Published online Dec 6, 2019. doi: 10.12998/wjcc.v7.i23.4063
Peer-review started: March 8, 2019
First decision: September 9, 2019
Revised: October 16, 2019
Accepted: November 14, 2019
Article in press: November 14, 2019
Published online: December 6, 2019
Processing time: 276 Days and 8 Hours
Micronodular thymic tumors with lymphoid stroma include micronodular thymoma with lymphoid stroma (MNT) and micronodular thymic carcinoma with lymphoid hyperplasia (MNC), whose micromorphological features are lymphoid stromal hyperplasia and nodular arrangement of tumor epithelial cells. This type of tumor is rare; therefore, the corresponding clinical guidelines, histopathological diagnostic criteria, prognostic factors, and therapeutic regimens have not been established.
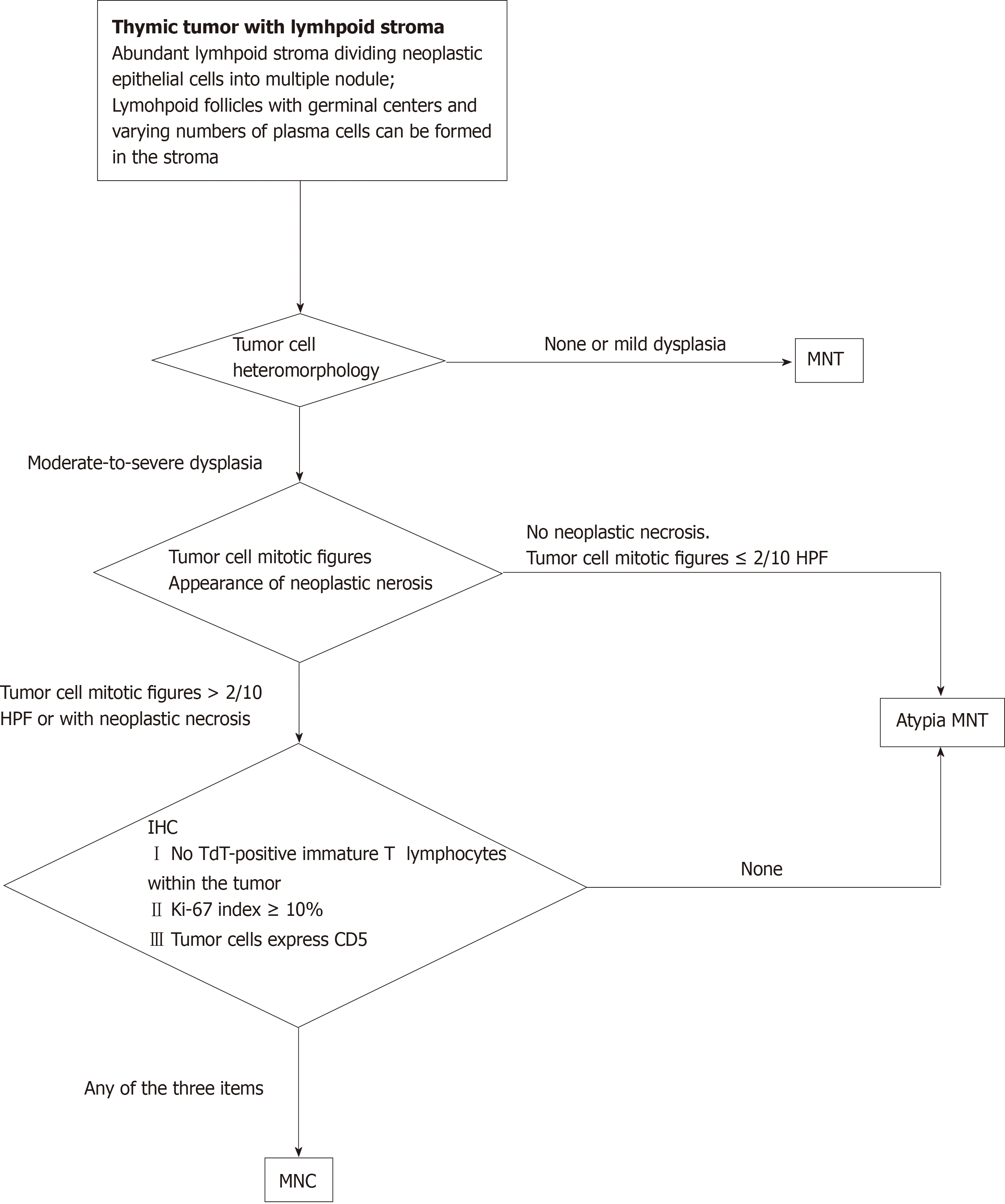
This study covers a novel presentation of MNC in a patient and summarizes the clinicopathological characteristics of this type of tumor by using pooled-analysis methods. Morphologically, this tumor type is a series of benign to malignant pedigrees. We establish the following criteria for the classification of micronodular thymic tumors with lymphoid stroma: (1) Tumor cells with moderate-to-severe dysplasia; (2) Tumor cell mitotic figures > 2/10 high-power fields; (3) Appearance of neoplastic necrosis; (4) No terminal deoxynucleotidyl transferase-positive immature T lymphocytes within the tumor; (5) Tumor cells with a Ki-67 index ≥ 10%; and (6) Tumor cells express CD5. Cases that fall into the borders of two categories in terms of morphology are attributed to atypical MNT. It is proposed that the diagnosis of MNT should be established on the diagnostic criteria mentioned above.
Our diagnostic algorithm can effectively distinguish malignant tumors from benign tumors and provides a potent basis for predicting a prognosis, which offers a practical reference for oncologists and pathologists.
Core tip: This study covers a novel presentation of micronodular thymoma with lymphoid stroma in a patient and summarizes the clinicopathological characteristics of this type of tumor by using pooled-analysis methods. Morphologically, this tumor type contains a series of benign to malignant pedigrees. We established criteria for the classification of micronodular thymic tumors with lymphoid stroma. Our diagnostic algorithm can effectively distinguish malignant tumors from benign tumors and provides a potent basis for predicting patient prognosis.
- Citation: Wang B, Li K, Song QK, Wang XH, Yang L, Zhang HL, Zhong DR. Micronodular thymic tumor with lymphoid stroma: A case report and review of the literature. World J Clin Cases 2019; 7(23): 4063-4074
- URL: https://www.wjgnet.com/2307-8960/full/v7/i23/4063.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i23.4063
The thymus is an organ that contains T lymphocytes. Immature T lymphocytes gradually mature in the process of migrating from the thymus cortex to the medulla, while B lymphocytes only exist in the thymus medulla. As the most common neoplasm of the thymus, thymus tumors are characterized by T lymphocyte infiltration. Nevertheless, it is rare for thymic neoplasms to form germinal centers within the thymic cortex with a heavy infiltration of B lymphocytes. In 1999, Suster et al[1] first reported and named this type of tumor as micronodular thymoma with lymphoid stroma (MNT). According to different tumor types, micronodular thymic tumors with lymphoid stroma include MNT and micronodular thymic carcinoma with lymphoid hyperplasia (MNC) and the presence of micronodules in tumor epithelial cells; the micromorphological feature of MNC is the proliferation of lymphoid follicle-infiltrating lymphoid stroma[2]. This type of tumor is very rare; therefore, the corresponding clinical guidelines, histopathological diagnostic criteria, prognostic factors, and therapeutic regimens have not been established. This paper reports a case of MNC, and pooled-analysis methods were used to summarize the classification and clinicopathological characteristics of this type of tumor, providing a practical reference for oncologists and pathologists.
On January 29, 2018, a 57-year-old female patient presented with symptoms of an upper respiratory tract infection, which were manifesting as cough and fever (up to 38°C).
Since the onset of the disease, the patient showed the following symptoms: Gradually changing sputum color, back pain, limb weakness, difficulty swallowing, coughing blood, wheezing, chest pain, and stool abnormality. Nevertheless, she was in good spirits, was in good health, was sleeping well, and had no substantial alterations in weight or physical manifestations on the upper face.
The patient had no history of hypertension, diabetes, nerve disease, cerebrovascular disease, neuropsychiatric disease, hepatitis, tuberculosis, malaria, mental process, trauma, blood transfusion, or food or drug allergies, and the history of vaccination was unknown.
The patient had no epidemic exposure and no exposure to chemical, radioactive, and toxic substances. The patient had no history of drug abuse, smoking, or drinking. The patient’s parents are still alive, and the investigation found that there was no family genetic history of lung cancer.
The patient’s body temperature was 37°C, pulse was 60/min, respiratory rate was 20/min, and blood pressure was 120/80 mmHg. There was no yellow staining, rash, subcutaneous bleeding, subcutaneous nodules, or scar ulcers. In addition, there were no masses in the superficial lymph nodes or eyelid edema. The patient had a normal conjunctiva, no yellow staining in the sclera, normal pupil, no abnormal secretions in the external auditory canal, no tenderness in the mastoid handling, and a normal sense of olfactory perception. At the time of admission, the patient had a normal gingiva and oral mucosa. The tongue color was normal, and the patient did not have enlarged tonsils or thyroid gland. In addition, there were no deformities in the chest, no veins in the chest wall, and no tapping pain in the breastbone. Respiratory movement, intercostal space, and tremor were normal. The patient had normal breathing, obvious respiratory sounds in both lungs, no dry or wet sounds, and no pleural friction sounds.
There was no uplift in the precordial region, the apical pulsatile position was normal, the heart rate was 60 beats/min, the heart sound was powerful and steady, the murmur was not heard in each periodical auscultation area, and there was no pericardial friction. The abdominal area of the patient was soft; the patient had no abdominal wall varicose veins, no tenderness, and no rebound pain. The liver and spleen were not touched, Murphy’s sign was negative, there was no mobile voiced sound, and there was no immediate pain in the retinal area. Bowel sounds were normal at 4 times/min.
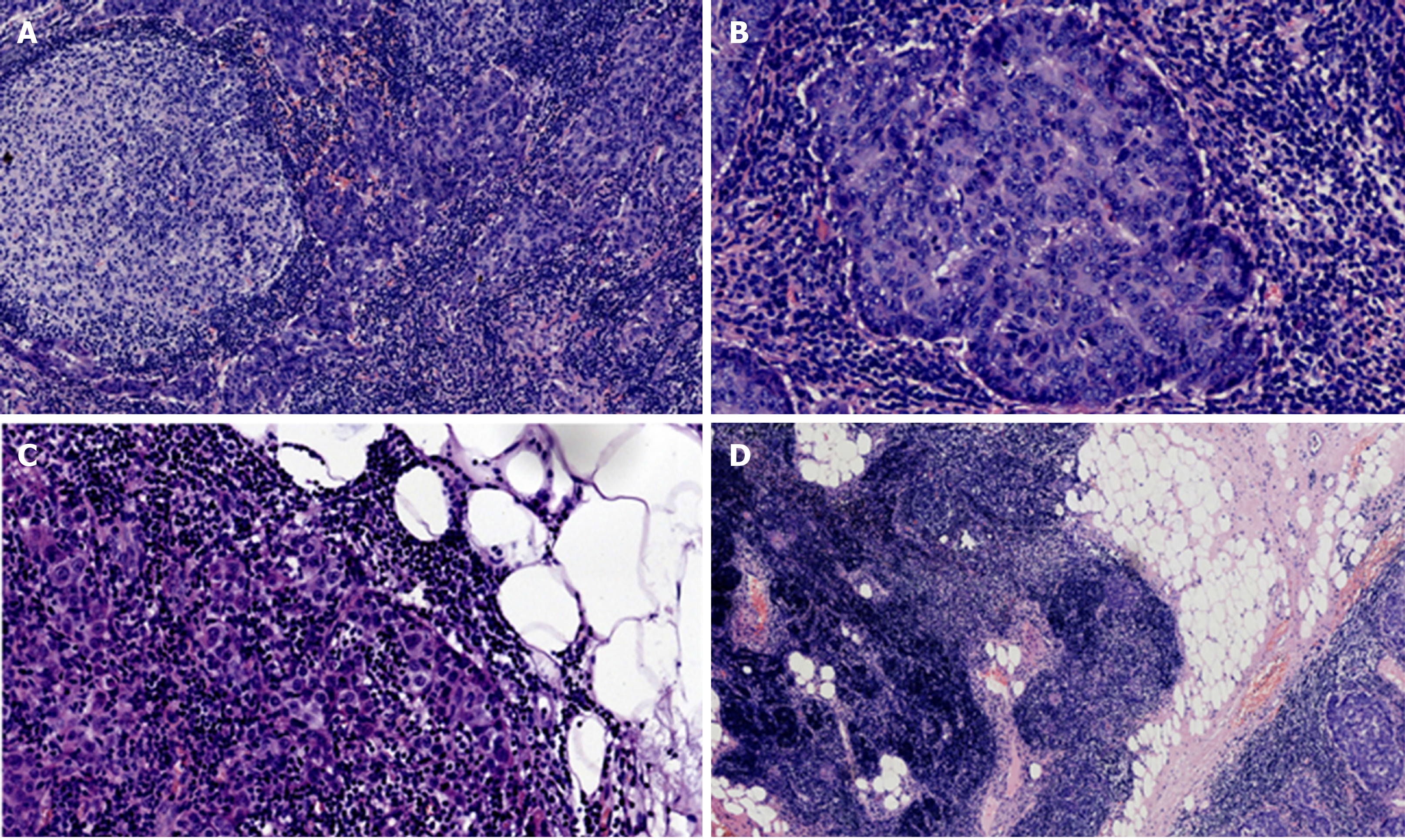
Hematoxylin and eosin staining was performed on the tumor tissue, and microscopic observation showed that the tumor presented with multiple micronodules, which were separated by abundant lymphocyte interstitium and formed lymphoid follicles. No necrotic structures were found in the tumor tissue, and tumor cells infiltrating the surrounding adipose tissue could be caught at the boundary of the neoplasm. Residual thymic tissue was observed around the adipose tissue. The neoplastic cells showed marked heteromorphic structures with nucleoli and mitotic figures in some areas (1 to 9/10 high power fields [HPF] at the high power field). The staining of different areas of the tumor is shown in Figure 1A-1D.
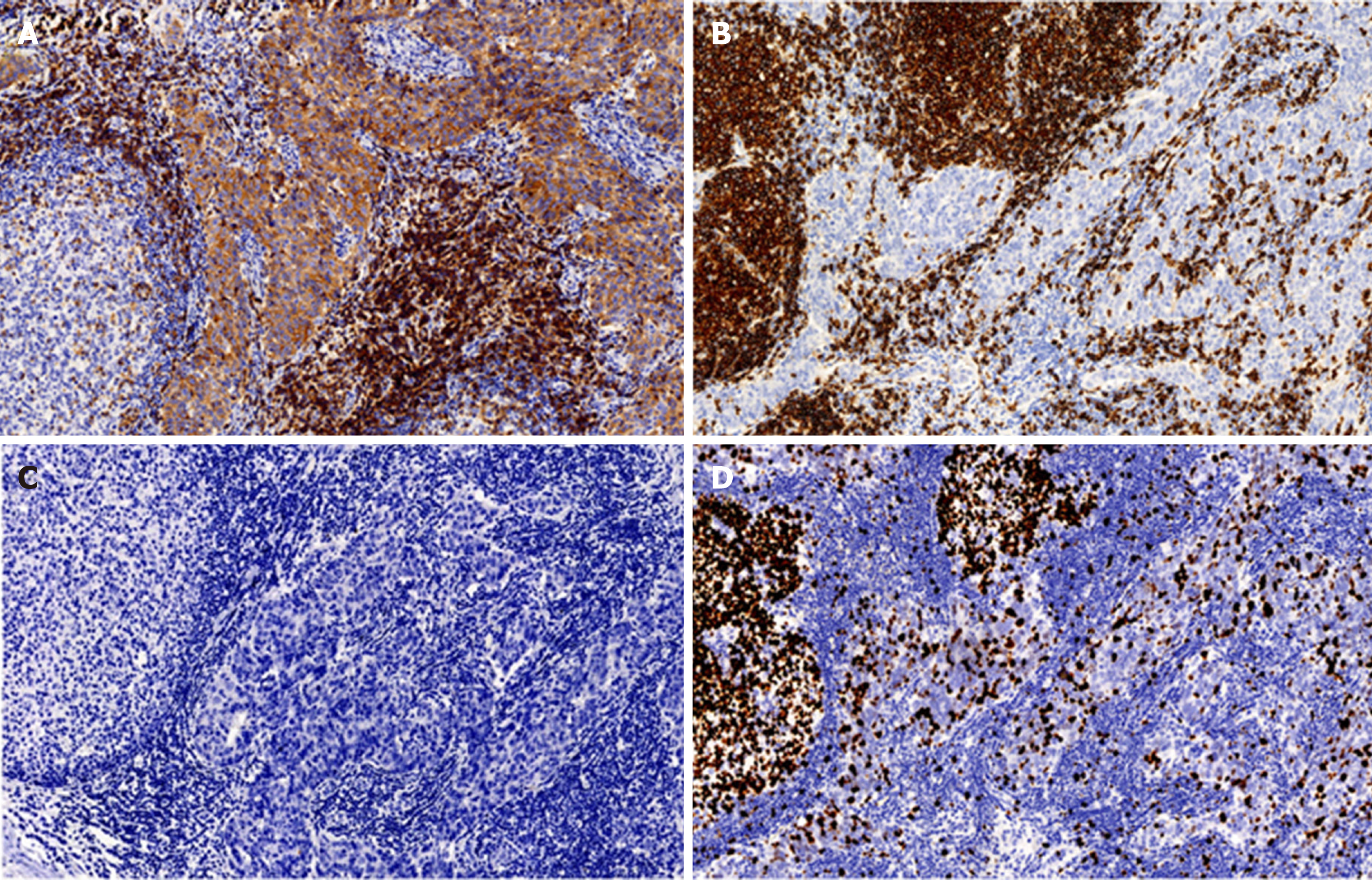
Immune status of T lymphocytes and B lymphocytes in the tumor was detected by the EnVision immunohistochemical method[3], and the results showed that there was strongly positive expression of CD5, CD117, pancytokeratin (CK-pan) (AE1-AE3), cytokeratin 19, and B-cell lymphoma-2 (Bcl-2) in tumor tissues. The rate of Ki-67 positive cells reached up to 30%. Human epidermal growth factor receptor 2 (HER2) and P53 were negative.
The expression of CD20 and CD3 in stromal lymphocytes was positive, the positive rate of CD20 was high, and lymphoid follicle formation was also noted. Terminal deoxynucleotidyl transferase (TdT)-positive immature lymphocytes were not found in tumor tissues (Figure 2A-2D, Table 1).
| Tumor cells | Lymphocytes in stroma | |
| CD5 | Diffuse, strong, cytomembrane | Focal, strong, cytomembrane |
| CD3 | Negative | Focal, strong, cytomembrane and cytoplasm |
| CD20 | Negative | Focal, strong, cytomembrane |
| CD117 | Diffuse, strong, cytomembrane and cytoplasm | Negative |
| CK-pan | Diffuse, strong, cytoplasm | Negative |
| CK-19 | Diffuse, strong, cytoplasm | Negative |
| Bcl-2 | Focal, strong, cytomembrane and cytoplasm | Focal, strong, cytomembrane and cytoplasm |
| Her-2 | Negative | Negative |
| P53 | Mottled expression | Negative |
| Ki-67 | Approximately 30% of cells, strong, nucleus | Focal, especially in the germinal center, strong, nucleus |
| TdT | Negative | Negative |
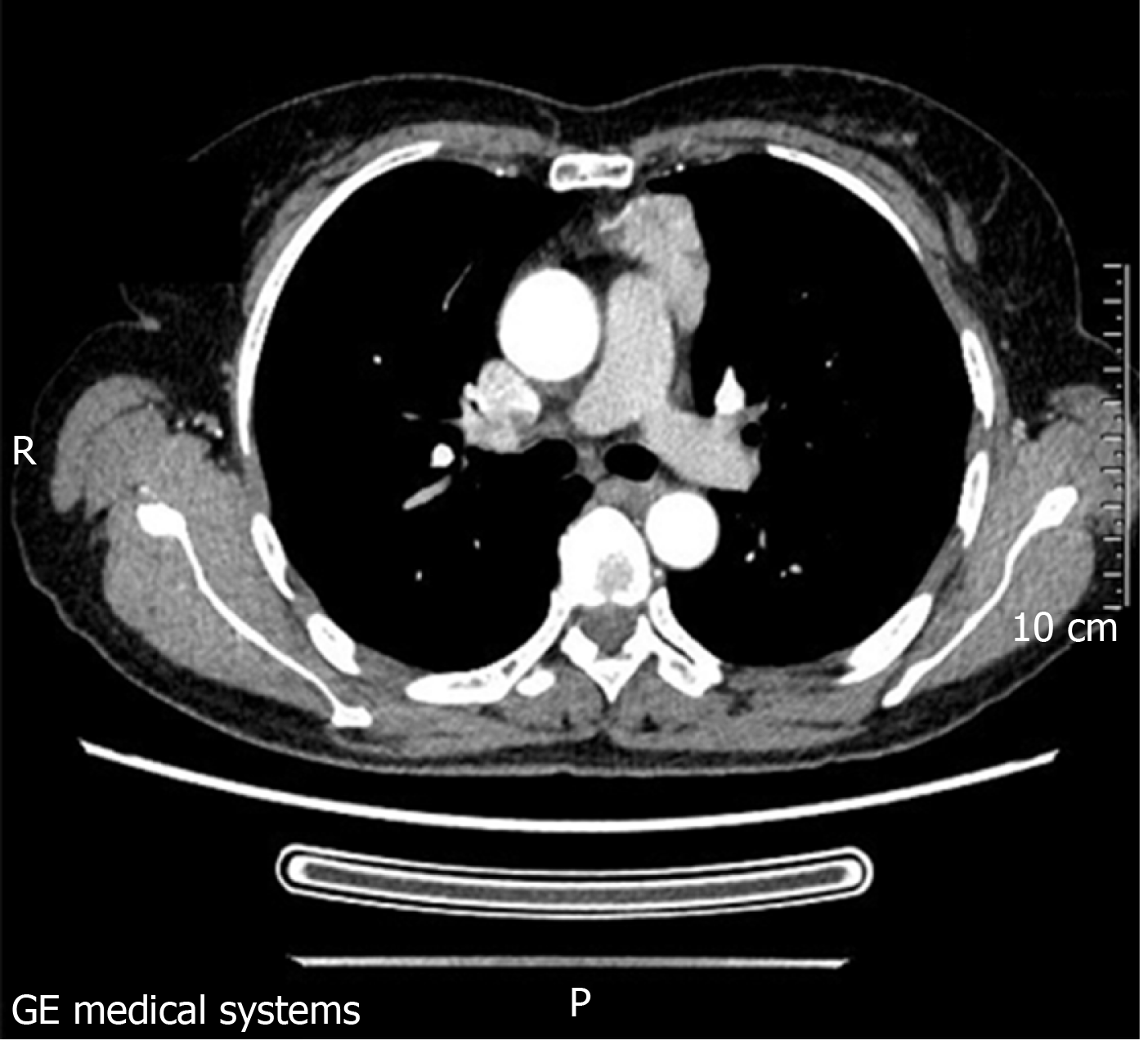
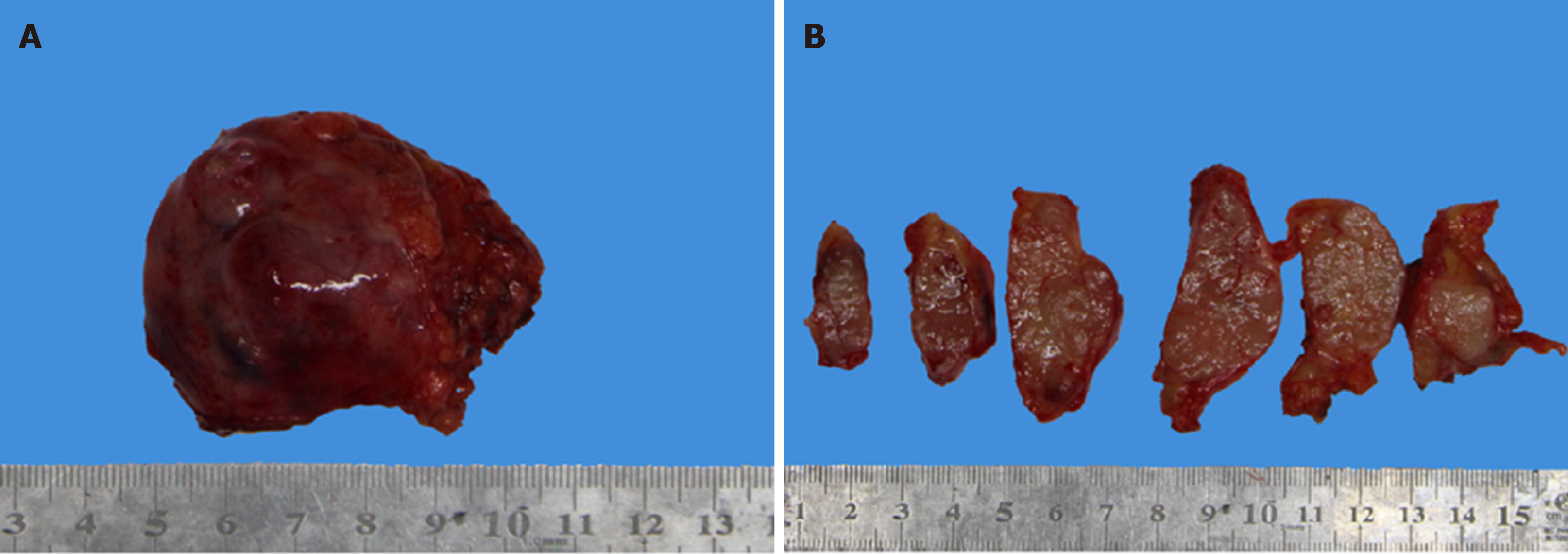
The patient’s computed tomography (CT) image is shown in Figure 3. The tumor was found to be a solid mass in the anterior mediastinum with pericardial invasion. However, there was adhesion between the tumor and pericardium, but at that stage, there was no definite pericardial invasion and the tumor should be classified as Masaoka stage IIb. The lump was completely resected under thoracoscopy, and the patient was not treated with radiotherapy or chemotherapy postoperatively. The nodular lump measured 5.5 cm × 5 cm × 1.8 cm in size. The tumor was encapsulated with a clear margin. The hybrid section was solid, grayish-white, grayish-brown, and somewhat textured. There was no clear border between the tumor and the surrounding adipose tissue (Figure 4A and 4B).
This case was pathologically diagnosed as MNC.
The tumor was completely resected by video-assisted thoracoscopic surgery.
No postoperative radiotherapy or chemotherapy was performed. Postoperatively, at the 12-mo follow-up, the patient’s condition was relatively stable without tumor relapse or metastasis.
PubMed and Chinainfo databases were searched for the relevant articles. The inclusion criteria were as follows: (1) The literature must contain case materials about micronodular thymic tumor with lymphoid stroma; (2) All patients were pathologically diagnosed with relatively complete pathological features; (3) When a case was reported more than once in multiple publications, the most recent literature with the largest sample size was selected; (4) Articles published in English or Chinese; and (5) Articles published during 1999-2018. The elimination criteria were as follows: (1) Cases reported repeatedly; and (2) Reviews, abstracts, and comments.
The retrieval was performed with “micronodular thym” as the English keyword and “Wei Jie Jie” and “Xiong Xian” as Chinese keywords to search the literature. The manual and keyword retrievals were performed at the same time. According to the inclusion and exclusion criteria, two researchers completed the literature retrieval independently and verified the retrieval results mutually. For any disputable articles, the two researchers discussed the article with a third researcher to decide whether to include it. The classification of benign and malignant cases was based on the Tateyama’s method[4] and Masaoka stage[2,5,6]. Ultimately, the clinicopathological and prognostic characteristics, treatment approaches, and prognoses were extracted.
SPSS23.0 statistical software was applied to eliminate the extreme values, t-test was employed to evaluate the data, and chi-squared test was employed to analyze the counting data. P < 0.05 indicated that the difference was statistically significant.
A total of 36 English articles and 12 Chinese articles were retrieved. According to the exclusion criteria, 30 English case reports and 8 Chinese case reports that met the requirements were selected. A total of 95 patients were recorded in these reports, of which 23 were reported in Chinese and 72 in English. There were 92 cases of primary thymic tumors and 3 cases of cervical ectopic thymic tumors. The detailed clinicopathological characteristics, treatment approaches, and prognoses of all included cases are shown in Table 2.
| Variable item | Number of cases | Percentage (%) |
| Age (median, age span) | 63, 41-83 | - |
| Sex | ||
| Male | 51 | 53.7 |
| Female | 44 | 46.3 |
| Benignity and malignancy[2,27] | ||
| Benign (MNT) | 77 | 81.1 |
| Malignancy (MNC) | 18 | 18.9 |
| Site | ||
| Thymic source | 92 | 96.8 |
| Extra-thymic source | 3 | 3.2 |
| Clinical symptoms | ||
| No obvious symptoms | 76 | 80.0 |
| Local symptoms | 11 | 11.6 |
| Immune related symptoms | 8 | 8.4 |
| Masaoka stage | ||
| 1 | 52 | 59.8 |
| 2a | 16 | 18.4 |
| 2b | 13 | 14.9 |
| 3 | 4 | 4.6 |
| 4 | 2 | 2.3 |
| Tumor size (cm) (mean, standard deviation, size span) | 5.14 ± 2.32, 1.2-10 | - |
| Tumor cross section | ||
| Cystic or cystic and solid | 35 | 44.3 |
| Solid | 44 | 55.7 |
| Tumor cell atypia | ||
| No | 58 | 66.7 |
| Mild | 6 | 6.9 |
| Moderate | 7 | 8.0 |
| Severe | 16 | 18.4 |
| Mitotic figures[25] | ||
| ≤ 2/10 HPF | 62 | 78.5 |
| 2/10 HPF | 17 | 21.5 |
| Necrosis | ||
| No | 38 | 77.6 |
| Yes | 11 | 22.4 |
| CD5 staining in tumor cells | ||
| Negative | 43 | 79.6 |
| Focally positive or diffusely positive | 11 | 20.4 |
| Ki-67 staining[25] | ||
| ≤ 2% of tumor cells | 15 | 51.7 |
| 2% to 10% of tumor cells | 5 | 17.2 |
| ≥ 10% of tumor cells | 9 | 31.0 |
| TdT positive lymphocytes | ||
| No | 12 | 42.9 |
| Few | 7 | 25.0 |
| Many | 9 | 32.1 |
| Treatment | ||
| Resection | 84 | 93.3 |
| Resection + radiotherapy or chemotherapy | 4 | 4.4 |
| Radiotherapy + chemotherapy | 1 | 1.1 |
| No treatment | 1 | 1.1 |
| Follow-up time | 2 d to 22 yr | |
| Follow-up results | ||
| Loss to follow-up | 18 | 21.2 |
| No relapse or metastasis | 60 | 70.6 |
| Tumor relapse | 1 | 1.2 |
| Death caused by nontumorous reason | 5 | 5.8 |
| Tumor-caused death | 1 | 1.2 |
To compare the differences in pathogenic factors between MNT and MNC, we analyzed the influencing factors between the MNT group and the MNC group; these factors included age, sex, clinical symptoms, Masaoka stage, mitotic figure, cellular atypia, tumor size, tumor necrosis, follow-up results, CD5-positive tumor cells, Ki-67-positive tumor cells, TdT-positive lymphocytes, and treatment. The results are shown in Table 3. There were no significant differences in age, sex, clinical symptoms, or tumor cystic size between the two groups (P > 0.05); however, there were significant differences in other influencing factors (Masaoka stage, mitotic number, cellular atypia, tumor size, tumor necrosis, follow-up results, CD5-positive tumor cells, Ki-67-positive tumor cells, TdT-positive lymphocytes, and treatments) between the two groups (P < 0.05).
| MNT group | MNC group | t or χ2 | P | |
| Age | 63.4 ± 9.45 | 63.4 ± 9.45 | 1.954 | 0.054 |
| Sex | 0.31 | 0.860 | ||
| Male | 41 | 10 | ||
| Female | 36 | 8 | ||
| Clinical symptoms | 0.577 | 0.749 | ||
| No | 65 | 14 | ||
| Local symptoms | 8 | 3 | ||
| Immune related symptoms | 4 | 1 | ||
| Masaoka stage | 18.980 | < 0.001 | ||
| I | 44 | 8 | < 0.001 | |
| II | 26 | 3 | ||
| III | 1 | 3 | ||
| IV | 0 | 2 | ||
| Mitotic figures | 41.214 | < 0.001 | ||
| ≤ 2/10HPF | 61 | 1 | ||
| > 2/10HPF | 6 | 11 | ||
| Cell atypia | 75.792 | < 0.001 | ||
| No | 57 | 1 | ||
| Mild | 6 | 0 | ||
| Moderate | 6 | 1 | ||
| Severe | 0 | 16 | ||
| Tumor size | 43.34 ± 43.094 | 53.00 ± 73.04 | 0.539 | 0.591 |
| Tumor necrosis | 12.459 | < 0.001 | ||
| No | 31 | 7 | ||
| Yes | 0 | 4 | ||
| Follow-up results | ||||
| No relapse or metastasis | 51 | 9 | 9.582 | 0.002 |
| Tumor relapse or tumor-caused death | 0 | 2 | ||
| CD5 staining in tumor cells | 5.264 | 0.022 | ||
| Negative | 44 | 12 | ||
| Positive | 1 | 4 | ||
| Ki67 staining in tumor cells | 13.426 | 0.001 | ||
| ≤ 2% | 15 | 0 | ||
| 2-10% | 5 | 0 | ||
| ≥ 10% | 4 | 5 | ||
| TdT positive lymphocytes | 14.933 | 000 | ||
| No | 4 | 8 | ||
| Yes | 16 | 0 | ||
| Treatment | 9.697 | 0.021 | ||
| Resection | 71 | 13 | ||
| Resection + radiotherapy or chemotherapy | 3 | 1 | ||
| Radiotherapy + chemotherapy | 0 | 1 | ||
| No treatment | 0 | 1 | ||
The incidence of micronodular thymic tumors with lymphoid stroma was low[7,8], accounting for 1% to 5% of all thymic tumors; most cases were reported in the form of case reports in Chinese and English journals. The research data for biological behaviors of such tumors are still insufficient; therefore, we integrated the existing cases and adopted a pooled-analysis method to summarize and analyze the clinicopathological materials of such rare tumors.
This type of disease mostly occurs in middle-aged and elderly patients, and the sex difference is not significant. Most patients have no obvious clinical symptoms; a few patients show local oppression, which is accompanied by thymus oppression, chest tightness, cough, and other symptoms. A very low number of patients may develop myasthenia gravis, but these clinical manifestations can be a simple forecaster for the disease.
Instead, this type of tumor involves an organic thymus epithelium characterized by large amounts of lymphoid stroma that divides tumor epithelial cells into multiple nodules. Lymphoid follicles with germinal centers and different numbers of plasma cells were formed in the stroma. The tumor cells were slight fusiform or oval, with oval nuclei, inconspicuous nucleoli, and mitotic appearance. In summary, a low number of cells are polygonal with different degrees of heteromorphism. The nuclei of the cells are round, the nucleoli are visible or obvious, and there are obvious mitotic figures.
In accordance with epithelial cells, tumor cells and epithelial cells can express CK-pan[9], CD5, CD117[10], and Bcl-2[1]. Previous studies have shown that CD5 is a specific marker of thymic carcinoma, but recent studies have found that CD5 is also expressed in other tumors and other types of thymoma[11]. CD117 plays an important role in the differential diagnosis of thymoma and thymic carcinoma. These markers are mostly negative in thymomas but positive in up to 86% of thymic carcinomas[12]. In thymoma, Bcl-2 is mainly expressed in lymphocytes, while in the fusiform and mixed thymoma, it is expressed in the focal sites[13]. In other studies, overexpression of Bcl-2 was associated with the invasiveness of thymic tumors[14,15]. CD5, CD117, and Bcl-2 were all positive in our study, which supported our diagnosis. The P53 protein may be a marker of a higher degree of malignancy of thymic tumors. Compared with thymoma, it has more expression in thymic carcinoma, and it also has higher expression levels in invasive thymoma than in noninvasive thymoma. Other studies have shown that P53 is also associated with shorter disease-free survival and overall survival[16]. Overexpression of HER2 is rare in thymoma but relatively common in thymic carcinoma[15]. In our case, both markers were negative, and we collected very limited data from the literature. Even without an indication of P53 expression, we can conclude that the patient we studied was diagnosed with MNC with a low degree of malignancy.
In the lymphoid stroma, B lymphocytes were mainly CD20-positive, while the number of mature T lymphocytes varied in different parts of the lesion but were mainly positive for CD3 and CD5. In some cases, immature T lymphocytes were mainly distributed at the edge of the tumor cell nodules or scattered in tumor cells, which were mainly positive for CD1a and TDT. These immunohistochemical staining results show that the lymphoid matrix is composed of mature B and T lymphocytes, which is the most important manifestation of this type of thymoma. Immature T cells (TDT+) are common in thymomas but may be dispersed or absent.
The accurate staging of thymic tumors has always been a challenge. Currently, there are two staging systems available for thymomas, which are classified as stages 1-4 according to the degree of tumor diffusion and invasion; tumors with a higher degree of malignancy often have a later stage. This staging system was developed by Masaoka in 1981 and updated by KenjiKoga in 1994[6,17]. The Masaoka-Koga system has been used to stage thymic tumors, and Masaoka stage is considered a good prognostic indicator in clinical practice. In 2017, the American Joint Committee on Cancer (AJCC)[18,19] proposed another method for staging thymic tumors. However, the progress of each staging system has not yet been confirmed[20]. In short, for this rare thymus tumor, the clinical course is indolent, and local excision is thought to be curative. Complete surgical removal of tumor tissues is critical to guarantee that no tumor residue remains at the incisal margins[21-23]. Whether further radiotherapy or chemotherapy is required for micronodular thymic carcinoma with lymphoid stroma warrants further investigation. The current study found that only 2 of the 18 patients reported recurrence or metastasis, and these patients were followed for 3 months to 22 years, indicating a lower degree of malignancy[2,24].
According to the WHO 2015 International Classification of Diseases, MNT belongs to lesions with low malignant potential or uncertainty (CADE, ICD-O 8580/1). MNCs are malignant tumors (ICD-O 8586/3). We found that tumor recurrence or tumor death was only found in MNCs. Therefore, from the pathological perspective, the differential diagnosis of these two diseases is of great significance to predict the prognosis. After a comprehensive analysis of these studies[4,7,25,26], we observed some polygonal tumor-like epithelial cells with a certain amount of type B2 thymomaism, which is similar to type B2 thymomas. As a result, it is important to distinguish that the classification of a thymoma is atypical MNT or B2 thymoma with lymphoid stroma. There are a few types of reports on this type of case; therefore, further studies are needed to determine whether MNTs and MNCs are continuous or completely independent diseases.
Current data indicate that thymic tumors with lymphoid stroma are benign or malignant and can be distinguished by the following criteria: (1) Moderate and severe dysplasia of tumor cells; (2) Tumor cell mitotic figures > 2/10 HPF; (3) Tumor necrosis; (4) No TdT-positive immature T lymphocytes in the tumor; (5) Ki-67 index of tumor cells ≥ 10%; and (6) Tumor cells express CD5.
Morphologically, for tumors on the boundary between the two types of disease, these cases are considered as atypical MNTs. We recommend the following algorithm to diagnose a micronodular thymic tumor with lymphoid stroma (Figure 5).
Differentiation from type A/AB thymoma: Ten percent of the epithelial tumor cells in type A and all type AB thymomas were arranged in a micronodular pattern[25]. Type AB thymoma is a mixture of types A and B thymomas with a small number of lymphocytes in different proportions. The characteristics that distinguish MNT from them are as follows: (1) T lymphocytes are mainly comprised of mature B and T lymphocytes. The number of TdT-positive cells in T lymphocytes decreases significantly; (2) The epithelial cells are absent in the lymphoid stroma; and (3) Nodular epithelial cells are negative for CD20 staining. The above points are helpful for differential diagnosis[25,26].
Differentiation from thymic carcinoma: For thymus cancer patients, especially lympho-epitheliomatous carcinoma patients, epithelial cancer cells have obvious heteromorphism, and an array of lymphocytes between cancer cells are mainly mature lymphocytes. Immunohistochemical staining showed that CK-5, CD5, and Bcl-2 were positive in epithelial cells, and the positive rate of Epstein-Barr virus (EBV) was 47%. The characteristics that distinguish MNT from thymic cancer are as follows: (1) The epithelium of the tumor is nodular rather than diffuse and has no uniform growth; (2) The stromal lymphocytes are adjacent to the nests of tumor cells, and there is no mixed growth with the tumor cells[2]; and (3) The positive rate of EBV is slightly lower (33.3%).
Differentiation from primary thymic mucosa-associated lymphoid tissue (MALT) lymphoma: Primary thymic MALT lymphoma is a mucosa-associated B-cell lymphoma located in the extranodal marginal zone. It is mainly comprised of centroid or monocyte-like small B cells, and residual thymic bodies can be detected. However, no nodular arrangements of tumor thymic epithelial components are found. MALT lymphoma can be identified by immunohistochemistry and detected by Ig gene rearrangement.
Differentiation from mediastinal lymph node metastasis or metastatic sarcoma: Because the mediastinal lymph nodes are mostly located in the middle mediastinum, metastatic tumors are also the most common in the middle mediastinum, and residual lymph node components are often observed in the peri-tumorous regions[27]. Therefore, to better diagnose the disease, the patient’s medical history must be reviewed, and whether a concomitant tumor can be found in other parts of the patient should be examined.
Micronodular thymic tumor with lymphoid stroma is a rare type of tumor. Morphologically, it contains a series of benign to malignant pedigrees. Its biological characteristics are relatively inert. The diagnosis algorithm established by us can be used to make the differential diagnosis between benign and malignant tumors and has appreciated guiding significance for prognosis, but the clinical treatment should not be radical.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhou M S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Suster S, Moran CA. Micronodular thymoma with lymphoid B-cell hyperplasia: clinicopathologic and immunohistochemical study of eighteen cases of a distinctive morphologic variant of thymic epithelial neoplasm. Am J Surg Pathol. 1999;23:955-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Weissferdt A, Moran CA. Micronodular thymic carcinoma with lymphoid hyperplasia: a clinicopathological and immunohistochemical study of five cases. Mod Pathol. 2012;25:993-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Lun X, Wells JC, Grinshtein N, King JC, Hao X, Dang NH, Wang X, Aman A, Uehling D, Datti A, Wrana JL, Easaw JC, Luchman A, Weiss S, Cairncross JG, Kaplan DR, Robbins SM, Senger DL. Disulfiram when Combined with Copper Enhances the Therapeutic Effects of Temozolomide for the Treatment of Glioblastoma. Clin Cancer Res. 2016;22:3860-3875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 4. | Tateyama H, Saito Y, Fujii Y, Okumura M, Nakamura K, Tada H, Yasumitsu T, Eimoto T. The spectrum of micronodular thymic epithelial tumours with lymphoid B-cell hyperplasia. Histopathology. 2001;38:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO Classification of Tumours of Lung, Pleura, Thymus and Heart. 4th ed. Lyon: International Agency for Research on Cancer (IARC). 2015;406-416. |
| 6. | Detterbeck FC, Nicholson AG, Kondo K, Van Schil P, Moran C. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol. 2011;6:S1710-S1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 7. | Wang B, Liu HY, Zhang HL, Wang XH, Da JP. Clinicopathological observation of micronodular thymic epithelial tumors with lymphoid stroma. Shoudu Yike Daxue Xuebao. 2018;39: 433-438. [DOI] [Full Text] |
| 8. | Wang XY, Xu M, Shi LG, Ding YZ, Cheng Q, Zhao YW. [Clinicopathologic analysis of micronodular thymoma with lymphoid stroma]. Zhonghua Bing Li Xue Za Zhi. 2017;46:837-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Weissferdt A, Moran CA. Immunohistochemistry in the diagnosis of thymic epithelial neoplasms. Appl Immunohistochem Mol Morphol. 2014;22:479-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Nakagawa K, Matsuno Y, Kunitoh H, Maeshima A, Asamura H, Tsuchiya R. Immunohistochemical KIT (CD117) expression in thymic epithelial tumors. Chest. 2005;128:140-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Tateyama H, Eimoto T, Tada T, Hattori H, Murase T, Takino H. Immunoreactivity of a new CD5 antibody with normal epithelium and malignant tumors including thymic carcinoma. Am J Clin Pathol. 1999;111:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Weissferdt A, Moran CA. Thymic carcinoma, part 1: a clinicopathologic and immunohistochemical study of 65 cases. Am J Clin Pathol. 2012;138:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Weissferdt A, Hernandez JC, Kalhor N, Moran CA. Spindle cell thymomas: an immunohistochemical study of 30 cases. Appl Immunohistochem Mol Morphol. 2011;19:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Engel P, Francis D, Graem N. Expression of bcl-2 in fetal thymus, thymomas and thymic carcinomas. Association with p53 expression and review of the literature. APMIS. 1998;106:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Van Kolen K, Pierrache L, Heyman S, Pauwels P, Van Schil P. Prognostic factors and genetic markers in thymoma. Thorac Cancer. 2010;1:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Mineo TC, Ambrogi V, Mineo D, Baldi A. Long-term disease-free survival of patients with radically resected thymomas: relevance of cell-cycle protein expression. Cancer. 2005;104:2063-2071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Roden AC, Yi ES, Jenkins SM, Edwards KK, Donovan JL, Cassivi SD, Marks RS, Garces YI, Aubry MC. Modified Masaoka stage and size are independent prognostic predictors in thymoma and modified Masaoka stage is superior to histopathologic classifications. J Thorac Oncol. 2015;10:691-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Detterbeck FC, Stratton K, Giroux D, Asamura H, Crowley J, Falkson C, Filosso PL, Frazier AA, Giaccone G, Huang J, Kim J, Kondo K, Lucchi M, Marino M, Marom EM, Nicholson AG, Okumura M, Ruffini E, Van Schil P; Staging and Prognostic Factors Committee; Members of the Advisory Boards; Participating Institutions of the Thymic Domain. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol. 2014;9:S65-S72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 295] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 19. | Detterbeck FC, Marom E. Thymus. In: Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. AJCC Cancer Staging Manual. 8th ed. Springer 2017; 423-429. [DOI] [Full Text] |
| 20. | Nicholson AG, Detterbeck F, Marx A, Roden AC, Marchevsky AM, Mukai K, Chen G, Marino M, den Bakker MA, Yang WI, Judge M, Hirschowitz L. Dataset for reporting of thymic epithelial tumours: recommendations from the International Collaboration on Cancer Reporting (ICCR). Histopathology. 2017;70:522-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Fang W, Fu J, Shen Y, Wei Y, Tan L, Zhang P, Han Y, Chen C, Zhang R, Li Y, Chen K, Chen H, Liu Y, Cui Y, Wang Y, Pang L, Yu Z, Zhou X, Liu Y, Chen G; Members of the Chinese Alliance for Research in Thymomas. Management of thymic tumors-consensus based on the Chinese Alliance for Research in Thymomas Multi-institutional retrospective studies. J Thorac Dis. 2016;8:641-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Wright CD. Management of thymomas. Crit Rev Oncol Hematol. 2008;65:109-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Ahmad U, Yao X, Detterbeck F, Huang J, Antonicelli A, Filosso PL, Ruffini E, Travis W, Jones DR, Zhan Y, Lucchi M, Rimner A. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg. 2015;149:95-100, 101.e1-101.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 24. | El MF, Braham E, Ayadi A, Ismail O, Kilani T. Micronodular thymoma with lymphoid stroma: report of two cases and particular association with thymic lymphoid hyperplasia in one case. Pathology. 2006;38:586-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Thomas De Montpréville V, Zemoura L, Dulmet E. [Thymoma with epithelial micronodules and lymphoid hyperplasia: six cases of a rare and equivocal subtype]. Ann Pathol. 2002;22:177-182. [PubMed] |
| 26. | Qu L, Xiong Y, Yao Q, Zhang B, Li T. Micronodular thymoma with lymphoid stroma: Two cases, one in a multilocular thymic cyst, and literature review. Thorac Cancer. 2017;8:734-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Janossy G, Bofill M, Trejdosiewicz LK, Willcox HN, Chilosi M. Cellular differentiation of lymphoid subpopulations and their microenvironments in the human thymus. Curr Top Pathol. 1986;75:89-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |