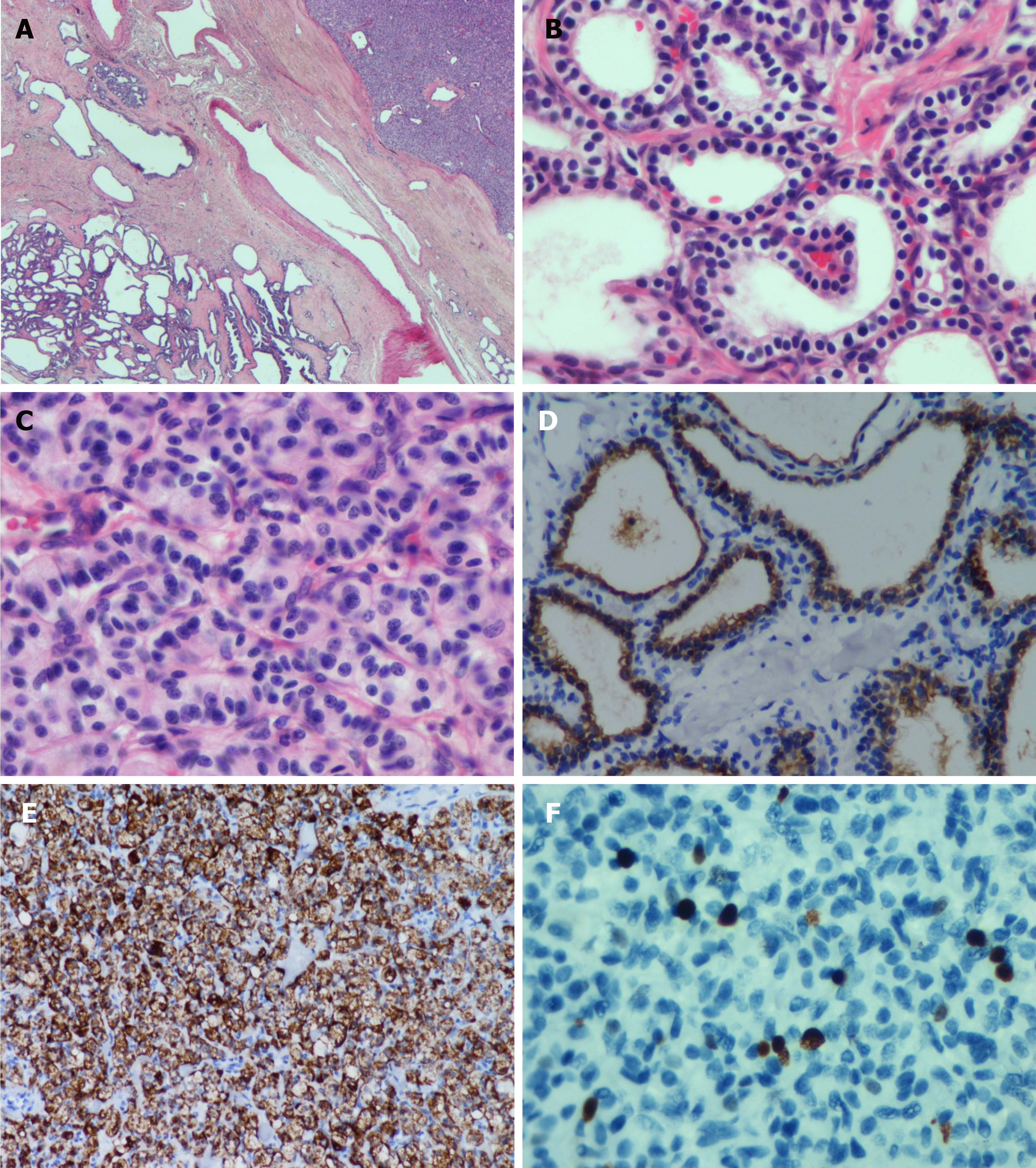
Microscopically, the histological morphology of the diffuse cystic lesions and the solid mass appeared significantly different, and the two components of the pancreatic tumor were separated by compressed fibrous tissue without any overlapping zone (Figure 3A). The diffuse cystic area was composed of prominent microcystic serous cystadenomas lined with a single layer of cubic to flattened epithelium with clear cytoplasm; the nuclei were uniformly small, round-to-oval, and bland with dense homogenous chromatin (Figure 3B). The pancreatic duct was normal, without any communication with the cysts. The solid tumor in the head of the pancreas demonstrated a well-differentiated grade 2 PanNET: The tumor cells were arranged in a ribbon-like and trabecular architecture with fine chromatin, and the mitotic count was 1 per 10 high power fields (HPF) (Figure 3C).
Immunohistochemically, the lining epithelium of the diffuse serous cystadenoma stained positive for cytokeratin (CK) 7 (Figure 3D) and CK19. The low-grade PanNET was positive for synaptophysin, chromogranin A (Figure 3E), and CD56. Both of the two pancreatic tumors were negative for HER-2 (Supplementary Figure 1). The Ki67 labeling index of the PanNET was 5% (Figure 3F). In addition, the tumor cells were hormone-negative (including insulin, glucagon, and gastrin), which, along with the patient’s clinical presentation, suggested a nonfunctional PanNET.
Based on these analyses, a diagnosis of pancreatic MSNN was established, consisting of diffuse SMA with a concomitant grade 2 PanNET. With this diagnosis in mind, we suspected that the patient may have VHL syndrome as she had clinical manifestations associated with VHL syndrome[11]: Pancreatic MSNN, bilateral renal cysts, and a hepatic cyst. However, no evidence of hemangioblastoma of the central nervous system has been found in this patient, and she refused to take a genetic test to confirm the VHL gene mutation.
A Whipple operation with total pancreatectomy and splenectomy was performed.
After surgery, the patient was followed for 30 mo with no evidence of disease recurrence or metastasis of pancreatic tumor.
PSCNs are rare benign tumors. Occasionally, other tumors could be found concurrently with PSCN in an excised pancreatic specimen, such as PanNET, pancreatic ductal adenocarcinoma, intraductal papillary mucinous neoplasm, and metastatic tumors[12]. PanNETs are the most common mixed tumor findings, and when found with PSCNs, the mixed tumor is termed MSNN according to the WHO classification[2]. In a large cohort of 193 PSCNs, the simultaneous occurrence of PSCN and PanNET appeared in 6% (12/193) of all cases[12]. Including the first report by Kamei et al[13] in 1991, 32 similar cases of MSNN were published in the English-language literature from PubMed (Table 2) until June 2019; however, only 22 cases, including ours, contain detailed clinicopathological data[3-10,13-22] to allow analysis. In the remaining specimens, the patients range in age from 25 to 78 years (mean, 50.1 years). There is a preponderance of females, with a female to male ratio of 19:3. The common tumor-related symptoms and signs include abdominal pain (7/22), nausea and vomiting (4/22), and obstructive jaundice (3/22), and one patient presented with watery diarrhea-hypokalemia-achlorhydria syndrome (WDHA) caused by a vasoactive intestinal polypeptide-secreting tumor (VIPoma)[21]. Other patients were asymptomatic (8/22) or had rare clinical manifestations, including abdominal distension, postprandial fullness, melena, anorexia, peptic ulcer, and weight loss.
| Ref. | Sex/age | Symptoms | Other conditions | PSCN | PanNET | Distribution pattern of PSCN and PanNET | Family history | VHL | Follow-up (mo) | |||||
| Location/ size(cm) | Pathologic diagnosis | Location/ size (cm) | Pathologic diagnosis | Invasion/metastasis | IHC hormone expression | Clinical function | ||||||||
| Diffuse PSCN | ||||||||||||||
| Kim et al[3] | F/67 | Melena | Diabetes mellitus | EP/16 | SMA | Head/2.8 | NET/NA | DWI and LYI | No | No | DT/NET separated from SMA | NA | NA | NA |
| Baek et al[4] | F/29 | Abdominal distension and vomiting | NA | EP/30 | SCN | Head/NA | NET/NA | PNI and LYI | Insulin | NA | DT/NET within SCN | No | Yes | NA |
| Jung et al[5] | F/29 | Nausea, vomiting, and weight loss | No | EP/NA | SMA and SOA | Head/2 | NET/NA | PNI and LYI | Insulin | NA | DT/NET within SCN | No | No | NA |
| Blandamura et al[6] | F/31 | Jaundice | Renal clear cell carcinoma, cerebellar hemangioblastoma, and multiple liver angiomas | EP/NA | SMA | Head/4 | NET/G2 | DWI and VI | Glucagon and VIP | NA | DT/NET collision with SMA | NA | Yes | NA |
| Agarwal et al[7] | F/35 | Abdominal pain, anorexia, and postprandial fullness | Small cysts in both kidneys and syringohydromyelia in the lower thoracic cord | EP/16 | SMA and SOA | Head/<0.5 | NET/NA | LNM | NA | NA | DT/NET collision with SCN | No | Suspected | 3 |
| Hsieh et al[8] | F/28 | No | Cerebellar hemangioblastoma | EP/NA | SOA | Uncinate process/2.8 | NET/NA | LYI, PSI, and PNI | NA | NA | DT/NET collision with SOA | No | Yes | 8 |
| Tewari et al[9] | F/25 | Abdominal pain | No | EP/NA | SOA | NA/NA | NET/NA | PNI | NA | NA | DT/NA | NA | No | 24 |
| Maeda et al[10] | F/39 | No | Cerebellar and retinal hemangioblastomas | EP/NA | SMA and SOA | Tail/3 | NET/G1 | N | NA | NA | DT/NET within SCN | No | Yes | 8 |
| Our case | F/45 | No | Multiple renal cysts in both kidneys | EP/16 | SMA | Head/3.5 | NET/G | PSI and PNI | No | NA | DT/NET separated from SMA | No | Suspected | 30 |
| Multiple PSCN | ||||||||||||||
| Kamei et al[13] | F/72 | Jaundice and abdominal distension | No | Six tumors involving head and body/0.5-10 | SMA | Tail/1.5 | NET/NA | No | NA | NA | ST/NET separated from SMA | No | No | NA |
| Kakkar et al[14] | M/38 | Abdominal pain and jaundice. | Bilateral adrenal pheochromocytomas, paravertebral paraganglioma, and lymph node tuberculosis | Multiple tumors involving head and body/NA | SMA | Head/5 | NET/G2 | DWI | No | NA | CT/NET collision with SMA | No | Yes | 10/dead of acute adrenal crisis |
| Isolated PSCN | ||||||||||||||
| Keel et al[15] | F/47 | Abdominal pain and jaundice | Lupus | Head/7.5 | SMA | Head/1.5 | NET/NA | NA | No | No | MT/NET within SMA | NA | NA | NA |
| Ustün et al[16] | F/49 | Abdominal pain, nausea, and vomiting | Diabetes mellitus | Head and body/13 | SMA | Head/NA | NET/NA | No | Glucagon and insulin | NA | MT/NET within SMA | NA | No | 12 |
| Slukvin et al[17] | M/53 | No | No | Head/4 | SMA | Head/1.2 | NET/NA | No | Somatostatin | NA | MT/NET within SMA | No | No | NA |
| Alasio et al[18] | F/78 | Abdominal pain and peptic ulcer | Hypertension, stable exertional angina, and chronic obstructive pulmonary disease | Body and tail/14 | SMA | Body/5 | NET/NA | No | NA | NA | MT/NET within SMA | NA | No | NA |
| Goh et al[19] | M/52 | No | Hepatitis B carrier | Head/1.5 | SMA | Tail/0.3 | NET/NA | No | Glucagon | No | ST/NET separated from SMA | No | No | 24 |
| Mohan et al[20] | F/52 | Abdominal pain, nausea, and vomiting | Diabetes mellitus. | Body/10 | SMA | Body/NA | NET/NA | NA | NA | NA | MT/NET within SMA | NA | No | 2 |
| Blandamura et al[6] | F/71 | No | Uterine leiomyomastosis and renal oncocytoma | Body/2.5 | SOA | Body/1.1 | NET/ G1 | No | Glucagon | NA | ST/NET separated from SOA | NA | No | NA |
| Blandamura et al[6] | F/57 | No | Left adnexal adenoma, gallbladder microlithiasis, and diabetes mellitus | Body/2.3 | SOA | Body/0.5 | NET/G1 | No | Insulin | NA | ST/NET separated from SOA | NA | No | NA |
| Hsieh et al[8] | F/64 | Abdominal pain | Diabetes mellitus and asthma | Body/1.3 | SMA | Body/0.6 | NET/NA | No | NA | NA | ST/NET separated from SMA | NA | No | 14 |
| Borka et al[21] | F/69 | WDHA syndrome | Parathyroid adenoma | Tail/NA | SCN | Tail/NA | NET/G1 | NA | VIP | VIPoma | MT/NET within SCN | NA | No | 36 |
| Li et al[22] | F/73 | No | Hypertension | Tail/2.5 | SOA | Tail/1.2 | NET/G1 | PNI and PSI | NA | NA | CT/NET collision with SOA | NA | No | 54 |
| NA | ||||||||||||||
| Reid et al[12] | 12 cases | NA | NA | NA | NA | NA | NET/NA | NA | NA | NA | NA | No | No | NA |
Although only a solitary PanNET component occurred in each analyzed MSNN case (Table 2), nine patients showed diffuse PSCN involving the whole pancreas, two had multiple PSCN, and the remaining 11 displayed an isolated PSCN. Most PSCNs were SMA or SMA with SOA component (15/22). SOA also appeared in five cases. Based on the distribution pattern of PSCN and PanNET, MSNN was categorized by Li et al[22] into four subtypes as follows: (1) Diffuse subtype: The entire pancreas is occupied by numerous PSCNs associated with one or more PanNETs, which could be located in any region of the pancreas; (2) Mixed subtype: The two different tumors combined with each other in a mass cannot be distinctly divided; (3) Solitary subtype: The two different components coincide in the pancreas without any intermingling area; and (4) Collision subtype: The two different lesions are separated from each other in most parts, but with a partially intermixed or overlapping zone. Among the analyzed MSNNs, 41% (9/22) were diffuse type, 27% (6/22) were mixed type, 23% (5/22) were solitary type, and 9% (2/22) were collision type. Histologically, most PSCNs can be easily differentiated from PanNETs. However, in rare cases, PSCN consists of small uniform nests or tubules with minimal lumen formation, specifically the solid variant of PSCN or solid serous adenoma[12]. These cases can be misdiagnosed as PanNET[23]. Indeed, the differential diagnosis of PSCNs and PanNETs can be challenging, especially in small biopsies. However, PSCN can be distinguished from PanNET based on the lack of reactivity for neuroendocrine markers (such as chromogranin A)[24], but it displays positive epithelial markers (such as CK19 and CK7) and possibly other markers (such as α-inhibin and calponin)[25,26].
The diagnostic nomenclature and classification criteria of PanNETs were inconsistent until 2010. However, the recent 2017 WHO classification classified pancreatic neuroendocrine neoplasms into well-differentiated NETs and poorly differentiated neuroendocrine carcinomas (NECs), as supported by genetic evidence[27]. PanNETs are further graded on a scale of 1-3 based on mitotic rates and Ki67 proliferation index as follows: PanNET G1 with <2 mitoses/10 HPF and a <3% Ki67 index; PanNET G2 with 2-20 mitoses/10 HPF and a 3%-20% Ki67 index; and PanNET G3 with >20 mitoses/10 HPF and a >20% Ki67 index. Accordingly, after reviewing the pathological images of the previous case reports of MSNNs, all the PanNET components diagnosed as “low-grade/well-differentiated malignant NET” or “well-differentiated NEC” should be reclassified as well-differentiated PanNET rather than a poorly differentiated PanNEC. In the analyzed cases, PanNETs favored the pancreatic head/uncinate process region over the body and the tail (11:5:5) of the pancreas and ranged in size from 0.3 to 5 cm (mean, 2.2 cm). Although nine PanNETs were hormone-positive by immunohistochemical examination, including insulin, glucagon, vasoactive intestinal polypeptide, and somatostatin, all but one case presented with a nonfunctional PanNET. As mentioned before, a 69-year-old woman with a history of parathyroid adenoma presented with typical symptoms of WDHA syndrome, and then histological examination revealed a PSCN coexisting with a VIPoma in the tail of the pancreas. Although MEN-1 syndrome was suspected, molecular genetic analysis did not show a MEN-1 gene mutation[21].
VHL syndrome is a rare autosomal dominant hereditary disease that is caused by mutations of the embryonic line of the VHL gene on chromosome 3p25-26[28]. The incidence of pancreatic involvement in VHL disease is 17%-77%[8,29]. PSCN and PanNET are classic presentations of VHL syndrome. In Hammel et al[29]’s study, 12.3% of VHL patients showed PSCN, 12.3% had PanNET, and 6.9% had MSNN. In addition, Cassol et al[30] reported that up to 17% of patients with VHL disease may develop PanNETs. In our reviewed cases from the literature, 14% (5/34) of MSNNs were confirmed to be associated with VHL syndrome, and two cases were suspected (Table 2). We categorized these patients into two groups according to the presence of VHL disease (Table 3). The mean age of VHL-related MSNN is less than that of the same VHL-unrelated association (33 years vs 55 years, P < 0.001). MSNN with a diffuse PSCN component is more likely to occur in VHL patients (P = 0.043). It has been reported that nearly 10% of PSCN patients could be demonstrated to have a germline mutation of the VHL gene[31]. Unfortunately, including our present case, none of the MSNNs reported previous genetic testing for VHL being performed, so the incidence of MSNN in VHL patients is still unclear. Even though the tumor size of the PanNET component in MSNN is larger in VHL patients than in its non-VHL counterpart (P = 0.016), PanNETs demonstrated signs of violation behavior in both VHL and non-VHL groups, such as duodenal wall/peripancreatic soft tissue invasion, lymphatic/venous permeation, perineural invasion, and lymph node metastasis. Patients with VHL syndrome are prone to suffering visceral cysts and markedly vascularized neoplasms in various organs[28]. As such, in addition to pancreatic tumors, patients in the VHL-related group were more likely to suffer from other tumors (P = 0.009), such as renal clear cell carcinoma, cerebellar/retina hemangioblastoma, liver angioma, adrenal pheochromocytoma, and paravertebral paraganglioma (Table 2).
| Characteristic | VHL | Non-VHL | P |
| Case number | 5 | 17 | |
| Sex (female:male) | 4:1 | 15:2 | 0.637 |
| Age | 33 (28-39) | 55 (25-78) | <0.001 |
| DT PSCN | 4/5 (80%) | 5/17 (29%) | 0.043 |
| PanNET size (cm) | 3.7 (2.8-5) | 1.7 (0.3-5) | 0.016 |
| Invasion | 4/5 (80%) | 6/17 (35%) | 0.078 |
| IHC hormone expression | 2/5 (40%) | 7/17 (41%) | 0.962 |
| Other tumors | 4/5 (80%) | 3/17 (18%) | 0.009 |
As all the PSCN components of MSNN are benign per our review, no recurrence of metastasis occurred during the follow-up period. Indeed, true malignant behavior is exceedingly rare in PSCN[12]. Nevertheless, PanNETs are considered low-grade malignancies[2], so the prognosis of MSNN is dependent on the PanNET component. As demonstrated by Agarwal et al[7], lymph node metastasis occurred even when the diameter of the PanNET component was less than 0.5 cm. Therefore, the pathologist must perform a careful gross examination and sampling to exclude the presence of a concomitant PanNET in PSCN specimens.
There are two hypotheses to explain the formation of MSNN: (1) Common progenitor cells; or (2) Distinct progenitor cells. For the first hypothesis, both PSCN and PanNET could derive from a common pluripotent stem cell[15], as supported by the observation of biphasic differentiation in a pancreatic lesion, such as nesidioblastosis, a nonneoplastic disorder with coincident epithelial and endocrine differentiation[32]. Additionally, Kakkar et al[14] identified neurosecretory granules, glycogen, and intermediate filaments within the same tumor cells by ultrastructural examination. This may explain cases in which both tumors are intermingled intimately but cannot explain those with separated lesions. For the second hypothesis, both counterparts in MSNN could derive from two different cell types, which would probably arise from a predisposing genetic disorder, such as VHL syndrome[6]. Further support for this hypothesis is that the genetic mutations of PSCN are different from those of PanNET[31], indicating a distinctive pathogenesis of the two tumors. Taken together, further studies, especially molecular analysis, are still needed to elucidate the pathogenesis and origin of MSNN.
We report a rare case of MSNN with a diffuse PSCN component involving the entire pancreas in a Chinese woman. Due to its close relationship with VHL syndrome and the malignant potential of the neuroendocrine component, patients with MSNN should undergo thorough systemic examination, including molecular genetic analysis, and close clinical follow-up is recommended.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fujino Y, Gurzu S S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Liu MY
| 1. | Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 490] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 2. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumors of the Digestive System. Lyon: World Health Organization; 2010; . |
| 3. | Kim YW, Park YK, Lee S, Park JH, Lee SM, Hong SW, Lee J, Yang MH. Pancreatic endocrine tumor admixed with a diffuse microcystic adenoma--a case report. J Korean Med Sci. 1997;12:469-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Baek SY, Kang BC, Choi HY, Lee SW. Pancreatic serous cystadenoma associated with islet cell tumour. Br J Radiol. 2000;73:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Jung HK, Son HY, Lee HC, Yi SY. Microcytic adenoma coexistent with low-grade malignant islet cell tumor of the pancreas. J Clin Gastroenterol. 2001;32:441-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Blandamura S, Parenti A, Famengo B, Canesso A, Moschino P, Pasquali C, Pizzi S, Guzzardo V, Ninfo V. Three cases of pancreatic serous cystadenoma and endocrine tumour. J Clin Pathol. 2007;60:278-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Agarwal N, Kumar S, Dass J, Arora VK, Rathi V. Diffuse pancreatic serous cystadenoma associated with neuroendocrine carcinoma: a case report and review of literature. JOP. 2009;10:55-58. [PubMed] |
| 8. | Hsieh MS, Liu KL, Tien YW, Shun CT. Combined pancreatic endocrine tumor and serous cystadenoma. J Formos Med Assoc. 2009;108:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Tewari M, Patne S, Katiyar R, Biswas D, Shukla HS. Co-existence of Diffuse Serous Cystadenoma and Pancreatic Neuroendocrine Tumor. Indian J Surg. 2017;79:455-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Maeda S, Motoi F, Oana S, Ariake K, Mizuma M, Morikawa T, Hayashi H, Nakagawa K, Kamei T, Naitoh T, Unno M. Pancreatic neuroendocrine tumor with complete replacement of the pancreas by serous cystic neoplasms in a patient with von Hippel-Lindau disease: a case report. Surg Case Rep. 2017;3:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Maher ER, Neumann HP, Richard S. von Hippel-Lindau disease: a clinical and scientific review. Eur J Hum Genet. 2011;19:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 437] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 12. | Reid MD, Choi HJ, Memis B, Krasinskas AM, Jang KT, Akkas G, Maithel SK, Sarmiento JM, Kooby DA, Basturk O, Adsay V. Serous Neoplasms of the Pancreas: A Clinicopathologic Analysis of 193 Cases and Literature Review With New Insights on Macrocystic and Solid Variants and Critical Reappraisal of So-called "Serous Cystadenocarcinoma". Am J Surg Pathol. 2015;39:1597-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Kamei K, Funabiki T, Ochiai M, Amano H, Kasahara M, Sakamoto T. Multifocal pancreatic serous cystadenoma with atypical cells and focal perineural invasion. Int J Pancreatol. 1991;10:161-172. [PubMed] |
| 14. | Kakkar A, Sharma MC, Yadav R, Panwar R, Mathur SR, Iyer VK, Sahni P. Pancreatic mixed serous neuroendocrine neoplasm with clear cells leading to diagnosis of von Hippel Lindau disease. Pathol Res Pract. 2016;212:747-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Keel SB, Zukerberg L, Graeme-Cook F, Compton CC. A pancreatic endocrine tumor arising within a serous cystadenoma of the pancreas. Am J Surg Pathol. 1996;20:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Ustün MO, Tuğyan N, Tunakan M. Coexistence of an endocrine tumour in a serous cystadenoma (microcystic adenoma) of the pancreas, an unusual association. J Clin Pathol. 2000;53:800-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Slukvin II, Hafez GR, Niederhuber JE, Warner TF. Combined serous microcystic adenoma and well-differentiated endocrine pancreatic neoplasm: a case report and review of the literature. Arch Pathol Lab Med. 2003;127:1369-1372. [PubMed] |
| 18. | Alasio TM, Vine A, Sanchez MA, Dardik H. Pancreatic endocrine tumor coexistent with serous microcystic adenoma: report of a case and review of the literature. Ann Diagn Pathol. 2005;9:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Goh BK, Tan YM, Kumarasinghe MP, Ooi LL. Synchronous serous cystadenoma and pancreatic endocrine tumor: a case report and literature review. Dig Dis Sci. 2006;51:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Mohan H, Garg S, Punia RP, Dalal A. Combined serous cystadenoma and pancreatic endocrine neoplasm. A case report with a brief review of the literature. JOP. 2007;8:453-457. [PubMed] |
| 21. | Borka K, Winternitz T, Tihanyi B, Zalatnai A, Lakatos P, Patócs A, Harsányi L, Tímár J. Coexistence of a pancreatic serous cystadenoma and a VIP-producing neuroendocrine tumor. Pancreatology. 2015;15:S110-S111. [DOI] [Full Text] |
| 22. | Li Y, Dai M, Chang X, Hu W, Chen J, Guo J, Wu W, Zhang T, Liao Q, Liu Z, Hu Y, Zhao Y. Mixed serous neuroendocrine neoplasm of the pancreas: Case report and literature review. Medicine (Baltimore). 2016;95:e4205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Geramizadeh B, Dabbaghmanesh MH, Nikeghbalian S, Soleimani N. Solid Serous Adenoma of Pancreas, Misdiagnosed as Neuroendocrine Tumor, a Rare Case Report and Review of the Literature. J Gastrointest Cancer. 2016;47:462-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Kanehira K, Khoury T. Neuroendocrine markers expression in pancreatic serous cystadenoma. Appl Immunohistochem Mol Morphol. 2011;19:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Kosmahl M, Wagner J, Peters K, Sipos B, Klöppel G. Serous cystic neoplasms of the pancreas: an immunohistochemical analysis revealing alpha-inhibin, neuron-specific enolase, and MUC6 as new markers. Am J Surg Pathol. 2004;28:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Marsh WL, Colonna J, Yearsley M, Bloomston M, Frankel WL. Calponin is expressed in serous cystadenomas of the pancreas but not in adenocarcinomas or endocrine tumors. Appl Immunohistochem Mol Morphol. 2009;17:216-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | González-Flores E, Serrano R, Sevilla I, Viúdez A, Barriuso J, Benavent M, CapdevilaJ, Jimenez-Fonseca P, López C, Garcia-Carbonero R. SEOM clinical guidelines for the diagnosis and treatment of gastroenteropancreatic and bronchial neuroendocrine neoplasms (NENs) (2018). Clin Transl Oncol. 2019;21:55-63. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Maher ER, Iselius L, Yates JR, Littler M, Benjamin C, Harris R, Sampson J, Williams A, Ferguson-Smith MA, Morton N. Von Hippel-Lindau disease: a genetic study. J Med Genet. 1991;28:443-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 358] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 29. | Hammel PR, Vilgrain V, Terris B, Penfornis A, Sauvanet A, Correas JM, Chauveau D, Balian A, Beigelman C, O'Toole D, Bernades P, Ruszniewski P, Richard S. Pancreatic involvement in von Hippel-Lindau disease. The Groupe Francophone d'Etude de la Maladie de von Hippel-Lindau. Gastroenterology. 2000;119:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 237] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 30. | Cassol C, Mete O. Endocrine manifestations of von Hippel-Lindau disease. Arch Pathol Lab Med. 2015;139:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Katabathina VS, Rikhtehgar OY, Dasyam AK, Manickam R, Prasad SR. Genetics of Pancreatic Neoplasms and Role of Screening. Magn Reson Imaging Clin N Am. 2018;26:375-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Zumkeller W. Nesidioblastosis. Endocr Relat Cancer. 1999;6:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |